Fluorescent Pore-Former Suggests an Approach for the Enhancement of Antibiotic Efficacy
Received: 03-Sep-2013 / Accepted Date: 27-Sep-2013 / Published Date: 08-Oct-2013 DOI: 10.4172/2090-4967.1000103
Abstract
A hydraphile channel having fluorescent dansyl side chains inserts into bacterial bilayers. The combination of nontoxic concentrations of hydraphiles and erythromycin enhances the efficacy of this antibiotic against Gram negative E. coli and P. aeruginosa.
Keywords: Amphiphile; Antibiotic; Bacteria; Erythromycin; Hydraphile; Synthetic channel
Introduction
It is remarkable to think that barely two decades ago, the possibility that a synthetic amphiphile could function as an ion channel was largely dismissed by many in the scientific community. It was thought that channel function required the exquisitely complex structure and organization of the integral membrane proteins that served this function in vital systems. It is certainly true that modern proteins are remarkable for their selectivity and efficiency as ion channels [1]. Still, it must not always have been so. There is no fossil record of the earliest ion channels and these must have been far simpler than modern proteins. Indeed, there is no evidence that the earliest channels were proteins at all. One possibility is that they were amphiphiles that caused defects in whatever served as membranes in the earliest organisms.
In fact, the amphiphile and well known detergent Triton X-100 had been shown to exhibit some properties of protein ion channels as early as 1977 [2]. This experiment was rather special, though. It involved the use of a planar bilayer conductance (voltage clamp) apparatus [3]. This device possesses two aqueous chambers separated by a pinhole (typically 200 μm). A bilayer membrane is painted into (over) the hole and no ions can pass between the two solutions. Normally, one inserts a potential channel-forming compound into one of the two buffer solutions, it insinuates itself into the membrane, and electron flow, carried by ions, is detected by using a sensitive ammeter. The Triton experiment referenced above was unusual in that the membrane that was formed in the pinhole was impregnated with the amphiphile. In later studies it was found that Triton X-100 added to a buffer solution did not show pore formation or channel-like activity [4]. Still, skepticism about synthetic amphiphiles forming channels remained high.
Of course, it was not only synthetic channels that were viewed askance by some in the biophysical community. The perspective held by many was the reasonable view that the complex structural elements of sophisticated proteins were required to afford the speed, selectivity, and rectification typical of natural channels. One example was the relatively small protein called IsK or MinK [5]. It is a protein having a little over 100 amino acids. The best known potassium selective channels known at that time had six transmembrane segments and IsK was proposed to have only one.
Another finding that aroused initial skepticism was the discovery of a bacterial channel comprised of poly (β-hydroxybutyrate) and calcium phosphate [6]. In this particular case, the classical method of total synthesis was used to confirm the structure. Comparison of the channel function with natural samples proved that this relatively simple oligomer was a true ion channel [7,8]. These channels simply did not fit the emerging understanding of the elaborate proteins that were known to function in higher organisms [9,10]. One may readily speculate that they that the earliest channels were far simpler than today’s complex protein channels. They were probably less effective and less selective, but they must have functioned or the existence of cellular boundary layers–cell walls and bilayer membranes–would have not only protected, but doomed the organism.
So it was with a ground-breaking attempt to prepare a synthetic ion channel by Tabushi and coworkers [11]. This attempt was based on the cyclodextrin scaffold and was conceptualized as a “half-channel” element. Cyclodextrin would serve as the amphiphile head group and hydrocarbon chains would secure the compound in one leaflet of the bilayer. Efficacy was demonstrated by transport of cobalt ion. This assay was used because detection was possible. Unfortunately, the lack of biological relevance of cobalt transport prevented this compound from attracting the attention it deserved.
By the late 1980s, several designs for alkali metal cation transporting structures were brought to reality [12-14]. These three efforts were all based on crown ethers in one way or another. Our own approach used crown ethers as amphiphilic head groups and as an element we referred to as a “central relay.” The point of the latter was to provide a polar element within the channel that would reside at the midplane of the bilayer. Chemical intuition suggested that the jump of an ion across a 30-35 Å nonpolar barrier was energetically unfavorable and could be aided by a “relay” point. It is interesting to note that when the MacKinnon’s Nobel Prize winning solid state structure of the KcsA voltage gated channel appeared [15], such an element was found to exist within that protein.
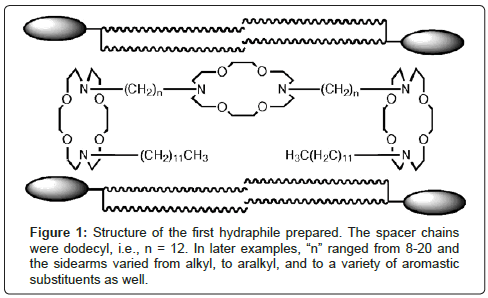
Our design for a hydraphile channel is shown in Figure 1 along with a stylized representation of phospholipid monomers. It is illustrated in the conformation that was deduced from numerous biophysical experiments including fluorescence [16] and planar bilayer conductance studies [17]. Much of the work that was done to characterize the behavior and position within the bilayer of these compounds is summarized in a short review [18].
In the following discussion, various applications of hydraphiles are presented that follow from their function as synthetic ion channels. One important consequence of their structure is that because they are symmetrical, they are non-rectifying. Since they can pass ions in either direction, they have the potential not only to create a pore or opening within a bilayer, they can disrupt the ion balance in a vital organism. Both of these properties have been used to good effect in applications described herein.
Results And Discussion
The first biological studies that we undertook were based on the fact that the hydraphiles inserted into liposomal membranes and functioned as ion channels. As noted above, the fact they these compounds are symmetrical prevents them from being rectifying. Ion concentrations are closely regulated in all vital cells, even in the simplest microbes. We reasoned, and later demonstrated [19], that hydraphiles can insert in microbial membranes [20]. If so, they should disrupt the natural ion balance (ion homeostasis) of these organisms, potentially with toxic effect. We studied Gram negative Escherichia coli, Gram positive Bacillus subtilis, and the primary eukaryote (yeast) Saccharomyces cerevisiae [21,22].
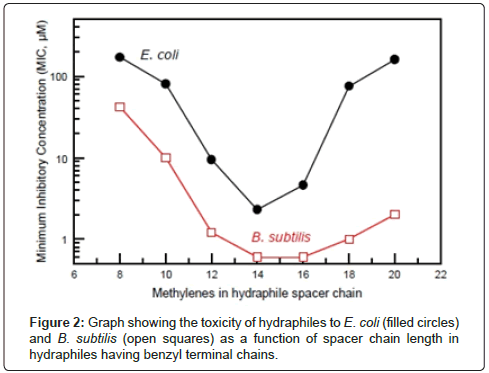
In studies involving release of Na+ ions from phospholipid liposomes, we found that the family of hydraphiles having benzyl terminal groups showed clear length dependence [23]. When the hydraphiles had spacers in the range n=12, 14 or 16 (Figure 1), they were excellent transporters. These same hydraphiles were toxic to E. coli or B. subtilis. When the spacer chains were as short as octylene (n=8 in Figure 1), they did not promote ion release from vesicles nor did they prove to be toxic to bacteria. Figure 2 shows the relationship of hydraphile spacer chain length to the toxicity determined in vitro.
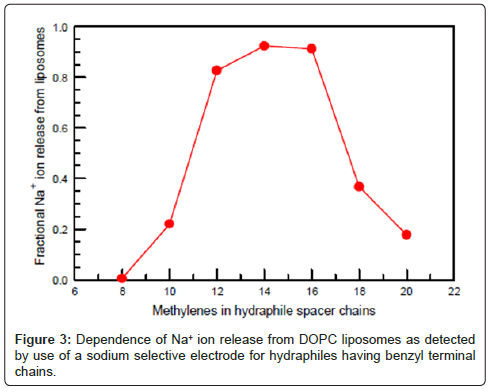
Figure 3 shows the Na+ transport efficacy of hydraphiles as a function of their chain length. The results shown are for dioleoylphosphatidylcholine (DOPC) liposomes. The transport efficiencies of the hydraphiles varied in liposomes having either longer or shorter fatty acid chain lengths. Essentially the same activity profile was obtained when Na+ ion release was studied by 23Na-NMR (23 is superscripted). Na-NMR methods or by using a Na+-selective electrode [23] to detect ion release from vesicles. The graphs of Figures 2 and 3 are essentially complementary: when transport is effective, toxicity is high [28].
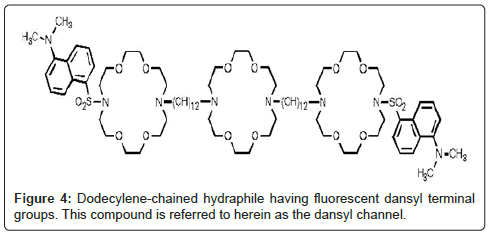
Two experiments were important in confirming that the hydraphiles’ ability to form channels was critical to toxicity. The first was the correlation of transport efficacy with toxicity (Figures 2 and 3). Especially telling was the failure of the hydraphile having octylene (C8) spacer chains either to transport Na+ or to kill bacteria. We inferred that the two were directly related. We prepared a fluorescent hydraphile to use as a biosensor to detect the position of the compound in a vital cell (E. coli). The sensor residue chosen was dimethylaminonaphthylsulfonyl or dansyl. The dansyl groups were attached directly to the hydraphile distal nitrogens as sulfonamides. Transport studies confirmed the activity of this compound, which was similar to the dodecylene (C12) spacer chain analog having benzyl terminal chains (Figure 4).
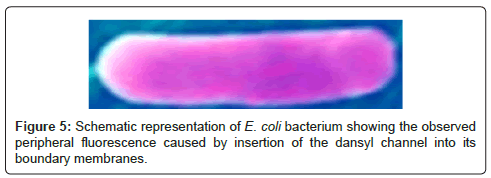
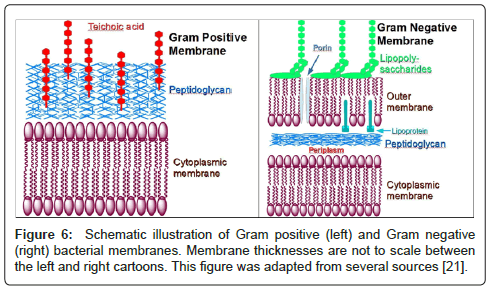
A suspension of E. coli was prepared and the dansyl channel was added to the mixture. E. coli are small (~0.5×2 μm), but they are readily detectable by optical microscopy. The suspension above was examined with a fluorescent microscope and fluorescence was clearly identified in the periphery of the organism and not within it to any significant extent [20]. This is illustrated in Figure 5. The colors in this organism have been adjusted to illustrate the peripheral fluorescence. We hypothesized that the formation of pores in the boundary membranes or permeability barriers of bacteria might foster the transport of substances other than alkali metal cations. We were encouraged in this hypothesis by the fact that hydraphiles of appropriate length are toxic both to Gram positive and Gram negative bacteria. These two types of bacteria have different boundary layers [28]. Bacillus subtilis is a Gram positive bacterium that possesses a rigid external cell wall that helps to maintain its shape. This peptidoglycan layer is essentially a polymeric matrix of amino acids and sugars. E. coli, a Gram negative organism, has a thin peptidoglycan layer along with an outer bilayer. Notwithstanding these differences, hydraphiles must penetrate the permeability barriers and disrupt ion homeostasis.
Figure 6 shows schematic representations of both Gram negative and Gram positive bacterial membranes that highlight the differences. The distinction is made experimentally by the Gram stain but the membranes are functionally different. As seen in Figure 6, a Gram positive bacterium will typically have a cytoplasmic membrane, covered by the rigid peptidoglycan layer, further elaborated by teichoic or lipoteichoic acid. This contrasts with the double membrane structure of Gram negative bacteria which have porins or openings in the outer membrane that permits permeability to species as large as 20 Å.
We had previously shown that hydraphiles are toxic at different levels to both types of bacteria. There seemed to be three likely possibilities. On the one hand, the presence of two toxins–the hydraphile and the antimicrobial agent–should be more effective than either separately. A second possibility is that that the hydraphile’s ability to form a pore, channel, or opening in the bilayer might enhance the passage of an antibiotic through the bilayer, effectively increasing the dose. A third possibility is that the hydraphiles might, by some unknown mechanism, interfere with the efflux pumps [29] that protect bacteria by ejecting foreign substances.
An initial, unsuccessful, attempt was made to assess the feasibility of assisting drug passage through an asolectin membrane. This experiment was attempted by using a planar bilayer conductance apparatus. Although the membrane in this device is characterized as filling a “pinhole,” the orifice is actually 200 μm in diameter. This is many thousands of times larger than even a large protein. Fluorescein was chosen as the “model drug” for enhanced delivery by an amphiphile. Unfortunately, even this highly fluorescent dye proved very difficult to detect at the concentrations typically used in such experiments.
The hydraphiles chosen for initial study were benzyl-terminated compounds that had spacer chains of 12, 14, and/or 16 methylenes. The similarity in their Na+ transport behavior is apparent in Figure 3 (above). The minimum inhibitory concentrations (MICs [30]) against E. coli that were previously determined for hydraphiles having 12, 14, and 16 methylene spacer units were 9.4 μM, 2.3 μM, and 4.6 μM, respectively. A study was done in which E. coli were grown in the absence and presence of C12 benzyl hydraphile. The hydraphile concentration was maintained at 1 μM, which is between about 50% and 10% of the concentration required by any of the hydraphiles to kill the bacterium. Simultaneous experiments were conducted for a duration of 800 min (>13 h). The life cycle of E. coli is generally stated to be approximately 20-25 min depending on conditions [31], so this experiment spanned >30 generations. The growth curves observed were identical within experimental error in the presence or absence of this concentration of hydraphile. Thus, at the half MIC value of 1 μM, E. coli growth was unaffected by the presence of hydraphile.
We studied the effect of erythromycin in combination with hydraphiles in two Gram negative bacteria: E. coli and Pseudomonas aeruginosa. We used the hydraphiles called benzyl-C14 and benzyl-C16, which are pictured in Figure 7, at concentrations of 0.5 μM or 1 μM in concert with erythromycin. Erythromycin is known to be toxic to bacteria by interacting with the ribosomal 50S subunit [32].
The data obtained in this study clearly showed a substantial increase in the activity of erythromycin against both E. coli and P. aeruginosa. These bacteria are Gram negative and although erythromycin is a broad spectrum antibiotic, it is most effective against Gram positive organisms [33]. The poorer efficacy of this drug against Gram negative bacteria makes the enhancement more substantial and therefore more amenable to study. Data for these two bacteria are recorded in Table 1.
| Bacterium | Amphiphile | [Amphiphile] | MICa |
|---|---|---|---|
| E. coli | None | 0 | 200 |
| E. coli | Benzyl-C16 | 1 μM | 25 |
| P. aeruginosa | None | 0 | 200 |
| P. aeruginosa | Benzyl-C14 | 0.5 μM | 50 |
| P. aeruginosa | Benzyl-C14 | 1 μM | 25 |
| P. aeruginosa | Benzyl-C16 | 0.5 μM | 50 |
| P. aeruginosa | Benzyl-C16 | 1 μM | 25 |
Table 1: Effect of added hydraphiles on the antimicrobial activity of erythromycin.
Experiments with other antibiotics and other amphiphiles have shown quite different results. An example is the detergent Triton X-100. As noted above, it shows channel-like behavior when embedded in a membrane but does not function in this way when added to the membrane-bathing suspension . We have repeated the experiments described above and found that Triton X-100 does not enhance antibiotic efficacy against E. coli. This begs the question of what properties amphiphiles, antibiotics, or microbes must possess for the types of activity enhancements we have observed to occur. In studies of other antibiotics and other bacteria, including Gram positive microbes, we have observed enhancements. Experiments are currently underway to elicit the mechanism(s) of this enhancement and in particular to develop new fluorescent probes that may permit us to observe the behavior of the drugs in concert with the amphiphiles.
Conclusion
The dansyl channel biosensor proved to be critical in demonstrating that hydraphiles penetrate the barrier membranes of bacteria. This led to co-administration of antibiotics and non-toxic doses of hydraphiles. Significant enhancements of antimicrobial activity were observed. Three possible mechanisms for this enhanced activity have been considered. First, the hydraphiles may create a pore that facilitates transport into bacteria. Second, the hydraphile’s toxicity to bacteria may be enhanced by the presence of the antibiotic. Third, the hydraphiles may block efflux pumps, making the lower dosages of antibiotics more effective. Evidence beyond the data presented here shows that enhancements occur for at least four different antibiotic structures. Enhancements also occur for Gram positive bacteria, which have very different barrier membrane structures. Finally, not all amphiphiles are effective suggesting that details of the mechanism or mechanisms will emerge in due course.
Acknowledgements
We thank the National Science Foundation for grants (CHE-0957535, CHE- 1307324) that supported this work.
References
- Hille B (2001) Ionic Channels of Excitable Membranes. (3rd edn) Sinauer Associates, Sunderland, MA, pp 814
- Schlieper P, De Robertis E (1977) Triton X-100 as a channel-forming substance in artificial lipid bilayer membranes. Arch. Biochem. Biophys 184: 204-208
- SakmannB, Neher E (1995) Single-channel Recording. Kluwer Academic Publishers, New York, pp700.
- Rostovtseva TK, Bashford CL, LevAA, Pasternak CA(1994) Triton channels are sensitive to divalent cations and protons.J. Membr. Biol 141: 83-90.
- Folander K, Smith JS, Antanavage J, BennettC and SteinRB, et al. (1990) Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci USA 87: 2975-2979.
- Reusch RN, Sadoff HL (1988) Putative structure and functions of a poly-ß-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc. Natl. Acad. Sci. 85: 4176-4180.
- Seebach D, Brunner A, Buerger HM, Reusch RN, Bramble L L(1996) Channel-forming activity of 3-hydroxybutanoic acid oligomers in planar lipid bilayers. Helv. Chim. Acta 79: 507-517.
- Das S, Lengweiler UD, Seebach D, Reusch R N (1997) Proof for a nonproteinaceeous calcium-selective channel in Escherichia coli by total synthesis for (R)-3-hydroxybutanoic acid and inorganic polyphosphate. Proc. Nat. Acad. Sci. 94: 9075-9079.
- Stein WD (1990) Channels, Carriers, and Pumps. Academic Press: New York, pp 344.
- Hille B (2001) Ionic Channels of Excitable Membranes. (3rd Edn). Sinauer Associates: Sunderland, MA, 814.
- Tabushi I, Kuroda Y, Yokota K (1982) A,B,D,F-Tetrasubstituted beta-cyclodextrin as artificial channel compound; Tetrahedron Lett. 4601-4604.
- Jullien L, Lehn JM (1988) The 'chundle' approach to molecular channels. Synthesis of a macrocycle-based molecular bundle; Tetrahedron Lett. 29: 3803-3806.
- Carmichael VE, Dutton PJ, Fyles T M, James TD, Swan JA, Zojaji M (1989) Biomimetic ion transport: a functional model of a unimolecular ion channel. J. Am. Chem. Soc 111: 767-769.
- Nakano A, XieQ, Mallen JV, Echegoyen L, Gokel, GW (1990) Synthesis of a membrane-insertable, sodium cation conducting channel: kinetic analysis by dynamic sodium-23 NMR. J. Am. Chem. Soc.112:1287-1289
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A and Gulbis JM, et al.(1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69-77.
- Abel E, Maguire GE M, Murillo O, Suzuki I, Gokel GW (1999) Hydraphile channels: Structural and fluorescent probes of position and function in a phospholipid bilayer. J. Am. Chem. Soc 121: 9043-9052.
- Murray CL, Meadows ES, Murillo O, Gokel GW (1997) Fluorobenzyl terminal residues as probes of function in tris(macrocycle) channels: evidence from NMR and planar bilayer conductance studies.J. Am. Chem. Soc 119:7887-7888.
- Gokel GW (2000) Hydraphiles: Design, Synthesis, and Analysis of a Family of Synthetic, Cation-Conducting Channels. Chem. Commun 1-9.
- Leevy WM, Huettner JE,Pajewski R, Schlesinger PH, Gokel GW (2004) Synthetic Ion Channel Activity Documented by Electrophysiological Methods in Living Cells. J. Am. Chem. Soc 126: 15747-15753.
- Leevy WM, Donato GM, Ferdani R, Goldman WE and Schlesinger PH, et al. (2002) Synthetic hydraphile channels of appropriate length kill Escherichia coli. J. Am. Chem. Soc 124: 9022-9023.
- Leevy WM, Gokel MR, Hughes-Strange G,Schlesinger PH, Gokel GW(2005) Structure and Medium Effects on Hydraphile Synthetic Ion Channel Toxicity to the Bacterium E. coli. New J. Chem 29: 205-209.
- Leevy WM, Gammon ST, Levchenko T,Daranciang DD and Murillo O, et al (2005) Structure-Activity Relationships, Kinetics, Selectivity, and Mechanistic Studies of Synthetic Hydraphile Channels in Bacterial and Mammalian Cells.Org. Biomol. Chem 3: 3544-3550.
- Weber ME, Schlesinger PH, Gokel GW (2005) Dynamic Assessment of Bilayer Thickness by Varying Phospholipid and Hydraphile Synthetic Channel Chain Lengths. J. Am. Chem. Soc 126: 636-642.
- Alberts B, Bray D, Lewin J, Raff M, Roberts K, Watson JD (1994) Molecular Biology of the Cell.( 3rd Edn) Garland Publishing, Inc.: New York, pp 503.
- Voet D, Voet JG (1995) Biochemistry. (2nd Edn) John Wiley and Sons, Inc.: New York, pp 269.
- Schaechter M (2004) The Desk Encyclopedia of Microbiology. Elsevier Academic Press, San Diego, pp249.
- Leevy WM, Weber ME, Schlesinger PH, Gokel GW (2005) NMR and ion selective electrode studies of hydraphile channels correlate with biological activity in E. coli and B. subtilis. Chem. Commun 7: 89-91.
- Petty HR ( 1993) Molecular Biology of Membranes: Structure and Function; Plenum Press, New York, 404.
- Pietras Z, Bavro V N, Furnham N,Pellegrini-Calace M and Milner-White E J, et al. (2008) Structure and mechanism of drug efflux machinery in Gram negative bacteria. Curr. Drug Targets 9: 719-728.
- Institute C. a. L. S., M07-A9 (2012) 22 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard 9th Edn.
- Zobell CE, Cobet AB (1962) Growth, Reproduction, And Death Rates Of Escherichia Coli At Increased Hydrostatic Pressures. J. Bacteriol 84: 1228-1236
- Le Goffic F,Capmau M L, Tangy F, Baillarge M (1979) Mechanism of action of aminoglycoside antibiotics. Binding studies of tobramycin and its 6'-N-acetyl derivative to the bacterial ribosome and its subunits. Eur. J. Biochem 102: 73-81
- Gennaro AR, Ed. Remington (2000) The Science and Practice of Pharmacy.(20th edn) Lippincott, Williams, & Wilkins Philadelphia, pp 1535.
Citation: Gokel GW, Curvey NS, Gokel MR, Lindsey Meisel JW, Meisel JL, et al. (2013) Fluorescent Pore-Former Suggests an Approach for the Enhancement of Antibiotic Efficacy. Biosens J 2:103. DOI: 10.4172/2090-4967.1000103
Copyright: © 2013 Gokel GW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 23470
- [From(publication date): 12-2013 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 18633
- PDF downloads: 4837