For an Efficient Integration of Palliative Care in Oncology Treatment Pathways: Guidelines of the French Speaking Association for Supportive Care in Cancer
Received: 24-Aug-2018 / Accepted Date: 17-Sep-2018 / Published Date: 24-Sep-2018 DOI: 10.4172/2165-7386.1000342
Keywords: Guidelines; Algorithms; Early palliative care; Timely integration; Supportive care; Cancer; Multidisciplinary
Introduction
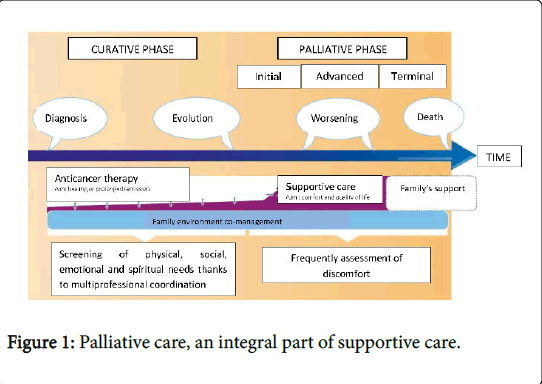
Palliative care is an integral part of supportive care in oncology. However, as current care in oncology evolves and therapeutic advances increase survival, new problems and symptoms are revealed, and the palliative phase extends (Figure 1) [1].
A study by Temel et al. [2] proved that an early integration of palliative care in lung cancer patients (non-operable and small cell) increased survival, improved quality of life and decreased anxiety and depression. Furthermore, the American Society of Clinical Oncology Smith et al. [3] drafted guidelines for the early integration of palliative care in oncology.
The National Comprehensive Cancer Network made the same recommendations Levy et al. [4]. The French group of experts agrees with the idea that the early introduction of palliative care in oncology is of benefit to all incurable patients, their families and healthcare professionals.
Though, these international guidelines are not entirely applicable to the French healthcare system. For example, early palliative and oncological co-management cannot be established at the time of an incurable diagnosis.
Hence, why our group of experts in oncology needed to more precisely define the modes and the time at which palliative care should be integrated in oncology based on using different scores to estimate a patient’s prognosis.
Methods
The development of national guidelines for oncological supportive care by the Association Francophone pour les Soins Oncologiques de Support (AFSOS) is based on inter-regional work carried out by hospital professionals working together within a network.
The French Palliative Care Association (SFAP) certified these recommendations.
The guidelines were drafted by a task force and a multidisciplinary expert panel. The national task force (47 healthcare professionals) and the national review group (24 healthcare professionals) were led by two scientific coordinators: A palliative care specialist and a psychologist who had methodological and administrative support.
The healthcare professionals involved included doctors (medical oncology, surgical oncology, anesthesia-resuscitation, palliative medicine, and general practitioners), psychologists, dieticians and nurses.
The guidelines were developed in four stages:
• An analysis and summary of the data in the literature was performed so as to draft the guidelines.
• The task force developed the guidelines by holding teleconference meetings and by exchanging information and ideas by electronic correspondence. Drafting of the guidelines was based on concise documents and straightforward and pragmatic flowcharts. A list of the most important bibliographic references was compiled.
• The guidelines produced by the task force were then presented, modified and subjected to an external validation process by a review panel comprised of a broader selection of professionals, as part of the AFSOS National Guidelines-Network Day (N2D) in cancer supportive care.
• The version of the guidelines validated during the workshop was finalized and formatted. The guidelines were made available in a downloadable and printable form through the AFSOS and regional oncology network’s webpages.
The guidelines for supportive care in cancer are not actionable from a regulatory point of view as a standard for appropriate care, and they do not take into account the individualized nature of treatment required for each patient.
With the fast pace of scientific knowledge, new scientific data may become publically available between the development of these guidelines and their publication. None of the members of the task force have any conflict of interest, such as employment relationships, consulting arrangements, stock ownership, honoraria, research funding and expert testimony in regard to this matter.
This experts’ agreement is based on a review of the literature which was carried out using PubMed. Retrieved articles were checked for additional references and a systematic review was undertaken, supplementary searches for secondary publications were also conducted.
Studies were included in the review of the literature if they contained the following key terms: early palliative care; quality of life; palliative care/models and administration; palliative care/ organization and methods; neoplasm/therapy; patient care team; end-of-life medical decisions; end-of-life care; palliative care access. Articles in a language other than English or French were excluded.
These guidelines are scheduled to be updated at least every three years.
Results
The multidisciplinary group found that, based on the literature review, a delayed intervention of palliative care can increase the risk of unreasonable obstinacy, and decrease survival and quality of life Temel et al. [2], Levy et al. [4], Earle et al. [5], Zimmerman et al. [6], Bakitas et al. [7].
In order to limit these risks, physicians need to:
•Conduct a clinical assessment.
•Perform an early assessment of prognosis.
•Respect the patient’s and family’s wishes including writing advanced decision
•Elaborate a quality of life project in a multi-professional and multidisciplinary team.
•Adjust decisions to the patient’s needs.
There are economic reasons, poor resources or inappropriate models that can slow down early palliative care intervention. Multiple assessment tools exist with a common goal: To estimate a patient’s prognosis and to administer adequate therapy.
The Karnofsky performance status (KPS) was developed by A. Karnofsky and Joseph H. Burchenal in 1949 [8]. Based on three items (displacement/ bed rest, activity level and care), it is considered as a reference for the overall assessment of a sick person Stenley [9]. It is quoted from 0 to 100%.
Scores that can be used are:
•The performance status or Karnofsky’s index,
•The Barbot score, which is French score (Table 1),
| BARBOT’s score or POITIER’s score | Partials scores | Prognostic profiles=Partial scores +3 |
|---|---|---|
| Karnofsky’s performance scale or Performans status (WHO) >60% 0-1 40 to 60% 2-3 <40% 4 |
0 2 4 |
Group A : overall score 0 to 3 Good prognosis Survival at 2 months=92% Group B : score total 4 to 7 Intermediate prognosis Survival at 2 months=43% Group C : score total 8 to 10 Poor prognosis Survival at 2 months=8.3% |
| Number of metastatic sites 0 or 1 ≥ 2 |
0 2 |
|
| Serum albumin (g/L) ≥ 33 24 to 33 <24 |
0 0 |
|
| LDH concentration (UI/L) <600 ≥ 600 |
0 1 |
Table 1: The Barbot’s score or Poitier’s score.
• The palliative prognostic score (Table 2).
| The Palliative Prognostic (Pap) score | Partials scores | Prognostic profiles |
|---|---|---|
| Anorexia | ||
| Yes | 1,5 | |
| No | 0 | |
| Dyspnea | ||
| Yes | 1 | |
| No | 0 | |
| Karnofsky’s performance scale | ||
| ≤ 20 % | 2,5 | |
| >20% | 0 | |
| Total WBC (cell/mm3) | ||
| ≤ 8.000 or 8.500 | 0 | |
| 8.000-11.500 or 8.500-11.000 | 0,5 | |
| >11.000 or 11.500 | 1,5 | |
| Lymphocytes percentage | ||
| ≥ 20% | 0 | |
| 12 to 20% | 1 | |
| <12 | 2,5 | |
| Clinical prediction of survival | ||
| ≤ 2 weeks | 8,5 | |
| 3-4 weeks | 6,5 | |
| 5-6 weeks | 4,5 | |
| 7-10 week s | 2,5 | |
| 11-12 weeks | 1,5 | |
| ≥ 12 weeks | 0 | |
Table 2: The palliative prognosis score (PaP score).
| Pallia 10 scale | Questions | Object | Yes/No |
|---|---|---|---|
| 1 | Patients with an incurable disease | A positive answer to his question=use pallia 10 scale | |
| 2 | There are pejorative prognostic factors | Low albuminemia, lymphopenia, Performance Status>3, Inflammatory syndrome | |
| 3 | The disease is quickly progressive | ||
| 4 | Patients or families who request palliative care and accompanying measures | Law n° 99-477/09.06.1999 | |
| 5 | There are uncontrolled symptoms | Pain, dyspnea, vomiting, confusion, occlusive syndrome | |
| 6 | Log factors of psychic vulnerabilities for the patient and/ or his entourage | Sadness, anxiety, behavioral disorders, communication disorders... | |
| 7 | Log factors of social vulnerabilities for the patient and/or his entourage | Remoteness, financial difficulties, young children... | |
| 8 | The patient or his entourage have difficulty integrating information on the disease and / or prognosis | Defensive mechanisms? | |
| 9 | You find any questions and / or differences within the team about the care plan | About: advanced prescriptions therapeutic indications palliative sedation place of care critical status |
|
| 10 | Treatment refusal by a patient? Treatment is discontinued or limited? Request for euthanasia? Presence of a conflict of values? |
Leonetti's Law | |
| NOTE: Beyond 3 positives responses, the use of a palliative care team must be considered. | |||
Table 3: The French “Pallia 10” scale.
• The French “pallia 10” questionnaire (Table 3).
The Eastern Cooperative Oncology Group score (ECOG score) or World Health Organization (WHO) score: Developed by Oken and his team in 1982, is rated from 0 (full health) to 5 (dead) Oken et al. [10]. It assesses a patient's general condition and level of autonomy.
The lower the KPS and the higher the ECOG score, the lower the health condition of the patient and the probability of survival are reduced.
For example, a study by Hwang et al. [11] of 429 patients with metastatic cancer showed that a KPS score below 50% led to a median survival of 29 days, whereas a score above 50% led to a median survival of 146 days.
The Barbot score was created by two French teams in Niort and Poitiers in 2008, based on two clinical items (KPS and the number of metastatic sites) and two biological items (albuminemia and lactate dehydrogenase (LDH) level) Barbot et al. (Table 1) [12].
The palliative prognostic score (PaP score) was defined by an Italian team Pironvano et al. [13], and takes into account several items. The items are both clinical (dyspnea, anorexia and KPS) and biological (total number of white blood cells (WBC) and percentage of lymphocytes), and it also includes a clinical prediction of survival (Table 2).
These two scores classify patients into three survival groups: Barbot et al. [12], Maltoni et al. [14], Glare et al. [15] good, intermediate, and poor prognosis.
The European Association of Palliative Care suggests that these scores should be monitored at 15-day intervals, to determine how the cancer is progressing.
The Pallia 10 questionnaire, created in 2010, is a decision-making tool to identify when the use of a palliative care team: it is used to identify the medico-psycho-social criteria of fragility (Table 3) [16].
It is based on 10 questions. Beyond three positive answers, the use of a palliative care team should be considered.
The Prepa 10 study Chvetzoff et al. [17] conducted by the Unicancer – French palliative care workgroup will be published soon, and shows that a score of three from the Pallia 10 questionnaire is probably not sufficiently discriminating but the questionnaire does have clear prognostic value.
However, these tools may have limits. Factors specific to the practitioner and the patient have a role to play. Therefore, an additional decision-making tool such as experts’ guidelines (AFSOS, SFAP, SFETD, SFPO…) is required [18].
Therefore, we have built two accurate and reflexive algorithms to be used, particularly by oncological care specialists to determine the timely integration of a palliative care team.
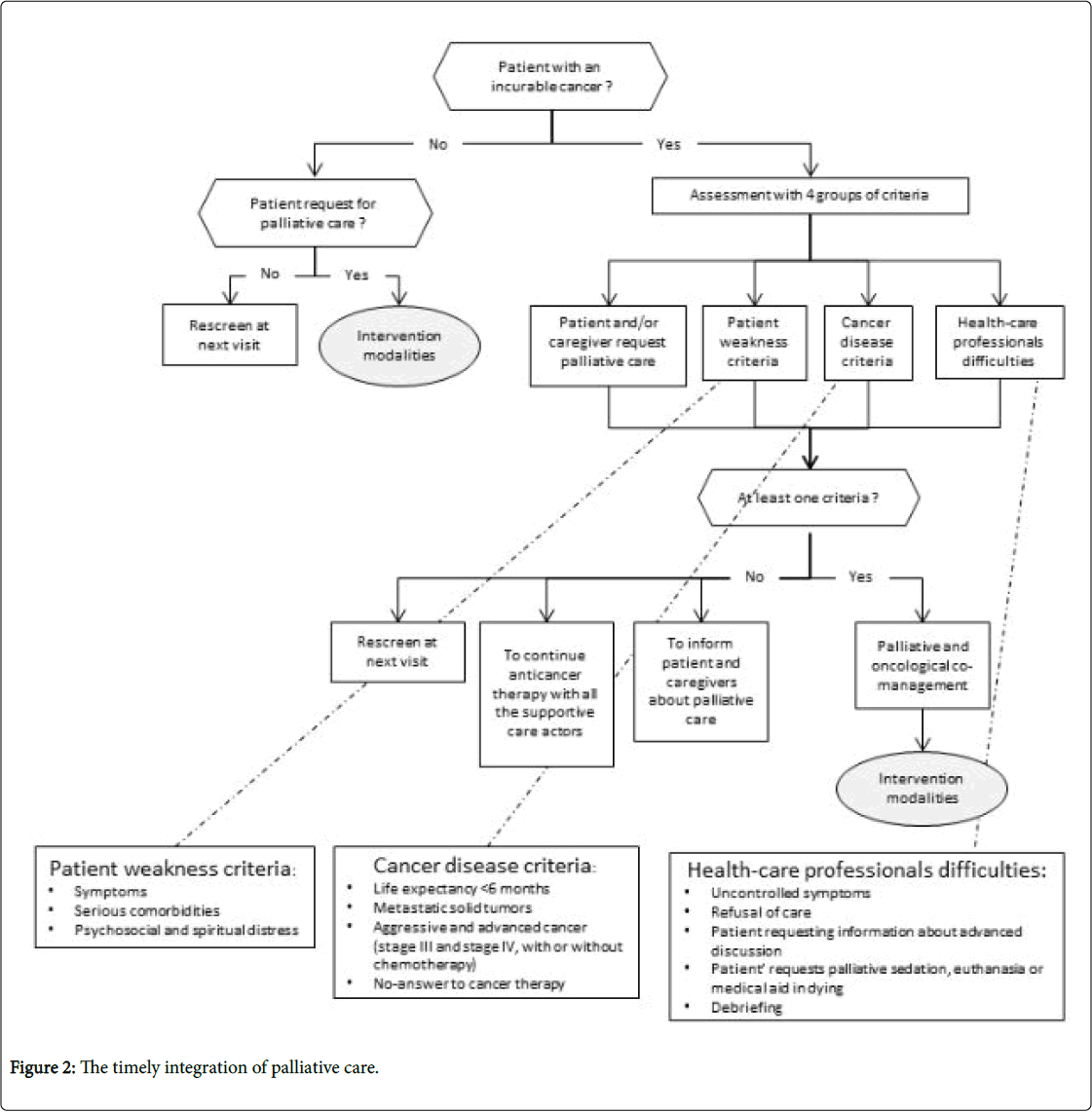
The first algorithm lists the criteria required for the concomitant integration of palliative care and oncological care. It aims to establish the timely integration of palliative care in oncology (Figure 2).
When caring for a patient with an incurable disease, we identified four groups of criteria for the integration of palliative care:
First group: Patient weakness criteria
• Aggressive symptoms.
• Serious comorbidities.
• Psychosocial and spiritual distress.
Second group: Cancer disease criteria
• Life expectancy of less than six months.
• Metastatic solid tumors.
• Aggressive and advanced cancer (Stage III and Stage IV, with or without palliative chemotherapy).
• The patient does not respond to anticancer therapy.
Third group
When the patient and/or their family request palliative care
Fourth group
When healthcare professionals experience are put in difficult situations
• For example a patient who has uncontrolled symptoms, or who refuses care, nursing etc.
• When the patient requests palliative sedation or euthanasia, or medical aid in dying.
• When the healthcare team requires a debriefing.
• If no palliative care intervention criterion is identified, reassessment of the medical situation is recommended.
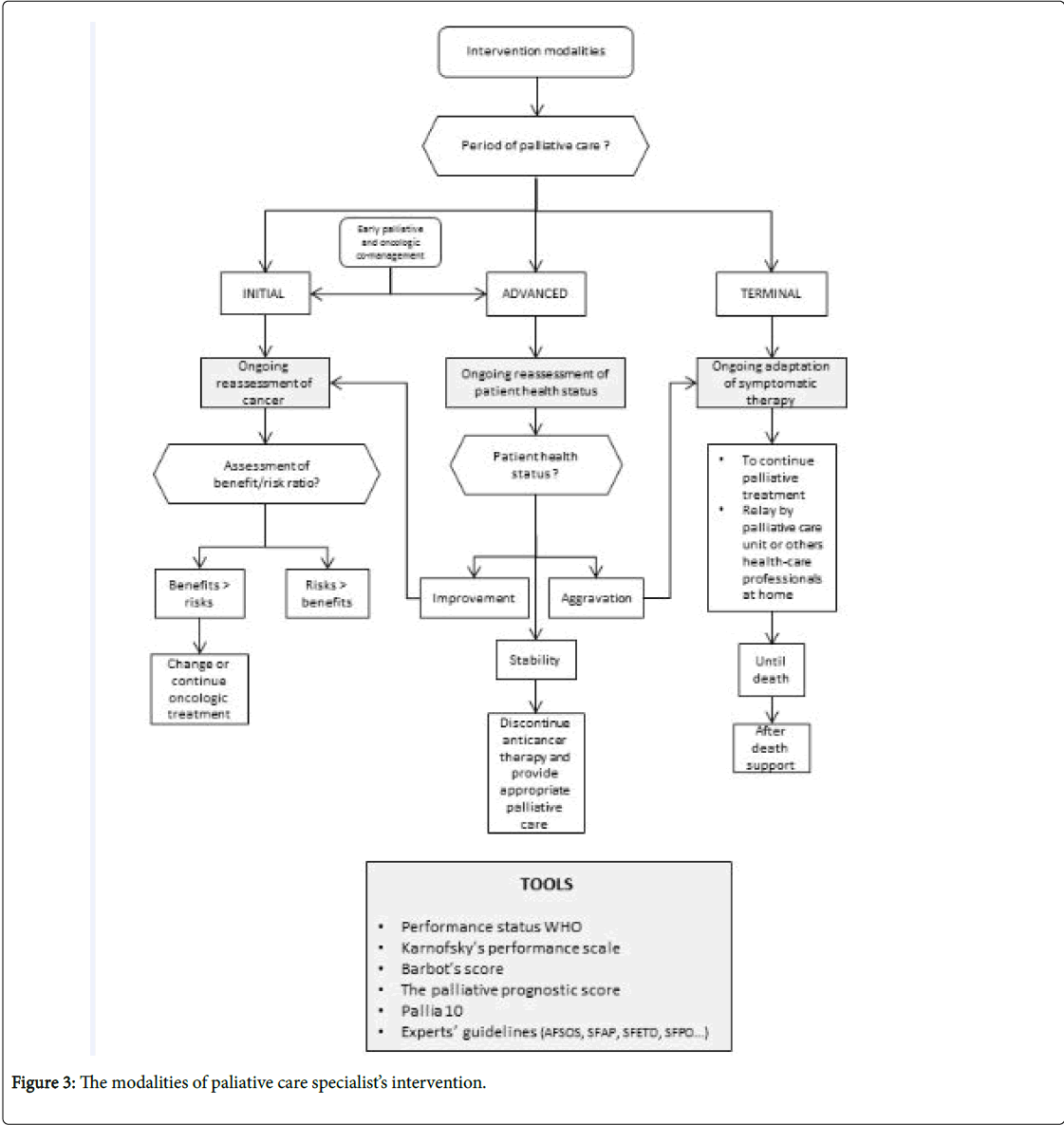
• The second algorithm describes how a palliative care team intervenes (Figure 3).
An essential prerequisite is knowledge of the treatment objectives of the various stages of care.
In the curative phase, recovery from cancer is possible. The objective is to cure, and provide symptomatic relief. Supportive care has a place in the management of pain, psychological, social and nutritional needs.
In the palliative phase, the cancer is considered impossible to cure. It can be divided into three steps:
• The initial palliative stage: Is often not very symptomatic. The prognosis can be counted in months or years. Specific treatments (such as chemotherapy) and supportive care (bisphosphonates, nutritional and psychological, and management of side effects, etc.) are used. The objective is to control symptoms and therefore improve the quality of life and/ or increase survival time.
• The advanced palliative stage: Is often more symptomatic. The prognosis can be counted in weeks or months. The objective is always to relieve symptoms (analgesics, vertebroplasty, radiotherapy with analgesic or hemostatic aim, digestive stent etc.) and attention is given to improving the quality and comfort of life.
In these stages, the benefit/risk ratio of the continuation of anticancer therapy must be jointly evaluated by oncology and palliative care teams (co-management) aware of the patient’s general condition, and their wishes and feelings.
• In the terminal palliative stage: Death is inevitable and imminent (days or weeks). The objective is only to relieve the patient's symptoms and to support caregivers. The palliative care teams will manage the patient in hospital (in palliative care units, in identified palliative care beds, in medical services) or at home (home visits from physicians and nurses, home hospitalization, oncological networks, geriatric retirement homes) until their death and will be able to support caregivers after the death.
Discussion
To determine the timely integration of palliative care in oncology physicians have to use these algorithms without forgetting requirements:
• That the patient remains informed throughout their cancer treatment (Golwasser et al. [19]).
This should occur as soon as the patient’s prognosis is determined, therefore therapeutic objectives can be clarified, and the patient and their oncologist can establish realistic objectives. The patient may adjust and adapt to realities, including some self-protection stages or defense mechanisms.
The notion of uncertainty can help the patient and his family to build a new life plan for the future and help caregivers to set a healthcare project.
As much as possible, try to discuss the inefficacy of anticancer therapy while respecting the spiritual progression of the patient.
• To work and collaborate with caregivers at home (Golwasser et al. [19]) (general practitioners, nurses etc.).
The referring physician has a better knowledge of the patient and their family environment. Objectives of the collaboration are:
• To ensure continuity of care and to establish, and evolve, in a team, a common project.
• To allow for advanced directives Wright et al. [20], Pennec et al. [21].
• To identify palliative care staff both in and outside of the hospital.
• To respect the patient's end-of-life choices.
To deliberate and form a decision in a multidisciplinary setting Goldwasser et al. [19].
Interdisciplinary deliberations such as onco-palliative multidisciplinary meetings or team meetings, are intended to Colombet et al. [22,23]:
• Overcome the emotional burden, often predominantly on the referring physician, and to consider the best treatment option.
• Exchange unknown information. It is the concept of shared medical confidentially.
• Repeat over time until the "timely integration of palliative care".
• Decide the best theoretical treatment.
Indeed, this interdisciplinarity is essential to:
• Evaluate and fully understand a patient’s complexity.
• Know, accept and respect a patient’s ambivalence.
• Promote working relationships between professionals in different medical fields [24].
When announcing the end of anticancer therapy (Goldwasser et al. [19]).
It is advisable that the end of anticancer therapy is announced by the oncologist during co-consultation with palliative care specialists to allow a relay of care.
It is essential to invest in other lines of care: Treatment of symptoms, nutrition, psychological care etc.
The end of anticancer therapy is not incompatible with clinical improvement, and it does not always mean that death is imminent.
Private practice physicians and paramedical professionals must be informed of the decision.
Conclusion
These two algorithms are intended to be used practically, especially, by oncological care specialists to more precisely define the time at which palliative care teams should intervene. Nurses, social workers, spiritual care providers or other advance practice providers are in a position to identify patients needing palliative care support. These algorithms can be an additional tool to detect these patients.
An early integration is only possible with the contribution of all involved (the patient, their family, caregivers, palliative care specialists and oncologists, but also nurses, social workers, psychologists, ethicists, other health care professionals, chaplains…).
This manuscript gives back the guidelines of the French speaking association for supportive care in cancer, accomplished in 2016. These guidelines will be updated in future.
We will think about integrating a fifth group of criteria. It will define distress screening in cancer population to identify those patients at risk for distress and needing support (NCCN guidelines for distress management [25]).
We will develop the patient weakness criteria, based on National Comprehensive Cancer Network palliative care guidelines, established in 2018 (NCCN palliative care guidelines [26]).
Finally, we will add tools like CARING criteria Fischer et al. [27], “the surprise question” Weissman et al. [28] and Palliative Performance Scale [29]).
Acknowledgement
AFSOS group that participated in the development of these guidelines.
Funding
None declared.
Disclosure
The authors have declared no conflict of interest.
References
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, et al. (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363: 733-741.
- Smith TJ, Temin S, Alesi ER Abernethy AP, Balboni TA, et al. (2012) American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol 30: 880-887.
- Levy MH, Smith T, Alvarez-Perez A Back A, Baker JN, et al. (2014) Palliative care, Version 1.2014. Featured updates to the NCCN Guidelines. J Natl Compr Cancer Netw 12: 1379-1388.
- Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, et al. (2008) Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 26: 3860.
- Zimmermann C, Swani N, Kryzanowska M, Hannon B, Leighl N, et al. (2014) Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. The Lancet 383: 1721-1730.
- Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, et al. (2015) Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J of Clin Oncol 33: 1438-1445.
- Karnofsky DA, Burchenal JH (1949) The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM (Ed), Evaluation of Chemotherapeutic Agents. Columbia University Press. p: 196.
- Stenley KE (1980) Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst 65: 25-32.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649-655.
- Hwang SS, Scott CB, Chang VT, Cogswell J, Srinivas S, et al. (2004) Prediction of survival for advanced cancer patients by recursive partitioning analysis: Role of Karnofsky performance status, quality of life, and symptom distress. Cancer invest 22: 678-687.
- Barbot AC, Mussault P, Ingrand P, Tourani JM (2008) Assessing 2-month clinical prognosis in hospitalized patients with advanced solid tumors. J Clin Oncol 26: 2538-2543.
- Pironvano M, Maltoni M, Nanni O, Marinari M, Indelli M, et al. (1999) A new Palliative Prognostic Score: A first step for staging of terminally ill cancer patients. J Pain Symptom Manage 17: 231-239.
- Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, et al. (2005) Prognostic factors in advanced cancer patients: Evidence-based- clinical recommendations. A study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol 23:6240-6248.
- Glare PA, Eychmueller S, McMahon P (2004) Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer. J Clin Oncol 22: 4823-4828.
- Société française d'accompagnement et de soins palliatifs. Guide "pallia 10 : Quand faire appel à une équipe de soins palliatifs". Paris: SFAP; 2010.
- Chvetzoff G, Molin Y, Gallay C PALLIA-10, a reliable tool to identify patients requiring palliative cares in comprehensive cancer centers: A prospective multicentric study (PREPA-10).Cancer
- Goldwasser F, Vinant P. Soins Palliatifs en cancérologie. DUNOD, Manuel de Soins Palliatifs 2014 ; 399-412.
- Wright AA, Zhang B, Ray A, Mack JW, Trice E, et al. (2008) Association between end-of- life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 300: 1665-1673.
- Pennec S, Monnier A, Pontone S, Aubry R (2012)End of life medical decisions in France: A death certificate follow-up survey 5 years after the 2005 Act of Parliament on Patients’ Rights and End of Life. BMC Palliat Care 11: 25.
- Colombet I, Montheil V, Durand JP, Gillaizeau F, Niarra R, et al. (2012) Effect of integrated palliative care on the quality of end of life care: Retrospective analysis of 521 cancers patients. BMJ Support Palliat Care 2: 239-247.
- Formarier M (2004) La place de l’interdisciplinarité dans les soins. Recherche en soins infirmiers N 79: 4-11.
- National Comprehensive Cancer Network (NCCN). NCCN guidelines for distress management version 2.2018: Distress management.
- National Comprehensive Cancer Network (NCCN). NCCN palliative care guidelines version 1.2018: Palliative care.
- Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, et al. (2006) A practical tool to identify patients who may benefit from a palliative approach: The CARING criteria. J Pain Symptom Manage 31: 285-292.
- Weissman DE, Meier DE (2011) Identifying patients in need of a palliative care assessment in the hospital setting a consensus report from the Center to Advance Palliative Care. J Palliat Med 14: 17-23.
- National Hospice and Palliative Care Organization (2018). Palliative Performance Scale (v2).
- Parcours de soins d’une personne ayant une maladie chronique en phase palliative. Note de cadrage. Haute Autorité de Santé, Mai 2013.
Citation: Henry A, Block V, Salleron J, Adam V (2018) For an Efficient Integration of Palliative Care in Oncology Treatment Pathways: Guidelines of the French Speaking Association for Supportive Care in Cancer. J Palliat Care Med 8: 342. DOI: 10.4172/2165-7386.1000342
Copyright: © 2018 Aline H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4839
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 3943
- PDF downloads: 896