Gaseous Pollutants Formation and Their Harmful Effects on Health and Environment
Received: 09-Dec-2010 / Accepted Date: 24-Jan-2011 / Published Date: 26-Jan-2011
Abstract
Many countries depend on oil for energy uses and development, hence import all of its oil and gas needs. Thereby, consequent harmful effects on the environment, such as global warming, ozone, smog, and acid rain, may result from the gases emanating from fossil fuel combustion. In this work, the scientific reasoning for the gaseous pollutant formation and their harmful effects on human health and environment, including biological effects of thermal pollution relevant to combustion processes, are discussed.
Keywords: Gaseous pollutants; Health and environment; Greenhouse effect; Economics of pollution
19688Introduction
Energy has strong impact on the environment, especially when fossil fuels such as coal, oil, gas, and wood are burned. The products will affect earth, buildings, water, and air. They consequently result in fog, smog, and global warming, which deteriorate vegetation, forests, and even human health. Human beings may be affected in the form of having asthma and even cancer [4,40]. Thousands of people in the world die each year due to heart and lung diseases that result from air pollution [10,30,33].
During the combustion of fossil fuels, which constitute nowadays 85% of the world energy resources, many harmful elements and gases are emitted from the vehicles, furnaces, stoves, and electric power plants [4,9,12,15,39,41].
Usually, the main combustion products of vehicular engines, gas turbines, and steam power plant furnaces are carbon monoxides (CO), unburned hydrocarbon UHC and nitrogen oxides (NOx) [16,20,22,38,46,47], sulfur oxides (SOx), and soot, in addition to volatile organic compounds (VOC) [8,11,14,23,37].
Usually, children are the most susceptible to harmful influences due to their tender tissues and their higher surface/ volume ratio, and the higher relative inhalation rate for healthier growth and buildup [19].
The Greenhouse Effect
Glass allows the solar radiation to enter freely but blocks the infrared radiation emitted by the interior surfaces. This causes a rise in the interior temperature as a result of the energy buildup in the car. This heating effect is known as the greenhouse effect, since it is utilized primarily in greenhouses.
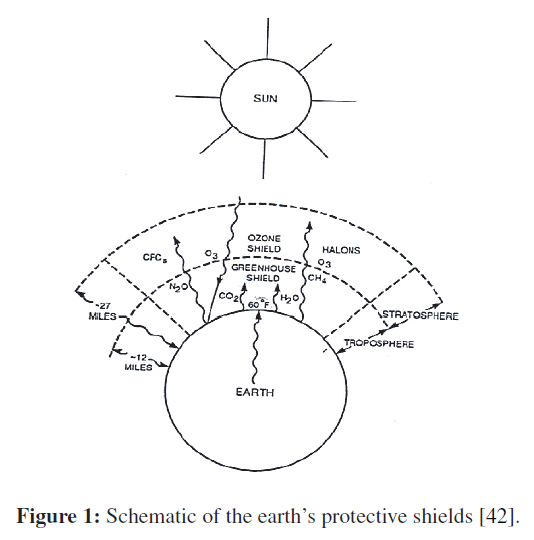
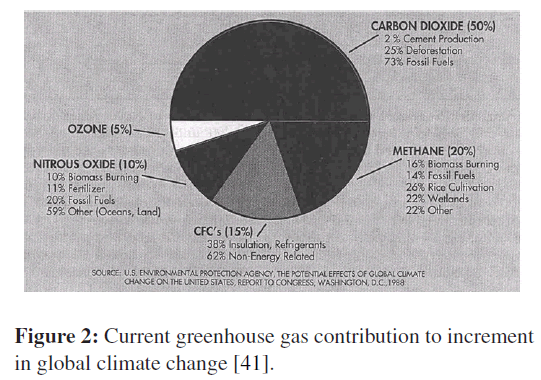
The greenhouse effect is also experienced on a larger scale on earth. The surface of the earth, which warms up during the day as a result of the absorption of solar energy, cools down at night by radiating part of its energy into deep space as infrared radiation. Carbon dioxide (CO2), water vapor, and trace amounts of some other gases such as methane and nitrogen oxides act like a blanket and keep the earth warm at night by blocking the heat radiated from the earth. Therefore, they are called “greenhouse gases,” with CO2 being the primary component (Figures 1 and 2).Water vapor is usually taken out of this list since it comes down as rain or snow as part of the water cycle and man’s activities in producing water. Each liter of gasoline burned produces about 2.5 kg of CO2. An average car is driven about 12,000 miles a year, and it consumes about 600 gallons of gasoline. Therefore, a car emits about 5,600 kg of CO2 to the atmosphere a year, which is about four times the weight of a typical car. This and other emissions can be reduced significantly by buying an energy-efficient car that burns less fuel over the same distance and by driving sensibly. Saving fuel also saves money and the environment. For example, choosing a vehicle that gets 30 rather than 20 miles per gallon will prevent 2 tons of CO2 from being released to the atmosphere, while reducing the fuel cost by $300 per year (under average driving conditions of 12,000 miles a year and at a fuel cost of $1.50/gal) [16,20,22,38].
Figure 1: Schematic of the earth’s protective shields [42].
Figure 2: Current greenhouse gas contribution to increment in global climate change [41].
In the Middle East, considerable amounts of pollutants are emitted as the chemical energy in fossil fuels is converted to thermal, mechanical, or electrical energy via combustion, such as power plants, motor vehicles, and even stoves.
Ozone And Smog
Urban smog is the dark yellow or brown haze that builds up in a large stagnant air mass and hangs over populated areas on calm hot summer days. Smog is made up mostly of ground-level ozone (O3); but it also contains numerous other chemicals, including carbon monoxide (CO), particulate matter such as soot and dust, and volatile organic compounds (VOC) such as benzene, butane, and other hydrocarbons. The harmful ground-level ozone should not be confused with the useful ozone layer high in the stratosphere that protects the earth from the sun’s harmful ultraviolet rays. Ozone at ground level is a pollutant with several adverse health effects [4,17,24,25,27].
The primary source of both nitrogen oxides and hydrocarbons is the motor vehicles. Hydrocarbons and nitrogen oxides react in the presence of sunlight in hot calm days to form ground-level ozone, which is the primary component of smog. The smog formation usually peaks when the temperatures are the highest and there is plenty of sunlight. This is generally noticed in the industrial areas. Although groundlevel smog and ozone form in urban areas with heavy traffic or industry, the prevailing winds can transport them several hundred miles to other cities. This shows that pollution knows no boundaries, and it is a global problem. This is generally noticed in the areas few miles away from refinery stacks.
Ozone irritates eyes and damages the air sacs in the lungs where oxygen and carbon dioxide are exchanged, causing eventual hardening of this soft and spongy tissue. It also causes shortness of breath, wheezing, fatigue, headaches, and nausea, and aggravates respiratory problems such as asthma.
The other serious pollutant in smog is carbon monoxide, which is a colorless, odorless, poisonous gas. It is mostly emitted by motor vehicles, and it can build up to dangerous levels in areas with heavy congested traffic in the capitals and major cities. It deprives the body’s organs from getting enough oxygen. At low levels, carbon monoxide decreases the amount of oxygen supplied to the brain and other organs and muscles, slows body reactions and reflexes, and impairs judgment. It poses a serious threat to people with heart disease because of the fragile condition of the circulatory system and to fetuses because of the oxygen needs of the developing brain.
Smog also contains suspended particulate matter such as dust and soot emitted by vehicles and industrial facilities. Such particles irritate the eyes and the lungs since they may carry compounds such as acids and metals, as noticed in the adjacent areas to the cement and steel factories in different cities in the region.
Acid rain
Fossil fuels are mixtures of various chemicals, including small amounts of sulfur. The sulfur in the fuel reacts with oxygen to form sulfur dioxide (SO2), which is an air pollutant. The main source of SO2 is the electric power plants that burn high-sulfur coal. Regulations should force the plants to install SO2 scrubbers, to switch to low-sulfur coal, or to gasify the coal and recover the sulfur. Motor vehicles also contribute to SO2 emissions since gasoline and diesel fuel also contain small amounts of sulfur. This gas is not tackled by the three-way catalyst installed on vehicles generally exported to the Middle East.
The sulfur oxides and nitric oxides react with water vapor and other chemicals high in the atmosphere in the presence of sunlight to form sulfuric and nitric acids. The acids formed usually dissolve in the suspended water droplets in clouds or fog. These acid-laden droplets, which can be as acidic as lemon juice, are washed from the air on to the soil by rain or snow. This is known as acid rain. The soil is capable of neutralizing a certain amount of acid, but the amounts produced by the power plants using inexpensive high-sulfur oil have exceeded this capability.
Gaseous Pollutants Effects On Health And Environment
Carbon dioxide
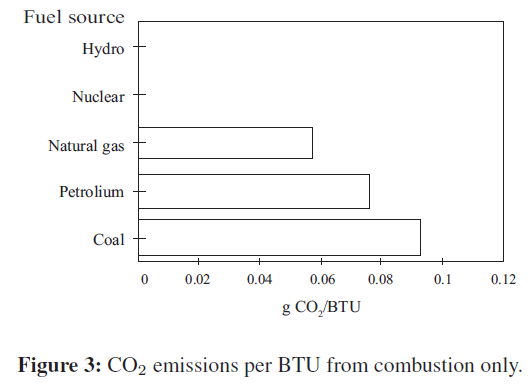
CO2 is produced as a waste product of human and animal respiration. Hence, carbon dioxide is naturally present in the atmosphere in reasonable quantities. A normal sample of air contains about 0.05% carbon dioxide by weight (Figure 3).
Not only is carbon dioxide consumed in photosynthesis; it also may dissolve in the vast oceans. When carbon dioxide dissolves in the ocean, carbonic acid is produced at a very low concentration. The carbonic acid dissociates in part to bicarbonate and carbonate ions. These ions combine with calcium and magnesium from the natural weathering of rocks which have been carried into the ocean. The reaction of calcium ion with carbonate ion produces calcium carbonate. We know this relatively insoluble substance as limestone. Magnesium and calcium may jointly react with the carbonate ion to produce dolomite.
These precipitation reactions remove carbonate from the water and make room for more carbon dioxide to dissolve. Thus, there are two natural mechanisms for carbon dioxide removal from the atmosphere: (1) solution in the ocean followed by precipitation and (2) the utilization of carbon dioxide by green plants in photosynthesis.
Carbon dioxide and monoxide from fossil fuels
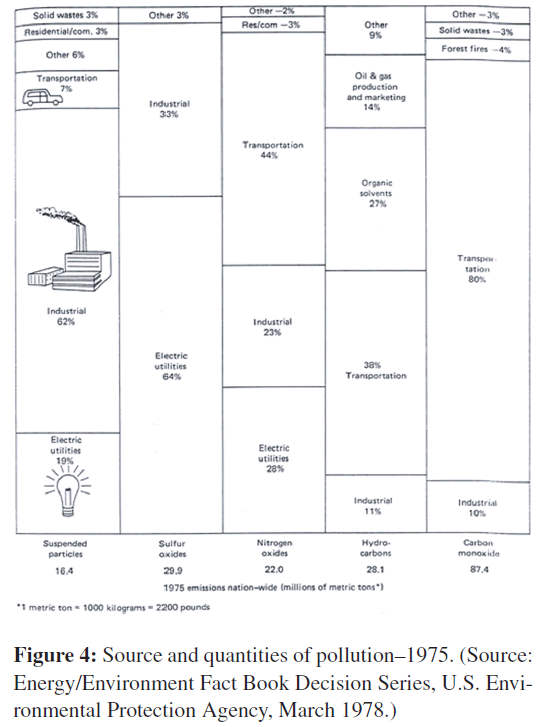
Carbon dioxide is now being produced in massive quantities from the burning of fossil fuels especially in the countries which depend heavily on fossil fuels whether in running vehicles, power systems, or heating appliances. The complete oxidation of carbon produces the gas carbon dioxide. The greatest source of carbon monoxide in cities is the motor vehicle (Figure 4). In most cities, over 90% of the carbon monoxide in the air comes from the incomplete combustion of carbon in motor fuels.
The exhaust gases are mixed with a stream of air in the presence of a catalyst. Further oxidation of the remaining carbon monoxide occurs in this “catalytic converter.” The catalyst system appears at present to be the chosen method to reduce carbon monoxide emissions. Nevertheless, automanufacturers have studied the potential of the stratified charge and dual carburetor engine system. This system has been used in Honda cars and has achieved the required reduction.
There is another source of carbon monoxide to which people are exposed. Only smokers and their neighbors, however, have this special privilege. We can compare the individual who smokes moderately and lives in a clean environment with an individual who does not smoke and lives in a highly polluted environment. The smoker will absorb daily twice as much carbon monoxide as his nonsmoking counterpart.
Carbon monoxide and hemoglobin
Carbon monoxide competes with oxygen for the molecule that carries oxygen to the cells. For this reason, at high enough concentrations, it is a deadly poison. Hemoglobin, a complex protein present in the blood, transports oxygen from the lungs to the cells and carries carbon dioxide from the cells back to the lungs.
Sulfur dioxide
Natural gas, in contrast to coal and oil, is almost free of sulfur. From this standpoint, it is an environmentally useful fuel. Burning oil in electric generating plants is used to produce 65% of the sulfur oxides emitted. Other oil burning accounted for half of the remainder. Sulfur compounds also enter the air from the smelting and refining industries as well as from burning municipal wastes.
Sulfur oxidation to a corrosive mist
When oil is burned, the sulfur in the fuel is oxidized. Two compounds are formed: sulfur dioxide and sulfur trioxide. Less than 3% of the sulfur is oxidized to the trioxide form during the initial burning.
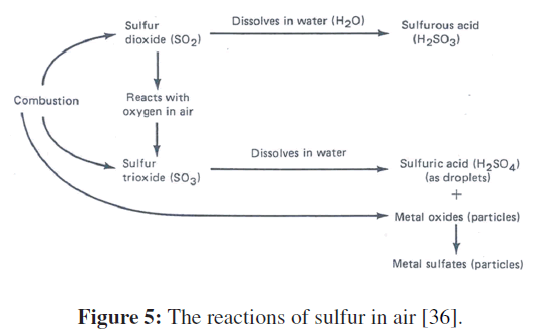
As an example, calcium oxide may react with sulfuric acid to produce calcium sulfate and water. Iron oxides may react in a similar fashion. The reactions are summarized in Figure 5. Metal sulfate particles plus sulfuric acid droplets usually account for between 5% and 20% of the particulate matter in urban air.
Figure 5: The reactions of sulfur in air [36].
Effects on materials, plants, and people
Many materials may be attacked by the mists of sulfuric acid, among them carbonate building materials such as marble and mortar. Calcium sulfate, water, and carbon dioxide are produced in the reaction. Metals such as steel, copper, and aluminum are corroded; and common cloth fabrics are damaged as well.
Respiratory illnesses such as bronchitis are seen to increase with sulfur oxide levels. One study found the illness rate increasing in an area where the average annual concentration was only 100 micrograms per cubic meter.
The health effects of sulfur oxides and particles
Sulfur oxides and particles aggravate respiratory disease. When it was first recognized that contaminants which fouled the air could cause death, sulfur oxides and particles were present at the scene. We have long known that particles act as a focus upon which water vapor condenses. We now understand that sulfur dioxide will quickly dissolve in the water droplet producing a highly acid, highly corrosive mist. It is this sulfurous acid mist that is thought to be responsible for so much damage to life and health.
The airborne substances under the greatest suspicion as potential carcinogens are polycyclic aromatic hydrocarbons, an example of which is benzpyrene. Known to cause cancer in experimental animals, these compounds have been found free or associated with particles of soot in urban air.
Ways to reduce sulfur dioxide emissions
It depends on whether it is more economical and efficient to remove particulate and SO2 from the end of the stack after the burning of coal or to clean the coal before it is consigned to the flames [28].
Cleaning up the coal itself
In addition to the possibility of using expensive low sulfur coal, emissions of sulfur dioxide from power plants can be reduced by cleansing the coal of its sulfur prior to use.
(a) Scrubbers: a number of methods are being and have been developed to “scrub” sulfur dioxide from the gas that exits the smokestack. A recent concept combines both coal cleaning and scrubbing.
(b) Fluidized bed: the limestone reacts with the sulfur dioxide coming from the burning coal to form fine particles of calcium sulfate, which are carried off in the stack gases and will be removed with the fly ash, probably by the electrostatic precipitator. The temperature of the burning coal in the fluidized bed is lower than the temperature of burning coal in a boiler; and, as a consequence, fewer nitrogen oxide are formed. Thus, both sulfur dioxide and oxides of nitrogen oxide are formed. Thus, both sulfur dioxide and oxides of nitrogen are lower for the fluidized bed combustion system.
Particulate matter
Particulate matter is another serious air pollutant. Unlike the pollutants discussed earlier, particles are not of a single chemical type. Instead, numerous solid and liquid compounds are dispersed in the air from many sources [1,3,7,13,18,21,26].
Particles that stem from combustion and industrial activities
The burning of coal produces not only ash particles (calcium silicates) and carbon particles, but also metal oxides such as calcium and ferric oxide. The metal oxide particles may react with the mist of sulfuric acid droplets. The reaction produces still other particles: the metal sulfates. The sulfuric acid droplets themselves are particles derived from the reaction of sulfur trioxide with water vapor. Both the acid droplets and the sulfate particles then are derived in part from fuel burning.
The quantity of particles derived from coal burning is enormous. Fortunately, however, a very large proportion of the particles is removed from the stack gases. In 1975, about 3.2 million metric tons of particles were released to the atmosphere from coal-burning electric plants in the United States. Probably seven times that quantity was generated, but most of it was removed.
Particles may settle on surfaces, leading to a dirty gray appearance. In addition to soiling, particles may cause corrosion, acting as centers from which corrosion spreads. The annual repair of these surfaces has a significant cost. The sulfuric acid mist may damage plant tissue. Compounds from photochemical reactions produce a burn on the leaves of many vegetables.
The health of human beings is also affected by particles. The frequency of respiratory infections such as colds and bronchitis is seen to increase with particle levels. At a particle concentration of 375 micrograms/cubic meter, an air pollution “alert” is to be declared. Under an alert, industries might be requested to curtail or postpone their activities.
Furthermore, particles may be reflecting solar energy away from the earth. As such, they may be responsible for the small but noticeable cooling in the Northern Hemisphere.
Numerous devices are available to reduce particle emissions. These include the settling chamber, the after-burner to ignite and burn particles, and the electrostatic precipitator.
An alternative to the electrostatic precipitator is the “baghouse” composed of fabric bags to capture the fly ash. It is a very large vacuum cleaner. Air is drawn up through the bags; the particles cannot pass out through the fine weave of the bag and are trapped inside it. Only clean air exits.
Lead compounds in the air
Lead has been in gasoline for many years as compounds of tetraethyl lead. These compounds are used to improve the antiknock quality of gasoline; they eliminate the knock during engine operation. These substances are not absolutely necessary. A slightly higher priced refining process produces a fuel with the required antiknock properties. In the past, lead levels have ranged from 2.5 to 4.23 grams of lead per gallon of gasoline. About 75% of this amount is released to the atmosphere in the exhaust stream of the cars. Over 142,000 metric tons of lead were added to the atmosphere from gasoline combustion in the world during the year 1975.
Children and lead poisoning
Although lead poisoning may lead to death, in milder cases, it causes mental retardation. Even levels below the poison threshold appear to cause subtle inadequacies in learning. The hazard to health is one of the reasons for the decision to eventually make gasoline lead-free. The other reason is that lead poisons the catalytic converter which further burns the hydrocarbons, carbon monoxide, and nitrogen oxides remaining in exhaust gases. Practical steps are being taken for conversion to unleaded petrol in most of the countries of the world.
Lead poisoning in the child is first seen as a variety of symptoms which include appetite loss, problems in discipline, and a lack of interest in play. The disease progresses to constipation, vomiting, seizures, and finally coma. A permanent loss of mental ability may result from a case of lead poisoning.
Lead poisoning is a public health problem of major importance. To guard against new cases, parents and children must be informed of the risk involved in eating paint chips. Detection programs are necessary to find those children already near the toxic limit. Where cases are found, the surface from which the paint peeled should be covered up or the paint should be completely removed.
Photochemical pollution
A photochemical reaction requires light energy to take place. Certain pollutants in the atmosphere, nitrogen oxides and hydrocarbons, undergo photochemical reactions. These reactions produce new pollutants, including ozone, aldehydes, and exotic organic compounds. The new pollutants are referred to, in sum, as “photochemical air pollution” because they arise from photochemical reactions [5,34,35].
The sources of photochemical air pollution then are the sources of nitrogen oxides and of hydrocarbons. By the same reasoning, the control of photochemical air pollution consists of controlling emissions of nitrogen oxides and of hydrocarbons.
The dark spots are the result of elevated ozone levels. Such levels result when excessive use of the automobile leads to high concentration of hydrocarbons and nitrogen oxides.
Nitrogen oxides and hydrocarbons
Approximately 90% of the annual emissions of nitrogen oxides stem from the combustion of fossil fuels. Probably about one-third of these emissions are from motor vehicle operation.
Most important, however, incomplete combustion left unburned hydrocarbons as droplets and gases in the exhaust stream. Smaller sources of hydrocarbons were petroleum refining and transfer operations.
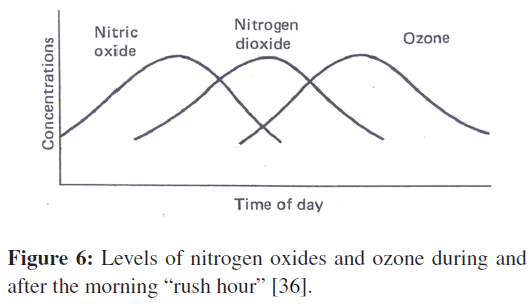
Nitrogen and oxygen unite during the high-temperature combustion of fuel in an automobile engine to produce the gas nitric oxide, which is then released to the atmosphere. In several hours’ time, the level of nitric oxide in the air decreases substantially. During the period of that decline, the level of nitrogen dioxide will be rising to a peak. Later, as the nitrogen dioxide level declines, the concentration of a third gas, ozone, will increase. Ozone levels then decrease (Figure 6).
Figure 6: Levels of nitrogen oxides and ozone during and after the morning “rush hour” [36].
Ozone compounds damage the leaves of common vegetables. Ozone also causes citrus trees to lose their fruit early. Ozone and aldehydes irritate the respiratory tract. The eyes may be irritated by aldehydes compounds. Individuals with asthma have found their disease aggravated by the oxidants.
Effects of nitrogen dioxide on human health
About 90% of the nitrogen oxides are produced in the form of nitric oxide (NO). The remaining 10% are in the form of nitrogen dioxide (NO2). Two effects of nitrogen dioxide on body functions have been noted. One is the greater effort required in breathing. Individuals with chronic lung diseases experienced breathing difficulty. Statistical analysis indicates a positive relation between nitrogen dioxide concentrations and a higher mortality rate from heart disease and cancer [36].
Biological effects of thermal pollution
The effect of excess heat on ecosystems
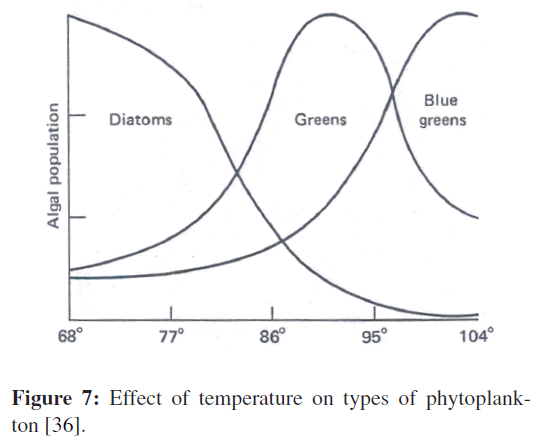
Temperature can affect the whole community structure of an aquatic environment. For instance, different species of freshwater algae compete for light, space, and nutrients. Temperature changes can alter the competitive position of different species, even though the changes are not severe enough to be lethal for any species. At low temperatures (around 21 °C), yellow-green algae may predominate in a lake community. As the temperature is raised to 26 °C or 32 °C, green algae become more abundant, and finally the blue-green algae begin to dominate at very high temperatures (Figure 7). In this manner, heat can seriously affect aquatic food chains, since the blue-green algae tend to be more resistant to grazing than other kinds of algae. In addition, blue-green algae characteristically have a greater mass than the species they replace.
Figure 7: Effect of temperature on types of phytoplankton [36].
One study found that fewer than half as many species were found at 31 °C than at 26 °C. Another 24% disappeared at a temperature of 34 °C. Such a simple ecosystem is generally believed to be less stable than the original more complex one. In freshwater, fish appear to be the most sensitive species. Protection of fish from excess heat would thus appear to protect freshwater ecosystems in general. In salt water, however, plant species may be more sensitive than fish are to heated discharges.
Factors that act in combination with temperature
There are many factors that act in combination with temperature to affect the survival and well-being of organisms, such as the following:
(i) Oxygen content of water: the amount of oxygen in water can affect how an organism reacts to heat.
(ii) Chemicals in water: increasing the salinity, or salt content, of water changes the effects of temperature, making some organisms more resistant to heat and cold and others less so.
(iii) Entrainment: microscopic floating plants, called phytoplankton, as well as fish, insects, and larval stages of various creatures, can be sucked into the condenser of a power plant along with cooling water [32,36,42].
Economic analysis of pollution due to SO2
Emissions and their impact on human health and ecosystems are hereby discussed by Nakada et al. [29]. According to Wang et al. [43], a system for SO2 emission charge of $24/t- SO2 has been applied in China for several years.
According to Park [31], SO2 emitted from industry mainly causes domestic problems because the emitted dry gas, produced mainly by large combustion plants, falls to the ground within about 300 km of the source and is deposited in a gaseous form called “dry deposition,” which directly damages human health and buildings. The burden of chronic obstructive pulmonary disease such as emphysema and chronic bronchitis could cause hundred thousands of premature deaths [44].
SO2 emissions also bring about regional or transboundary problems. Colls [6] describes that sulfate is deposited to land via cloud droplets, known as wet deposition, which develops in acid rain; for instance, SO2 is oxidized into H2SO4 with the help of OH into which ozone decomposes. It disperses around 1,000 km across the boundaries in 24 h. The sulphur oxidants dissolve in clouds and rain droplets, and produce acids and SO4. Eventually, acid rain falls by wet deposition. In average, 38% of the sulfur deposition is of domestic origin, 45% comes from non-anthropocentric sources such as volcanoes, and 17% from trans-borders; see Calori et al. [2].
SO2 emissions directly damage human health by dry deposition, and indirectly damage buildings, forests and crops due to wet deposition (acid rain). Damage cost estimates in China are $221 and $128/t-SO2 for health and acid rain, respectively; see Xu [45].
Conclusions
Most countries depend on fossil fuels in their energy uses and development, hence being exposed especially in industrial regions to the resulting gaseous pollutants, namely, CO2, CO, SO2, NOx, UHC, particulate matter, and lead compounds in addition to chlorofluorocarbons. They generally cause ozone layer depletion, smog, acid rain, and health hazards to all creatures.
Thermal pollution causes problems to ecosystems. Therefore, developing countries should be aware of the harmful effects of these gaseous pollutants and try to reduce them. The proposed technologies for reducing gaseous pollutants are detailed in a separate publication by the author [45]. These technologies are briefly mentioned here, namely, fluidized bed combustion, combined cycles, fuel cells, nuclear power, natural gas, renewable energy, energy conservation, energy storage, oxy-fuel combustion, and chemical looping combustion.
References
- J. B¨unger, J. Krahl, A. Munack, Y. Ruschel, O. Schr¨oder, B. Emmert, et al., Strong mutagenic effects of diesel engine emissions using vegetable oil as fuel, Arch Toxicol, 81 (2007), 599–603.
- G. Calori, G. R. Carmichael, D. Street, N. Thongboonchoo, and S. K. Guttikunda, Inter-annual variability in sulfur deposition in Asia, J Global Environ Eng, 7 (2001), 1–16.
- F. Cavalli, M. Viana, K. E. Yttri, J. Genberg, and J.-P. Putaud, Toward a standardised thermal-optical protocol for measuring atmospheric organic and elemental carbon: the EUSAAR protocol, Atmos Meas Tech Discuss, 2 (2009), 2321–2345.
- Y. A. Cengel and M. A. Boles, Thermodynamics: An Engineering Approach, McGraw-Hill, Boston, MA, 6th ed., 2007.
- Y.-L. Cheng, Y.-H. Bai, J.-L. Li, and Z.-R. Liu, Modeling of air quality with a modified two-dimensional Eulerian model: A case study in the Pearl River Delta (PRD) region of China, Journal of Environmental Sciences, 19 (2007), 572–577.
- M. P. Dorado, E. Ballesteros, J. M. Arnal, J. Gomez, and F. J. Lopez, Exhaust emissions from a Diesel engine fueled with transesterified waste olive oil, Fuel, 82 (2003), 1311–1315.
- S. Duflo, M. Greenstone, and R. Hanna, Indoor air pollution, health and economic well-being, Surveys and Perspectives Integrating Environment and Society, 1 (2008), 1–10.
- S. Fan, B.Wang, M. Tesche, R. Engelmann, A. Althausen, J. Liu, et al., Meteorological conditions and structures of atmospheric boundary layer in October 2004 over Pearl River Delta area, Atmospheric Environment, 42 (2008), 6174–6186.
- A. Foster and N. Kumar, Health effects of air quality regulations in Delhi, India, Atmospheric Environment, 45 (2011), 1675– 1683.
- C. Frankenberg, J. F. Meirink, M. van Weele, U. Platt, and T. Wagner, Assessing methane emissions from global spaceborne observations, Science, 308 (2005), 1010–1014.
- H. C. Frey, H. Zhai, and N. M. Rouphail, Regional onroad vehicle running emissions modeling and evaluation for conventional and alternative vehicle technologies, Environ Sci and Technol, 43 (2009), 8449–8445.
- J. S. Gaffney and N. A. Marley, The impacts of combustion emissions on air quality and climate – From coal to biofuels and beyond, Atmospheric Environment, 43 (2009), 23–36.
- M. Greenstone and T. Gayer, Quasi-experimental and experimental approaches to environmental economics, J Environ Econ Manag, 57 (2009), 21–44.
- H. Guo, T. Wang, D. R. Blake, I. J. Simpson, Y. H. Kwok, and Y. S. Li, Regional and local contributions to ambient nonmethane volatile organic compounds at a polluted rural/coastal site in Pearl River Delta, China, Atmospheric Environment, 40 (2006), 2345–2359.
- H. Huo, Q. Zhang, M. Q. Wang, D. G. Streets, and K. He, Environmental implication of electric vehicles in China, Environmental Science and Technology, 44 (2010), 4856–4861.
- J. Jackson, S. Choudrie, D. G. Thistlethwaite, N. Passant, T. Murells, J. Watterson, et al., UK greenhouse gas inventory, 1990 to 2007: annual report for submission under the framework convention on climate change, AEA Technology plc., 2009, http://uk-air.defra.gov.uk/reports/cat07/0905131425 ukghgi-90- 07 main chapters Issue2 UNFCCC CA v5 Final.pdf.
- I. Kooter, M. van Vugt, A. Jedynska, P. Tromp, M. Houtzager, R. Verbeek, et al., Toxicological characterization of diesel engine emissions using biodiesel and a closed soot filter, Atmospheric Environment, 45 (2011), 1574–1580.
- N. Kumar, A. Chu, and A. Foster, Remote sensing of ambient particles in Delhi and its environs: estimation and validation, International Journal of Remote Sensing, 29 (2008), 3383–3405.
- R. H. F. Kwok, J. C. H. Fung, A. K. H. Lau, and J. S. Fu, Numerical study on seasonal variations of gaseous pollutants and particulate matters in Hong Kong and Pearl River Delta Region, Journal of Geographic Research, 115 (2010), D16308.
- N. Li, C. Sioutas, A. Cho, D. Schmitz, C. Misra, J. Sempf, et al., Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage, Environ Health Perspect, 111 (2003), 455–460.
- X.-H. Liu, Y. Zhang, S.-H. Cheng, J. Xing, Q. Zhang, D. G. Streets, et al., Understanding of regional air pollution over China using CMAQ, part I performance evaluation and seasonal variation, Atmospheric Environment, 44 (2010), 2415–2426.
- S. L. Mabit and M. Fosgerau, Demand for alternative-fuel vehicles when registration taxes are high, Transportation Research Part D: Transport and Environment, 16 (2011), 225–231.
- A. J. Manning and R. G. Derwent, Interpretation of longterm measurements of radiatively active trace gases and ozone depleting substances, Defra Contract: CPEG1 Quarterly Report, The Met Office, Devon, United Kingdom, 2006.
- A. J. Manning, D. B. Ryall, R. G. Derwent, P. G. Simmonds, and S. O’Doherty, Estimating European emissions of ozone-depleting and greenhouse gases using observations and a modeling backattribution technique, Journal of Geophysical Research, 108 (2003), 4405.
- J. D. McDonald, I. Eide, J. Seagrave, B. Zielinska, K. Whitney, D. R. Lawson, et al., Relationship between composition and toxicity of motor vehicle emission samples, Environ Health Perspect, 112 (2004), 1527–1538.
- R. Milne and D. C. Mobbs, UK emissions by sources and removals by sinks due to land use, land use change and forestry activities report, April 2006. DEFRA contract EPG 1/1/160 CEH No. C02275.
- Y. S. H. Najjar, Modern and appropriate technologies for the reduction of gaseous pollutants and their effects on the environment, Clean Techn Environ Policy, 10 (2008), 269–278.
- M. Nakada and K. Ueta, Sulphur emission control in China: domestic policy and regional cooperative strategy, Energ Environ, 18 (2007), 195–206.
- H. A. Nasrallah and R. C. Balling, The Heated Debate: Greenhouse Predictions Versus Climate Reality, Pacific Research Institute for Public Policy, San Francisco, CA, 1995.
- D. Polson, D. Fowler, E. Nemitz, U. Skiba, A. McDonald, D. Famulari, et al., Estimation of spatial apportionment of greenhouse gas emissions for the UK using boundary layer measurements and inverse modelling technique, Atmospheric Environment, 45 (2011), 1042–1049.
- C. A. Pope III, R. T. Burnett, M. J. Thun, E. E. Calle, D. Krewski, and K. Ito, Lung cancer, cardiopulmonary mortality, and longterm exposure to fine particulate air pollution, Journal of the American Medical Association, 287 (2002), 1132–1141.
- B. Pun, S.-Y. Wu, C. Seigneur, J. Seinfeld, R. Griffin, and S. Pandis, Uncertainties in modeling secondary organic aerosols: Three-dimensional modeling studies in Nashville/western Tennessee, Environ Sci Technol, 37 (2003), 3647–3661.
- B. K. Pun, R. J. Griffin, C. Seigneur, and J. H. Seinfeld, Secondary organic aerosol 2. Thermodynamic model for gas/particle partitioning of molecular constituents, J Geophys Res, 107 (2002), 4333.
- P. Revelle and C. Revelle, The Environment: Issues and Choices for Society, Van Nostrand Reinhold, New York, 1981.
- C. O. Reynolds, M. Kandlikar, and G. M. Badami, Determinants of PM and GHG emissions from natural gas-fueled autorickshaws in Delhi, Transportation Research Part D: Transport and Environment, 16 (2011), 160–165.
- J. Romm, The car and fuel of the future, Energy Policy, 34 (2006), 2609–2614.
- D. S. Shen, S. Y. Cheng, L. Liu, T. Chen, and X. R. Guo, An integrated MM5-CMAQ modeling approach for assessing transboundary PM10 contribution to the host city of 2008 olympic summer games—Beijing, China, Atmospheric Environment, 22 (2007), 1237–1250.
- K. Slezakova, J. C. M. Pires, F. G. Martins, M. C. Pereira, and M. C. Alvim-Ferraz, Identification of tobacco smoke components in indoor breathable particles by SEM-EDS, Atmospheric Environment, 45 (2011), 863–872.
- D.W. South, Coal and CO2: What are the options, Private Power Executive, September-October (1993), 25–31.
- K. J. Springer, Global what? Control possibilities of CO2 and other greenhouse gases, J Eng Gas Turbines Power, 113 (1991), 440–447.
- J. Wang, ed., SO2 Emissions Trading Program: US Experience and China’s Perspective, China Environmental Science Press, Beijing, China, 2000.
- World Bank, ClearWater, Blue Skies: China’s Environment in the New Century, World Bank, Washington, DC, 1997.
- T. Xu, Economic Damage of Environmental Pollution in China, China Environmental Science Press, Beijing, China, 1998.
- J. Zhang, T. Wang, W. L. Chameides, C. Cardelino, D. R. Blake, and D. G. Streets, Source characteristics of volatile organic compounds during high ozone episodes in Hong Kong, Southern China, Atmos Chem Phys, 8 (2008), 4983–4996.
- J. Zheng, M. Shao, W. Che, L. Zhang, L. Zhong, Y. Zhang, et al., Speciated VOC emission inventory and spatial patterns of ozone formation potential in the Pearl River Delta, China, Environmental Science and Technology, 43 (2009), 8580–8586.
Citation: Najjar YSH (2011) Gaseous Pollutants Formation and Their Harmful Effects on Health and Environment. InnovativeEnergy Policies 1: 101.
Copyright: ©2011 Najjar YSH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 33998
- [From(publication date): 12-2011 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 29007
- PDF downloads: 4991