GMEssential Role of GM-CSF-Dependent Macrophages in Human Autoimmune and Inflammatory Responses
Received: 25-Jun-2019 / Accepted Date: 10-Jul-2019 / Published Date: 24-Jul-2019
Abstract
Macrophages are important cells in the innate immune system that express toll-like receptors, produce various cytokines, and have a major role in both inflammation and autoimmune diseases. These cells undergo differentiation into GM-CSF-dependent or M-CSF-dependent macrophages in response to influences in the microenvironment. Because there are marked physiological and immunological differences between mice and humans, the inflammatory response of human macrophages is not accurately reproduced by murine models. GM-CSF and M-CSF are factors that promote the differentiation of bone marrow progenitor cells and induce various changes of human macrophage lineages. GM-CSF also promotes activation of pathways involved in immunity by upregulating the expression of various receptors. While cross-talk among these receptor-mediated signaling pathways is complicated, it is known that binding of different receptor ligands results in quantitative/qualitative changes of cytokine production. GM-CSFdependent macrophages produce pro-inflammatory cytokines that are known as type 1 T helper cell (Th1) cytokines. Among them, IL-23 is a pro-inflammatory cytokine required for differentiation of Th17 cells, which are involved in autoimmunity and inflammation. Pathogenic IL-23 signaling is considered to initiate autoimmune processes that are driven by GM-CSF-dependent macrophages. This review focuses on the complex intracellular signaling pathways activated in GM-CSF-dependent human macrophages by several receptors. A model is proposed, in which crosstalk among multiple signal transduction pathways leads to reactivation of autoimmune and inflammatory responses.
Keywords: Cross talk; GM-CSF; PAR-2; TLR7/8; IL-23; Signal regulatory protein α
Introduction
Macrophages have an important role in innate immunity and produce many of the cytokines involved in regulating immune responses. Granulocyte macrophage-colony stimulating factor (GM-CSF) induces the differentiation of proinflammatory macrophages and has a major role in both inflammation [1] and autoimmunity [2-5] Macrophages are also involved in adaptive immune responses. Activation of the adaptive immune system is a complex process regulated by multiples stimuli from the innate immune system, as part of which T cells are activated by cytokines released from macrophages. It was reported that T helper 17 cells (Th17 cells) are involved in the pathogenesis of autoimmune diseases6. Differentiation of Th17 cells is regulated by the IL-23/IL-17 axis, with IL-23 inducing and activating these cells [3-6]. The IL-23/IL-17 axis has been suggested to have a crucial role in the development of psoriasis [7]. Activated antigen-presenting cells (APC), such as dendritic cells and phagocytic cells, are the main source of IL- 23. We previously reported that IL-23 was produced by human GMCSF- dependent macrophages in response to stimulation with a toll-like receptor 7/8 (TLR7/8) agonist, but not a toll-like receptor 4 agonist [8]. Accordingly, these macrophages may be important for induction of Th17 cells via the IL-23/IL-17 axis [3,4]. The phenotypic features of murine GM-CSF-dependent macrophages are well known,but those of human GM-CSF-dependent macrophages are less clear. Because there are considerable physiological and immunological differences between mice and humans, murine models do not closely reproduce the inflammatory responses of human macrophages, which means direct examination of human cells is required [9].
Role Of Human GM-CSF-Dependent Macrophages
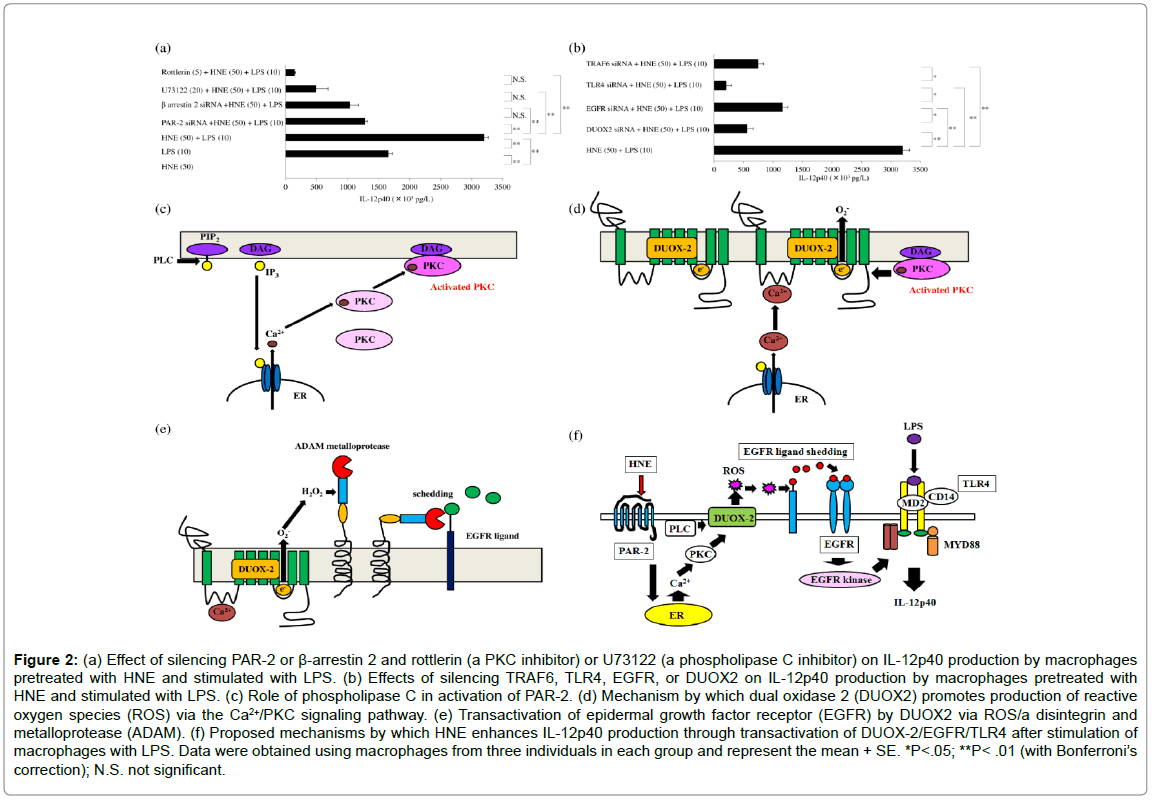
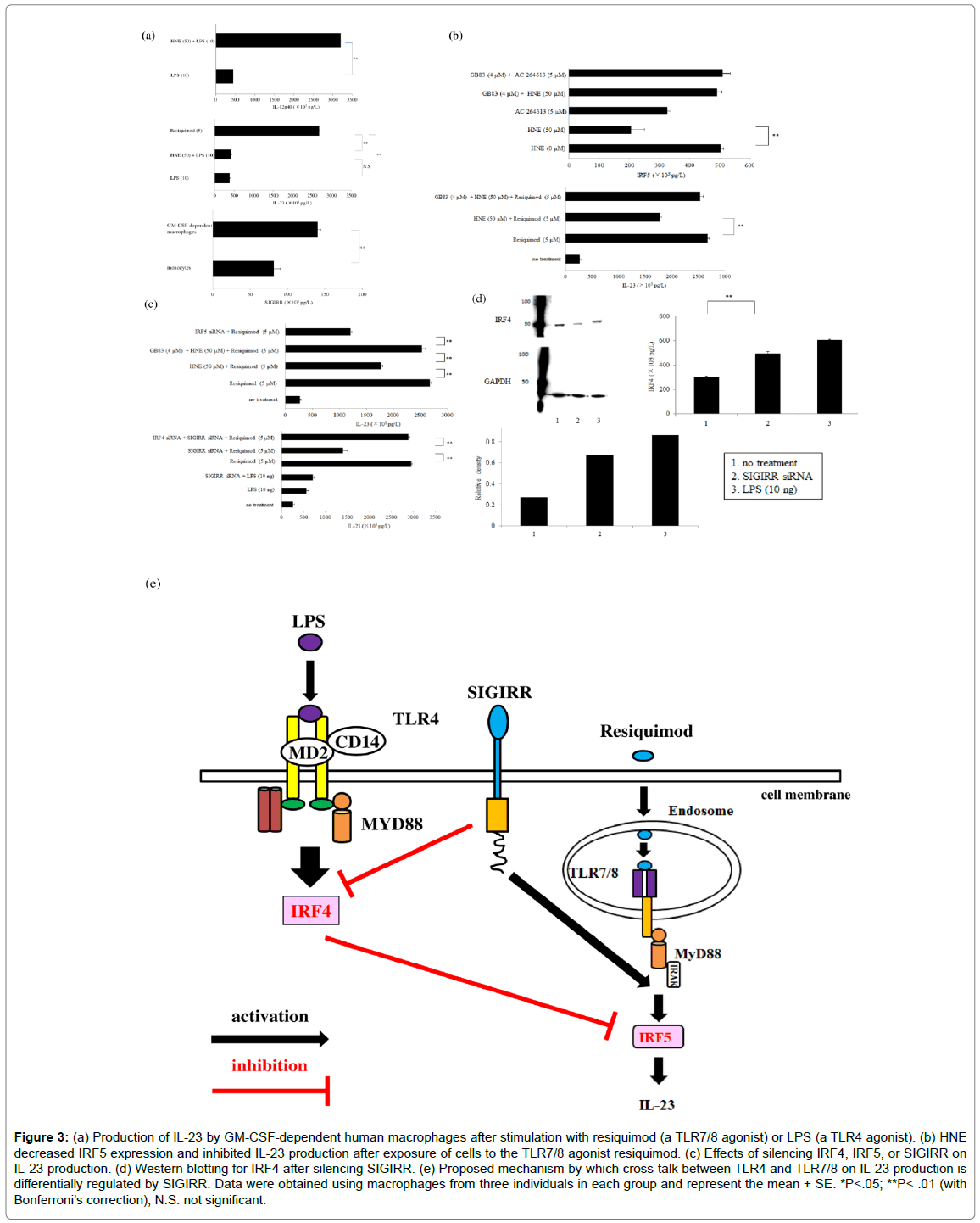
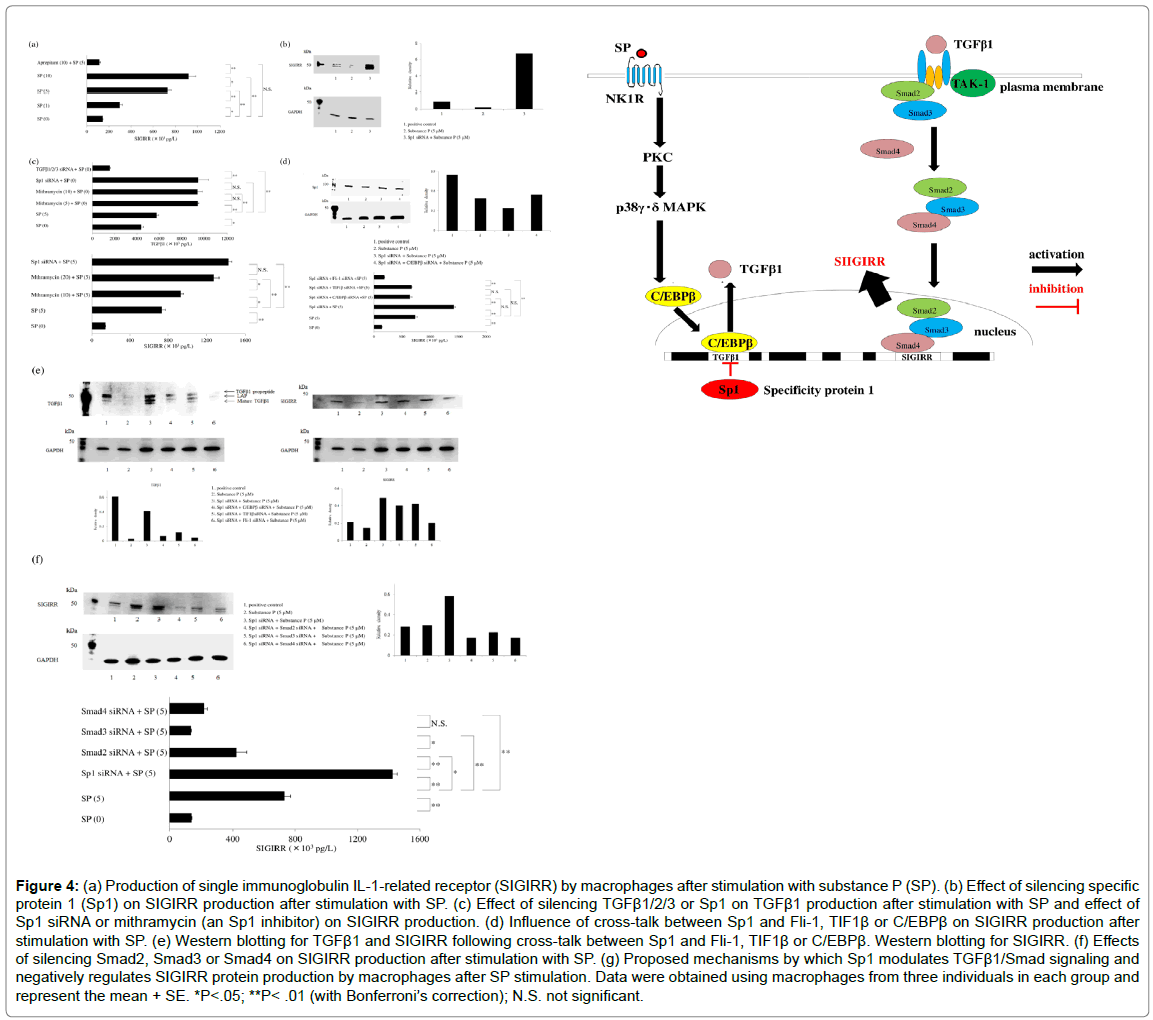
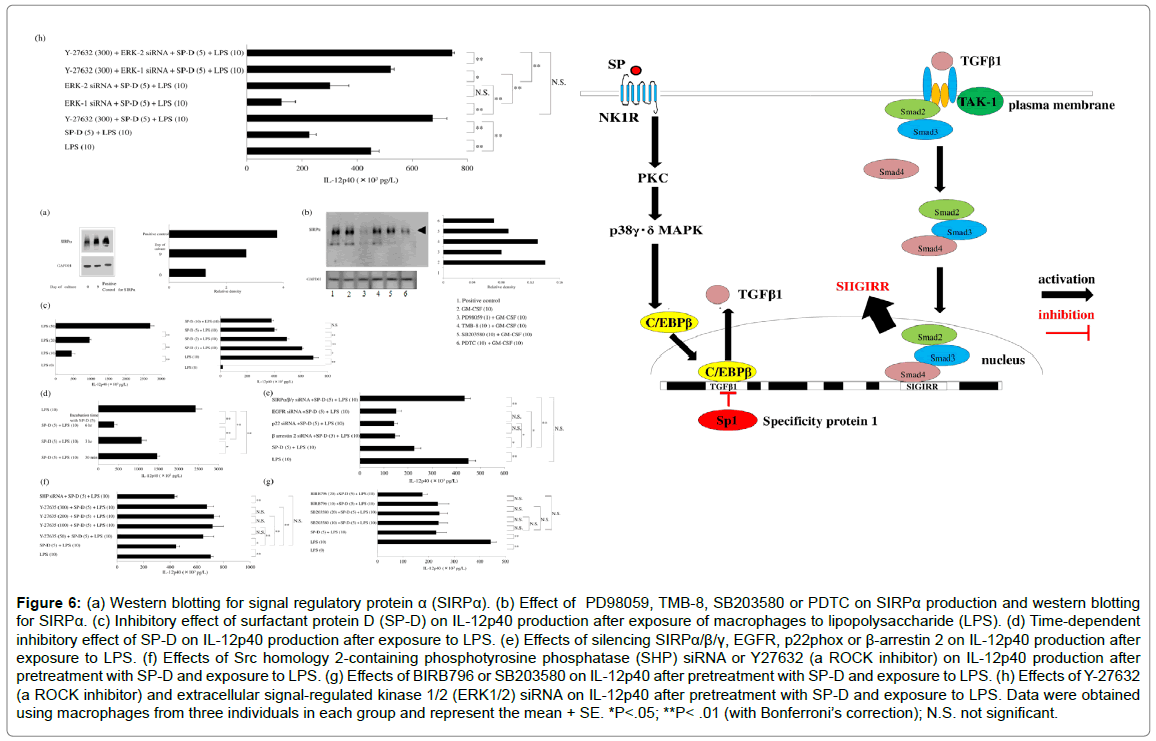
This review discusses recent findings about the role of human GMCSF-dependent macrophages in autoimmunity and inflammation. Induction of human GM-CSF-dependent macrophages Peripheral blood mononuclear cells (PBMCs) were harvested from heparinized blood samples using Lymphoprep gradients (Axis-Shield PoC As, Norway) and stored frozen.Cells were suspended in Lymphocyte medium for thawing (BBLYMPH1, Zen-Bio, Inc. Research Triangle Park, NC). Monocytes were stained with a phycoerythrin (PE)-labeled CD14 mouse anti-human monoclonal antibody (Life Technologies, Staley Road Grand Island, NY) and subjected to fluorescence activated cell sorting (FACS) analysis, revealing 86.08 + 0.11 % purity (mean + SE, n=42, 85.0-87.6). Then the monocytes were cultured and stimulated with recombinant human GM-CSF on days 1, 3, and 6 of incubation to obtain GM-CSF-dependent macrophages. Cells were harvested on day 9 of culture for use as GM-CSF-dependent macrophages in all studies. Identification of protein bands during macrophage differentiation After proteins are separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, Coomassie Brilliant Blue (CBB) staining can be employed to visualize protein bands. Therefore, CBB staining was investigated as a method for identifying proteins associated with differentiation of monocytes into macrophages after GM-CSF stimulation. CBB staining of polyacrylamide gels showed differences of protein bands between control monocytes (day 0 of culture) and GMCSF-dependent macrophages (day 9 of culture) (Figure 1a). Protein bands also differed between GM-CSF-dependent macrophages incubated with or without human neutrophil elastase (HNE) and harvested on day 9 of culture (Figure 1b). Protease-activated receptor-2 The protease-activated receptors (PARs) are a family of G proteincoupled receptors that undergo activation following proteolytic cleavage of the amino terminal by extracellular proteases [10]. PAR-2 is found in many tissues of the body and may be an important player in inflammation. Western blotting showed that GM-CSF stimulation increased PAR-2 expression by macrophages (Figure 1c), with upregulation of PAR-2 protein over time (Figure 1d). It has been reported that PAR-2 is activated in macrophages by various serine proteases [11], including HNE [12]. When GM-CSF-dependent human macrophages were stimulated with HNE (50 μM) for 6 h on day 9 of culture, production of the Th2 cytokine IL-13 was increased significantly compared to that after stimulation on days 0 or 7 (Figure1e) [13]. PAR-2 is activated by proteases that are involved in signaling by mitogenactivated protein kinases (MAPKs), with the MAPK pathways being controlled by extracellular signal-regulated kinases (ERKs), c-Jun- NH2-terminal kinase (JNK), and various p38 protein kinases [14]. However, SB203580 (a p38α and p38β inhibitor) did not inhibit IL- 13production by macrophages after stimulation with HNE, and neither did BIRB796 (ap38γand p38δ inhibitor). ERK1 and ERK2 are two protein kinases from the MAPK cascade. While an ERK1 inhibitor (PD98059) failed to inhibit IL-13 production by macrophages after PAR-2 activation, U0126 (an ERK1/2 inhibitor) markedly reduced IL- 13 production by macrophages following HNE stimulation. These findings suggested that PAR-2 undergoes activation by proteases and then is involved in ERK2 signaling. Reactive oxygen species (ROS) produced by the mitochondria are key mediators in signaling pathways triggered by PAR-2 [15]. Calcium is required for ROS production in the mitochondria, with elevation of intracellular calcium resulting in activation of ROS-generating enzymes that create free radicals in the respiratory chain [16]. It was found that an intracellular calcium antagonist (TMB-8) blocked the upregulation of IL-13 production by macrophages (Figure 1f), suggesting PAR-2-mediated IL-13 production was dependent on Ca2+/ERK2 signaling [17]. Th1 and Th2 cytokines stimulate the differentiation of macrophages into GM-CSF-dependent andM-CSF-dependent subsets, respectively, after which these cells promote Th1 and Th2 responses. IL-13 is a Th2 type cytokine and protease-mediated activation of PAR-2 stimulates production of IL-13 by GM-CSF-dependent macrophages. IL-13 has a central role in certain chronic inflammatory diseases, including asthma and ulcerative colitis [18,19]. IL-13 also seems to be an important player in tissue fibrosis [20]. After an episode of acute pancreatitis, complete recovery may occur or chronic pancreatitis may develop and HNE seems to be associated with progression of acute pancreatitis [21,22]. In patients with chronic pancreatitis, progressive fibrosis and inflammation cause permanent damage to the pancreas, with both exocrine and endocrine function showing impairment [23]. Pancreatic stellate cells are myofibroblast-like cells that have a role indevelopment of fibrosis [24]. Activated myofibroblasts show increased expression of α-smooth muscle actin (α-SMA) [25], and western blotting demonstrated a concentration-dependent increase of α-SMA expression by human pancreatic stellate cells stimulated with IL-13 (Figure 1g). It was reported that M-CSF-dependent macrophages promote pancreatic fibrosis in patients with chronic pancreatitis [26], and GM-CSFdependent macrophages may also participate in the fibrotic process by producing IL-13 in response to HNE/PAR-2 signaling. Cross-talk between PAR-2 and toll-like receptor 4 PAR-2 and toll-like receptor 4 (TLR4) are biosensors in the innate immune system [27] and are involved in immune responses, suggesting that cross-talk between these receptors could promote inflammation. HNE is a PAR-2 agonist, and pretreatment of GM-CSF-dependent macrophages with HNE synergistically increased production of the p40 subunit of IL-12 (IL- 12p40) after stimulation with lipopolysaccharide (LPS), a TLR4 agonist, while HNE alone did not induce IL-12p40 [28]. In macrophages stimulated with both HNE and LPS, IL-12p40 production was attenuated by a phospholipase C inhibitor (U73122) or a protein kinase C (PKC) inhibitor (rottlerin). β-arrestin 2 is a G-protein-coupled receptor (GPCR) adaptor protein that modulates proinflammatory responses, and silencing of PAR-2 or β-arrestin 2 with small interfering RNA (siRNA) decreased IL-12p40 production (Figure 2a). Epidermal growth factor receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases, with EGFR kinase activity being required for TLR4 signaling and having an important role in septic shock [29]. Downregulation of EGFR was reported to suppress the activation of nuclear factor-kappa B (NF-κB) by TLR4 following LPS stimulation, indicating that this receptor may be required for LPS to induce signaling via TLR4 [30]. Cross-talk between cell surface receptors is crucial for intercellular communication. Production of ROS by NADPH oxidase leads to activation of EGFR. The dual oxidases (DUOX-1 and DUOX- 2) are members of the NADPH oxidase family that produce H2O2. TLR4 activation is necessary for induction of DUOX-2 [31], while activation of PAR-2 also upregulates the DUOX-2/ROS pathway [32]. Treatment of GM-CSF-dependent macrophages with siRNA for TLR4 blunted the synergistic effect of HNE and LPS to enhance IL-12p40 production. Therefore, HNE promotes transactivation of TLR4 via activation of DUOX-2/EGFR along with synergistic upregulation of IL- 12p40 production in LPS-stimulated macrophages (Figure 2b). HNE cleaves PAR-2 at non-canonical sites to trigger various signaling cascades. PAR-2 activates Gq and phospholipase C to promote the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), forming diacylglycerol and inositol triphosphate (IP3). IP3 is a second messenger and it induces release of Ca2+ from the endoplasmic reticulum (ER). PKC is activated by various signals, including elevation of the intracellular concentration of diacylglycerol or Ca2+ (Figure 2c), after which production of DUOX-2 is induced by activation of PKC or phospholipase C. DUOX-2 generates H2O2 in a Ca2+-dependent manner (Figure 2d), and DUOX-2 activation is also involved in H2O2-dependent EGFR ligand shedding, which results in EGFR activation (Figure 2e). EGFR kinase activity is required for TLR4 signaling. Accordingly, HNE stimulation of macrophages leads to transactivation of the DUOX-2/ EGFR/TLR4 pathway. The possible mechanisms through which HNE enhances IL-12p40 production by macrophages stimulated with LPS are depicted in (Figure 2f). IL-23 production by GM-CSF-dependent macrophages. On day 9 of culture, stimulating GM-CSF-dependent macrophages with HNE and LPS led to synergistic upregulation of IL- 12p40 production. IL-12 and IL-23 share the IL-12p40 subunit, but only IL-23 targets the p19 subunit. Unexpectedly, stimulation of macrophages with both HNE and LPS did not result in a synergistic increase of IL-23 production. A TLR4 agonist (LPS) only slightly increased IL-23 production by macrophages, but it was a significantly upregulated in response to a TLR7/8 agonist (resiquimod) (Figure 3a). PAR-2 agonists (HNE or AC264613) attenuated the production of interferon regulatory factor 5 (IRF5) by GM-CSF-dependent macrophages, while IRF5 production was restored by a PAR-2 antagonist (GB83). When GM-CSF-dependent macrophages were pretreated with HNE or AC264613, production of IL-23 was suppressed after resiquimod stimulation, whereas it was restored by GB83 (Figure 3b). GM-CSF-dependent macrophages show elevated expression of IRF5 and it activates transcription of IL-23p19 [33]. However, HNE suppressed IRF5 expression in a concentration-dependent manner and it was also significantly decreased by the PAR-2 agonist AC-264613 [34]. IRF5 is a downstream mediator in the TLR7/8 signaling pathway [35]. After exposure of macrophages to resiquimod, HNE or AC- 264613 significantly attenuated IL-23 production, whereas it was restored by the PAR-2 antagonist GB83 (Figure 3c). Single immunoglobulin IL-1-related receptor (SIGIRR) is a membrane protein involved in negative regulation of TLR4 signaling [36]. SIGIRR protein expression was upregulated in GM-CSF-dependent macrophages compared with monocytes, while treatment with SIGIRR siRNA increased IL-23 expression by LPS-stimulated macrophages. Interestingly, macrophages treated with SIGIRR siRNA showed significant downregulation of IL-23 production after stimulation with resiquimod to activate the TLR7/8 pathway [8] (Figure 3c), suggesting that SIGIRR may promote TLR7/8-mediated signaling. Expression of SIGIRR protein was also found to be significantly higher in GM-CSFdependent macrophages than in monocytes (Figure3a). SIGIRR deficiency is associated with significant enhancement of IRF4 expression [37] (Figure 3d), along with repression of IRF5 [38]. LPS also upregulates the expression of IRF4 [39]. Therefore, SIGIRR differentially influences the effect of cross-talk between TLR4 and TLR7/8 on IL-23 production, since it negatively regulates TLR4 and positively regulates TLR7/8 (Figure 3e). Stimulation of SIGIRR production by substance P. It was reported that SIGIRR expression by human monocytes increases in response to sepsis or sterile inflammation [40], but the stimuli promoting SIGIRR production and the signal transduction mechanisms involved are not well defined. Substance P (SP) is a neuropeptide that is involved in pro-inflammation responses [41] and induces sterile inflammation [42]. In addition to being produced by neurons, SP is secreted by inflammatory cells, including dendritic cells and macrophages. SP was found to cause concentrationdependent upregulation of SIGIRR protein production bymacrophages, while this increase of SIGIRR was inhibited by aprepitant, which is aneurokinin 1 receptor antagonist. SP was reported to induce the expression oftransforming growth factor-β1 (TGFβ1) [43], and TGFβ1 enhances neurokinin 1 receptor signaling by delaying its internalization [44]. When macrophages were transfected with siRNAs for TGFβ1/2/3, SIGIRR protein expression was reduced markedly to the level in untreated control cells [45]. Investigation of the influence of various transcription factors on SIGIRR expression by SP-stimulated macrophages unexpectedly revealed that transfection with siRNA for transcription factor specificity protein 1 (Sp1) led to significant upregulation of SIGIRR protein expression, while siRNA for Kruppellike factor 2 (KLF2) only induced a slight increase of SIGIRR expression and siRNA for Friend leukemia integration 1 (Fli-1) actually reduced the SIGIRR level (Figure 4b).
Figure 1: (a) and (b) Coomassie Brilliant Blue (CBB) staining. (c) Western blotting for PAR-2. (d) Time-dependent changes of PAR-2 expression. (e) Production of IL- 13 after stimulation with HNE. (f) Effect of MAPK inhibitors and TMB-8 on IL-13 production. (g) Western blotting for α-SMA after human pancreatic stellate cells were stimulated with IL-13. Data were obtained using macrophages from three individuals in each group and represent the mean + SE. *P<.05; **P< .01 (with Bonferroni’s correction); N.S. not significant.
Figure 2: (a) Effect of silencing PAR-2 or β-arrestin 2 and rottlerin (a PKC inhibitor) or U73122 (a phospholipase C inhibitor) on IL-12p40 production by macrophages pretreated with HNE and stimulated with LPS. (b) Effects of silencing TRAF6, TLR4, EGFR, or DUOX2 on IL-12p40 production by macrophages pretreated with HNE and stimulated with LPS. (c) Role of phospholipase C in activation of PAR-2. (d) Mechanism by which dual oxidase 2 (DUOX2) promotes production of reactive oxygen species (ROS) via the Ca2+/PKC signaling pathway. (e) Transactivation of epidermal growth factor receptor (EGFR) by DUOX2 via ROS/a disintegrin and metalloprotease (ADAM). (f) Proposed mechanisms by which HNE enhances IL-12p40 production through transactivation of DUOX-2/EGFR/TLR4 after stimulation of macrophages with LPS. Data were obtained using macrophages from three individuals in each group and represent the mean + SE. *P<.05; **P< .01 (with Bonferroni’s correction); N.S. not significant.
Figure 3: (a) Production of IL-23 by GM-CSF-dependent human macrophages after stimulation with resiquimod (a TLR7/8 agonist) or LPS (a TLR4 agonist). (b) HNE decreased IRF5 expression and inhibited IL-23 production after exposure of cells to the TLR7/8 agonist resiquimod. (c) Effects of silencing IRF4, IRF5, or SIGIRR on IL-23 production. (d) Western blotting for IRF4 after silencing SIGIRR. (e) Proposed mechanism by which cross-talk between TLR4 and TLR7/8 on IL-23 production is differentially regulated by SIGIRR. Data were obtained using macrophages from three individuals in each group and represent the mean + SE. *P<.05; **P< .01 (with Bonferroni’s correction); N.S. not significant.
Figure 4: (a) Production of single immunoglobulin IL-1-related receptor (SIGIRR) by macrophages after stimulation with substance P (SP). (b) Effect of silencing specific protein 1 (Sp1) on SIGIRR production after stimulation with SP. (c) Effect of silencing TGFβ1/2/3 or Sp1 on TGFβ1 production after stimulation with SP and effect of Sp1 siRNA or mithramycin (an Sp1 inhibitor) on SIGIRR production. (d) Influence of cross-talk between Sp1 and Fli-1, TIF1β or C/EBPβ on SIGIRR production after stimulation with SP. (e) Western blotting for TGFβ1 and SIGIRR following cross-talk between Sp1 and Fli-1, TIF1β or C/EBPβ. Western blotting for SIGIRR. (f) Effects of silencing Smad2, Smad3 or Smad4 on SIGIRR production after stimulation with SP. (g) Proposed mechanisms by which Sp1 modulates TGFβ1/Smad signaling and negatively regulates SIGIRR protein production by macrophages after SP stimulation. Data were obtained using macrophages from three individuals in each group and represent the mean + SE. *P<.05; **P< .01 (with Bonferroni’s correction); N.S. not significant.
Various combinations of transcription factors can have synergistic, stimulatory, or inhibitory effects. Investigation of the influence of crosstalk among transcription factors (Sp1, C/EBPβ, TIF1β, or Fli-1) on SIGIRR production by SP-stimulated macrophages demonstrated that transfection with Sp1 siRNA led to significant upregulation of TGFβ1 expression and SIGIRR protein production after SP stimulation, while SIGIRR production was reduced by co-transfection with siRNAs for Sp1 and C/EBPβ or TIF1β. These findings can be explained because C/EBPβ activates TGFβ/Smad3 signaling [46], while TIF1β acts as a cofactor of C/EBPβ [47]. Unexpectedly, transfection with siRNA for Sp1 or addition of mithramycin (a gene-selective Sp1 inhibitor) to cultures significantly upregulated TGFβ1 protein production by SP-stimulated macrophages and increased SIGIRR expression (Figure 4c). When macrophages were transfected with siRNAs for Sp1 and Smad2, Smad3, or Smad4 before SP stimulation, the SIGIRR protein level detected by ELISA and western blotting was significantly reduced (Figure 4d), suggesting that Sp1 negatively regulates SIGIRR production via the TGFβ1/Smad signaling pathway. Silencing of Fli-1, a member of the E26 transformation-specific (Ets) transcription factor family, led to a significant decrease of TGFβ1 and SIGIRR protein production by SP-stimulated macrophages, consistent with a report that Ets binding elements contain abundant Smad2/Smad3 binding sites and promote activation of the TGFβ/Smad signaling pathway [48].
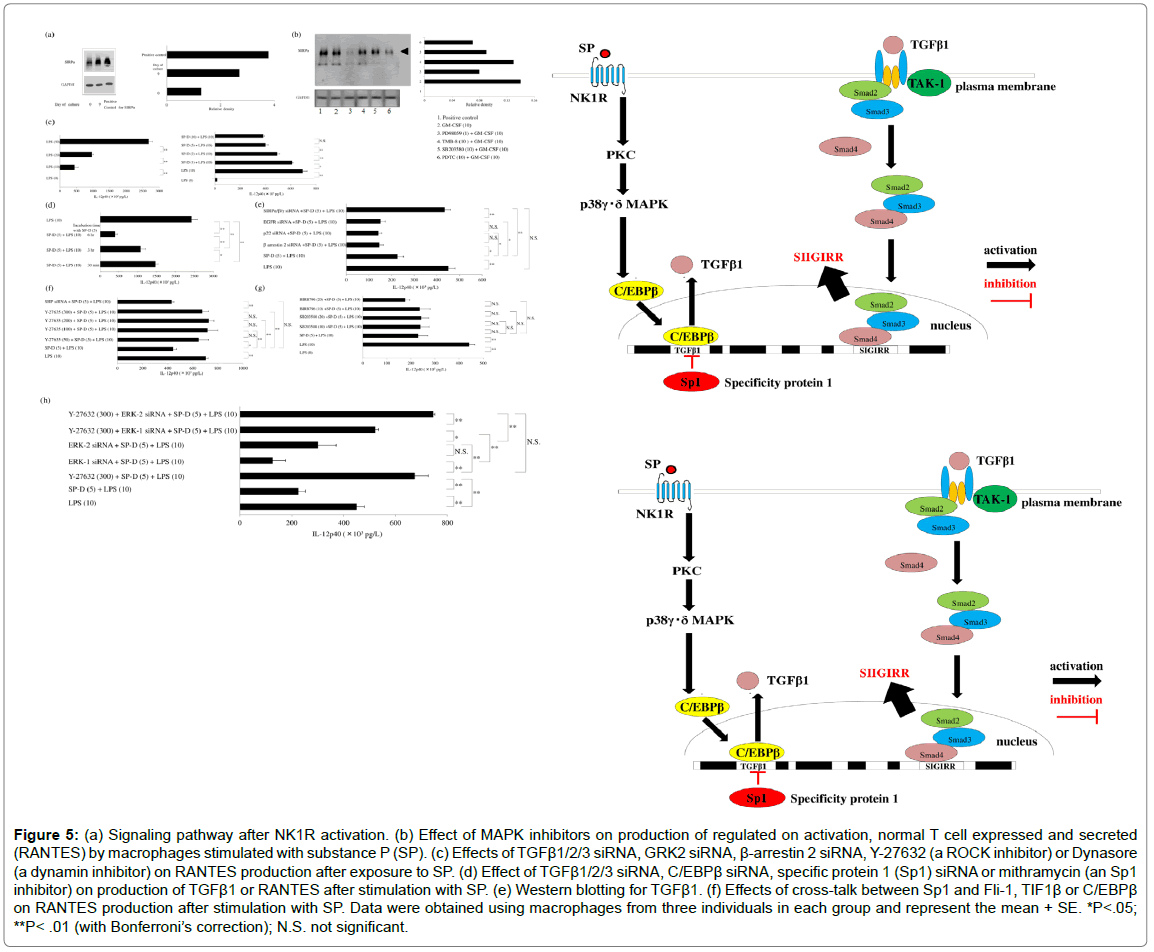
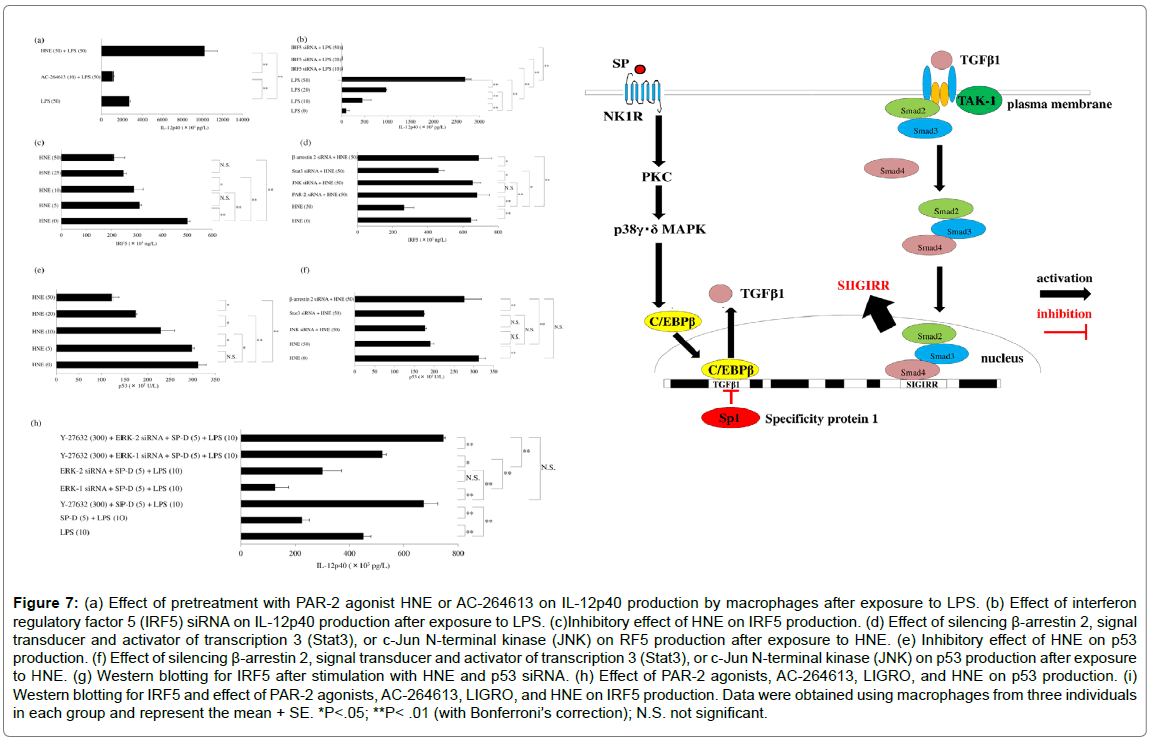
The receptor for SP is called neurokinin 1 receptor (NK1R). Recycling and resensitization of NK1R occur after its internalization following binding with SP. Because signal transduction is essential for trafficking of NK1R, it is important to investigate the molecular mechanisms underlying SP-induced internalization of this receptor. NK1R signaling does not only occur at the plasma membrane, but also at the endosomal membrane during internalization. The process of SPinduced internalization of NK1R is mediated by dynamin [49], which is a GTPase required for clathrin-mediated endocytosis of NK1R via membrane fission [50]. NK1R is co-localized with dynamin on the plasma membrane. In addition, the β-arrestins act as adaptors during internalization of NK1R after binding to SP [51]. Interestingly, TGFβ1 modulates phosphorylation of NK1R and delays its internalization after activation of this receptor by SP [44-52], and delayed NK1R internalization results in considerable enhancement of SP-induced cellular signaling pathways [53]. It was reported that SP causes upregulation of TGFβ1 at the mRNA and protein levels [52], while TGFβ1 acts to downregulate NK1R gene expression [54]. Interestingly, silencing of Sp1 was found to result in significantly increased TGFβ1 protein production by SP-stimulated macrophages [55]. Mithramycin inhibits the binding of Sp1 family members to DNA. In SP-stimulated macrophages, inhibition of Sp1 by mithramycin resulted in significantly higher levels of TGFβ1 and SIGIRR protein (Figure 4c). When the influence of cross-talk among Sp1 and C/EBPβ, TIF1β, or Fli-1 on SIGIRR protein production by SP-stimulated macrophages was investigated, co-transfection of macrophages with siRNAs for Sp1 and C/EBP was found to increase the Sp1 level (detected by western blotting) relative to transfection with Sp1 siRNA alone, while cotransfection with siRNAs for Sp1 and C/EBPβ reduced the production of both Sp1 and SIGIRR protein. In addition, co-transfection of macrophages with siRNAs for Sp1 and Fli-1 led to dramatic reduction of SIGIRR protein production (detected by ELISA) after SP stimulation (Figure 4d), while transfection with Sp1 siRNA alone increased the levels of TGFβ1 and SIGIRR protein (detected by western blotting). Cotransfection with siRNAs for Sp1 and C/EBPβ or TIF1β partially attenuated TGFβ1 and SIGIRR production by macrophages after SP stimulation, while co-transfection with siRNAs for Sp1 and Fli-1 led to marked inhibition of the production of these proteins (Figure 4e). Fli-1 is a member of the E26 transformation-specific (Ets) family of transcription factors. Ets binding elements contain abundant Smad2/ Smad3 binding sites and are involved in activating the TGFβ/Smad signaling pathway. Investigation of the influence of TGFβ1/Smad signaling on SIGIRR expression by macrophages revealed that transfection with siRNAs for Sp1 and Smad2, Smad3, or Smad4 significantly reduced the SIGIRR protein level (detected by ELISA and western blotting) after stimulation with SP (Figure 4f). Accordingly, it can be suggested that Sp1 acts as a negative regulator of SIGIRR production by macrophages in response to NK1R activation via the TGFβ1/Smad pathway (Figure 4g). Enhancement of NK1R-mediated cellular signaling by TGFβ1NK1R is a GPCR-like PAR-2 that is expressed by human macrophages [56] and modulates immune responses. NK1R signaling is mediated via two separate pathways: a β-arrestin 2-dependent pathway and a G-protein/Ca2+ pathway. Recycling and resensitization NK1R can only occur after its internalization in response to various stimuli and β-arrestins act as adaptors during the internalization process. NK1R signaling at the plasma membrane can be terminated by β-arrestin 2-dependent desensitization and internalization of the receptor. Interestingly, TGFβ1 modulates phosphorylation of NK1R and delays its internalization after activation of this receptor [44], leading to marked enhancement of NK1R-mediated signaling [54]. Figure 5a displays the three signaling pathways initiated by activation of NK1R. Interaction between SP and NK1R has been widely reported to have a role in regulating the immune response to infection. SP causes a significant increase of tissue factor release by GM-CSF-dependent human macrophages via the p22phox/ β-arrestin 2/Rho A signaling pathway [57]. M1 macrophages were reported to produce regulated on activation, normal T cell expressed and secreted (RANTES) [58] and RANTES seems to be involved in the progression of atherosclerosis [59]. Expression of RANTES (also known as the proatherogenic chemokine (C-C motif) ligand 5: CCL5) by human M1 macrophages is upregulated after SP stimulation through enhancement of TGFβ1-mediated NK1R signaling. Pretreatment of macrophages with a p38γ/p38δMAPK inhibitor (BIRB796) significantly decreased the expression of RANTES protein by SP-stimulated macrophages, while neither a p38α/p38β inhibitor (SB203580) nor an ERK1 inhibitor (PD98059) altered RANTES expression. Treatment of macrophages with a high concentration of an ERK1/2 inhibitor (U0126) also had no effect on RANTES production after stimulation with SP (Figure 5b). Interestingly, SP has been reported to promote the phosphorylation of p38MAPK and ERK1/2 [60]. RANTES production by SP-stimulated macrophages was significantly suppressed by a p38γ/ p38δ inhibitor (BIRB796). Treating macrophages with β-arrestin 2 siRNA and GRK2 siRNA resulted in significant upregulation of the production of RANTES protein, whereas TGFβ1/2/3 siRNA or a dynamin inhibitor (dynasore) attenuated RANTES production and a Rho-associated coiled-coil forming kinase [ROCK] inhibitor (Y -27632) had no effect (Figure 5c). G protein-coupled receptor kinases (GRKs) regulate GPCRs by causing receptor desensitization and internalization through phosphorylation of the intracellular domain of the active receptor. GRK2 also mediates receptor internalization via β-arrestin-independent mechanisms. It was found that treating macrophages with siRNA for β-arrestin 2 or GRK2 delayed internalization of NK1R and enhanced its signaling. Enhancement of RANTES production was blunted by transfection of macrophages with siRNAs for TGFβ1/2/3, suggesting that TGFβ1 increases RANTES expression in response to activation of NK1R by SP. Delayed internalization promotes NK1R signaling and TGFβ1 was reported to delay SP-induced internalization of NK1R, resulting in enhancement of its signaling [61]. Interestingly, silencing of Sp1 led to a significant increase of TGFβ1 protein production by SP-stimulated macrophages. Mithramycin inhibits the binding of Sp1 family members to DNA and adding mithramycin to macrophage cultures resulted in a significant concentration-dependent increase of TGFβ1 protein production. Surprisingly, transfection of macrophages with C/EBPβ siRNA attenuated TGFβ1production, unlike the effect of Sp1 siRNA. On the other hand, Sp1 siRNA significantly upregulated RANTES protein production by SP-stimulated macrophages compared to untreated macrophages. Mithramycin also caused concentration-dependent elevation of the RANTES protein level in response to stimulation with SP, while silencing of C/EBPβ attenuated RANTES production (Figure 5d). These findings showed that SP upregulates TGFβ1 expression in SP-stimulated macrophages, along with increased RANTES production. Western blotting confirmed that TGFβ1 expression was elevated by Sp1 siRNA or mithramycin, but not by C/EBPβ siRNA (Figure 5e). Both transcription factor Sp1 and C/EBPβ are promoters of TGFβ1 [56,62]. When the effect of Sp1 or C/EBPβ on TGFβ1 protein production by SPstimulated macrophages was investigated, it was unexpectedly found that silencing of Sp1 led to significant upregulation of TGFβ1 expression and a consequent increase of RANTES protein. Mithramycin is a geneselective inhibitor of Sp1 that binds to GC-rich DNA sequences and displaces Sp [63] or modulates Sp1 protein levels by regulating proteasome-dependent degradation [64]. Inhibition of Sp1 by mithramycin led to a concentration-dependent increase of TGFβ1 and RANTES protein levels. Various cytokine/chemokine genes are induced or repressed by the transcription factor C/EBPβ, and transfection of macrophages with C/EBPβ siRNA inhibited TGFβ1 production. The influence of cross-talk among four transcription factors (Sp1 and C/ EBPβ, TIF1β, or Fli-1) on RANTES expression by macrophages was investigated after double transfection with siRNAs for these factors. Compared with siRNAs for TIF1β or Fli-1, C/EBPβ siRNA caused significant inhibition of RANTES production by Sp1 siRNA-transfected macrophages after stimulation with SP (Figure 5f). Activated p38 MAPK regulates C/EBPβ via phosphorylation [65], so these findings suggested that SP may increase TGFβ1 expression via the NK1R/ p38γδMAPK/C/EBPβ signaling pathway. Accordingly, Sp1 and C/ EBPβ have opposite influences on expression of TGFβ1. Cross-talk among transcription factor pathways is complicated, with different combinations of transcription factors having additive, synergistic, or antagonistic effects. It is known that C/EBP-β binds to various response elements and forms heteromeric complexes with other transcription factors, including Sp1. The C/EBPβ promoter contains a TATA box and has binding sites for several transcription factors regulating its mRNA expression, including C/EBPβ itself [66], signal transducer and activator of transcription 3 (STAT3) [67], and Sp1 [68]. Inhibition of IL-12p40 production via the signal regulatory protein α (SIRPα)/surfactant protein D (SP-D) signaling pathway SIRPα is a highly glycosylated type-1 transmembrane protein comprising three immunoglobulin-like extracellular loops and a cytoplasmic tail that has three classical tyrosine-based inhibitory motifs. Western blotting showed that GMCSF upregulates SIRPα expression by macrophages (Figure 6a). It was found that an ERK inhibitor (PD98059) significantly suppressed the response of SIRPα to GM-CSF, whereas this response was only partially inhibited by a p38α/βMAPK inhibitor (SB203580), an intracellular Ca2+ antagonist (TMB-8), or an NF-κB inhibitor (PDTC) (Figure 6b). All SIRPs possess extracellular domains with a distal immunoglobulin variable-like fold (D1) and two proximal immunoglobulin constantlike folds (D2-D3) [69]. CD47-SIRPα signaling was reported to downregulate responsiveness to IL-12 and inhibit the activation of dendritic cells [70]. The epithelium of pulmonary alveoli is largely composed of type I and type II alveolar cells, with type II cells producing GM-CSF and SP-D. It was reported that SP-D binds to the proximal domain (D3) of SIRPα, which is distant from the binding domain D1 of CD47 [71]. Binding of CD47 to SIRPα initiates signaling that inhibits phagocytosis [72] via several downstream molecules, including Src homology 2-containing phosphotyrosine phosphatase (SHP) and Ras homolog gene family member A (RhoA). GM-CSF was initially found in conditioned lung tissue medium after injection of LPS into mice [73]. Recruitment of monocytes to the lungs is required for normal immune function and the inflammatory response to pulmonary injury, and resident pulmonary macrophages are reported to exist in close proximity to the respiratory epithelium [74]. The IL-12 receptor (IL- 12R) has two known subunits, which are IL-12R β 1 and IL-12R β 2 [75]. In humans, IL-12R β2 is expressed by airway and parenchymal fibroblasts, and IL-12 signaling via its β2 subunit leads to the phosphorylation and activation of signal transducer and activator of transcription 4 (STAT4), promoting pulmonary fibrosis. IL-12 also promotes the expression of type 1α1 collagen and transforming growth factor-β1 by fibroblasts, which are involved in remodeling small airways, and the serum level of IL-12p40 is elevated in idiopathic pulmonary fibrosis [76]. These reports suggest that by GM-CSFdependent macrophages infiltrating or residing in the lungs of patients with recurrent pulmonary infection due to gram-negative bacteria might produce IL-12p40. Exposure of GM-CSF-dependent macrophages to LPS caused a concentration-dependent increase of IL- 12p40 production, while SP-D caused concentration-dependent suppression of the response of IL-12p40 to LPS (Figure 6c). When GMCSF- dependent macrophages were pretreated with SP-D (5 μM) on day 9 of culture and then were exposed to LPS (10 ng) after 30 min, 3 h, or 6 h, the inhibitory effect of SP-D on IL-12p40 production became stronger over time (Figure 6d). On the other hand, silencing of SIRPα/ β/γ led to significant blunting of this effect of SP-D. Preincubation of macrophages with SP-D suppressed the phagocytosis of apoptotic cells via interaction with SIRPα involving several downstream molecules, including SHP and RhoA [77]. Phagocytosis was not suppressed by SP-D in SHP-deficient mice, and it was also blocked by sodium stibogluconate and by a ROCK inhibitor (Y27632). Transfection of macrophages with siRNA for SHP did not affect the response of these cells to SP-D, which is primarily a positive effector of receptor tyrosine kinase signaling that interacts with EGFR via tyrosine-phosphorylated adaptor proteins through its SH2 domains [78]. However, silencing EGFR did not influence the inhibition of IL-12p40 production by SP-D in LPS-stimulated macrophages. SHP-2 positively regulates the oxidative burst in macrophages [79]. The NADPH oxidase family is important for ROS production and p22phox protein is an essential component of membrane-associated NADPH oxidase. In addition, β-arrestin 2 mediates recruitment of SHP-1 and SHP-2 [80], and protein-tyrosine phosphatase Shp2 positively regulates the oxidative burst in macrophages. However, transfection of macrophages with siRNAs for β-arrestin 2, p22phox, or EGFR did not blunt the inhibitory effect of SP-D on IL-12p40 production after stimulation with LPS (Figure 6e). Interestingly, suppression of IL-12p40 production when LPS-stimulated macrophages were treated with SP-D was significantly attenuated by Y-27632, but not by SHP siRNA (Figure 6f). ERK and p38MAPK play different roles in regulating IL-12 gene expression in response to LPS stimulation. Activation of p38MAPK promotes the expression of IL-12p40 mRNA after LPS stimulation, whereas ERK activation suppresses transcription of IL-12 [81]. Neither a p38α/β MAPK inhibitor (SB203580) nor a p38δ/γ MAPK inhibitor (BIRB796) influenced the inhibition of IL-12p40 production by SP-D in LPSstimulated macrophages (Figure 6g). On the other hand, treatment of macrophages with ERK1/2 siRNA blunted the restoration of IL-12p40 production by Y-27632. The ROCK inhibitor Y-27632 was shown to restore IL-12p40 production by SP-D-treated macrophages (Figure 6h). Y-27632 was reported to suppress the activation of ERK1/2 [82]. Silencing ERK1/2 blunted the restoration of IL-12p40 production by Y-27632 in SP-D-treated macrophages after LPS stimulation. ERK shows an anti-inflammatory effect by suppressing the expression of NF- κB-dependent inflammatory genes through inhibition of IκB kinase activity [83]. These findings indicate that SP-D inhibits the production of IL-12p40 by LPS-stimulated macrophages via the SIRPα/ROCK/ ERK signaling pathway. PAR-2 agonists (HNE or AC-264613) differentially regulate IL-12p40 production by GM-CSF-dependent human macrophages after LPS stimulationPretreatment with HNE synergistically increased the IL-12p40 protein level after LPS stimulation of GM-CSF-dependent macrophages [28]. The influence of PAR2 activation was compared among HNE (a native peptide agonist), 2-furoyl-LIGRLO-amide (a synthetic peptide agonist), and AC-264613 (a non-peptide agonist). It was unexpectedly found that pretreatment with AC-264613 attenuated IL-12p40 production by macrophages after LPS stimulation compared to pretreatment with HNE (Figure 7a). Tumor necrosis factor receptor associated factor 6 (TRAF6) is the key adaptor in the TLR4 signaling pathway [84]. TLR4 induces IL-12p40 expression in macrophages [85], while HNE activates both TLR4 [86,87] and PAR-2, so HNE-TLR4 interaction may influence IL-12p40 production. HNE also stimulates MyD88, IRAK, and TRAF6 signal transduction, leading to NF-κB activation and induction of various cytokines [88]. The IRF transcription factor family is a member of the winged helix-turn-helix DNA-binding domain superfamily [89]. IRF-5 is important for innate antiviral and inflammatory responses, and is activated by TLR4 [90]. Because IRF5 expression is upregulated by GMCSF [91], it shows higher expression in GM-CSF-dependent macrophages than M2 macrophages. IRF5 directly activates transcription of genes encoding IL-12p4, IL-12p35, and IL-23p19 [33]. Treatment of macrophages with siRNA for IRF5 significantly reduced IL-12p40 production after stimulation with LPS (Figure 7b). Treating macrophages with HNE caused a concentration-dependent decrease of IRF5 protein expression (Figure 7c), while siRNA for PAR-2 or betaarrestin 2 blunted this effect. Silencing SPAK/JNK also suppressed the effect of HNE on macrophages, but STAT3 siRNA had a weaker influence (Figure 7d). PAR-2 is involved in the regulation of apoptosis [92], and PAR-2 signaling is independently mediated via a β-arrestin 2-dependent pathway and a G-protein/Ca2+ pathway. β-arrestin interacts with mouse double minute 2 homolog
Figure 5: (a) Signaling pathway after NK1R activation. (b) Effect of MAPK inhibitors on production of regulated on activation, normal T cell expressed and secreted (RANTES) by macrophages stimulated with substance P (SP). (c) Effects of TGFβ1/2/3 siRNA, GRK2 siRNA, β-arrestin 2 siRNA, Y-27632 (a ROCK inhibitor) or Dynasore (a dynamin inhibitor) on RANTES production after exposure to SP. (d) Effect of TGFβ1/2/3 siRNA, C/EBPβ siRNA, specific protein 1 (Sp1) siRNA or mithramycin (an Sp1 inhibitor) on production of TGFβ1 or RANTES after stimulation with SP. (e) Western blotting for TGFβ1. (f) Effects of cross-talk between Sp1 and Fli-1, TIF1β or C/EBPβ on RANTES production after stimulation with SP. Data were obtained using macrophages from three individuals in each group and represent the mean + SE. *P<.05; **P< .01 (with Bonferroni’s correction); N.S. not significant.
Figure 6: (a) Western blotting for signal regulatory protein α (SIRPα). (b) Effect of PD98059, TMB-8, SB203580 or PDTC on SIRPα production and western blotting for SIRPα. (c) Inhibitory effect of surfactant protein D (SP-D) on IL-12p40 production after exposure of macrophages to lipopolysaccharide (LPS). (d) Time-dependent inhibitory effect of SP-D on IL-12p40 production after exposure to LPS. (e) Effects of silencing SIRPα/β/γ, EGFR, p22phox or β-arrestin 2 on IL-12p40 production after exposure to LPS. (f) Effects of Src homology 2-containing phosphotyrosine phosphatase (SHP) siRNA or Y27632 (a ROCK inhibitor) on IL-12p40 production after pretreatment with SP-D and exposure to LPS. (g) Effects of BIRB796 or SB203580 on IL-12p40 after pretreatment with SP-D and exposure to LPS. (h) Effects of Y-27632 (a ROCK inhibitor) and extracellular signal-regulated kinase 1/2 (ERK1/2) siRNA on IL-12p40 after pretreatment with SP-D and exposure to LPS. Data were obtained using macrophages from three individuals in each group and represent the mean + SE. *P<.05; **P< .01 (with Bonferroni’s correction); N.S. not significant.
Figure 7: (a) Effect of pretreatment with PAR-2 agonist HNE or AC-264613 on IL-12p40 production by macrophages after exposure to LPS. (b) Effect of interferon regulatory factor 5 (IRF5) siRNA on IL-12p40 production after exposure to LPS. (c)Inhibitory effect of HNE on IRF5 production. (d) Effect of silencing β-arrestin 2, signal transducer and activator of transcription 3 (Stat3), or c-Jun N-terminal kinase (JNK) on RF5 production after exposure to HNE. (e) Inhibitory effect of HNE on p53 production. (f) Effect of silencing β-arrestin 2, signal transducer and activator of transcription 3 (Stat3), or c-Jun N-terminal kinase (JNK) on p53 production after exposure to HNE. (g) Western blotting for IRF5 after stimulation with HNE and p53 siRNA. (h) Effect of PAR-2 agonists, AC-264613, LIGRO, and HNE on p53 production. (i) Western blotting for IRF5 and effect of PAR-2 agonists, AC-264613, LIGRO, and HNE on IRF5 production. Data were obtained using macrophages from three individuals in each group and represent the mean + SE. *P<.05; **P< .01 (with Bonferroni’s correction); N.S. not significant.
(MDM2), anE3 ubiquitin-protein ligase that ubiquitinates p53 and thus promotes its degradation by the ubiquitin-proteasome system [93]. Therefore, HNE may reduce the p53 level in macrophages by activating the PAR2/β-arrestin 2/MDM2 signaling pathway. In fact, a concentration-dependent decrease of p53 protein expression was noted when macrophages were incubated with HNE (Figure 7e). It was found that siRNA for beta-arrestin 2 blunted this effect of HNE, but silencing STAT3 did not (Figure 7f). Furthermore, treatment with HNE led to a marked and concentration-dependent decrease of IRF5 expression in GM-CSF-dependent macrophages that had been transfected with siRNA for p53 [34]. Degradation of p53 is mediated by either MDM2 or JNK [94]. ELISA showed that treatment with HNE or AC-264613 significantly reduced the p53 protein level in GM-CSF-dependent macrophages, whereas 2-furoyl-LIGRLO-amide had little effect [34]. Furthermore, AC-264613 reduced IRF5 expression significantly more than the peptide PAR-2 agonists LIGRO or HNE (Figure 7g). IRF5 is a direct target of p53 that may mediate the immune effects of p53 [95]. TRAF6 is required for expression of the target genes of p53 [96]. Incubation of GM-CSF-dependent macrophages with either HNE or a non-peptide PAR2 agonist (AC-264613) reduced both IRF5 and p53expression. However, HNE promoted TLR4 transactivation via upregulation of TRAF6, while AC-264613 had little influence on TLR4 transactivation. Accordingly, LPS-stimulated macrophages treated with AC-264613 showed significantly lower IL-12p40 protein expression than macrophages treated with HNE. Transformation from GM-CSFdependent to M-CSF-dependent macrophages GM-CSF and M-CSF induce different changes in cells of the macrophage lineage. Basal levels of GM-CSF are low, but elevation occurs during immune/inflammatory reactions. Transformation from proinflammatory to anti-inflammatory macrophages has been reported in experimental studies, e.g., treating murine RAW 264.7 cells with substance P induces the M-CSF dependent like macrophage phenotype via HO-1 expression [97]. It was been reported that SP induces transformation of GM-CSF-dependent rat macrophages to an M-CSF-dependent like phenotype [98,99]. GMCSF- dependent human macrophages and M-CSF-dependent human macrophages were exposed to substance P for 6 h, followed by western blotting to assess cell markers. Before stimulation with SP, GM-CSFdependent macrophages were CD80highCD163low, while M-CSFdependent macrophages were CD80lowCD163high. Incubation with SP increased expression of both CD163 and CD80, soCD80lowCD163high M-CSF-dependent like macrophages were not induced.
Conclusion
Therefore, incubation of human GM-CSF-dependent macrophages with substance P for 6 h did not result in a shift to the M-CSF-dependent like phenotype, unlike murine and rat M1 macrophages.
Acknowledgement
This study was partly supported by a Kumamoto Health Science University special fellowship grant (No. 2018-C-2).
Role of the Funding Source
The funding source had no role in the design of this study, in collection, analysis, and interpretation of the data, in writing the report, or in the decision to submit this manuscript.
References
- Wicks IP, Roberts AW (2016) Targeting GM-CSF in inflammatory diseases. Na Rev Rheumatol 12: 37-48.
- Hamilton JA (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat Rev mmunol 8: 533-544
- El-Behi M, Ciric B, Dai H (2011) The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 12: 568-575.
- Codarri L, Gyülvészi G, Tosevski V (2011) RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12 :560-567.
- Rasouli J, Ciric B, Imitola J (2015) Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-β Therapy. J Immunol 194: 5085-5093.
- Tesmer LA, Lundy SK, Sarkar S (2008) Th17 cells in human disease. Immunol Rev 223: 87-113.
- Puig L (2017) The role of IL 23 in the treatment of psoriasis. Expert Rev Cli Immunol 13: 525-534
- Yamaguchi R, Sakamoto A, Yamamoto T (2017) Differential regulation of IL-23 production in M1 macrophages by TIR8/SIGIRR through TLR4- or TLR7/8-mediated  signaling. Cytokine 99: 310-315.
- Schneemann M, Schoeden G (2007) Macrophage biology and immunology: man is not a mouse. J Leukoc Biol 81: 579.
- Zabel BA, Zuniga L, Ohyama T (2006) Chemoattractants, extracellular proteases, and the integrated host defense response. Exp Hematol 34: 1021-1032.
- Rothmeier AS, Ruf W (2012) Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol 34: 133-149.
- Meyer-Hoffert U, Wiedow O (2011) Neutrophil serine proteases: mediators of innate immune responses. Curr Opin Hematol 18: 19-24.
- Aoki M, Yamaguchi R, Yamamoto T (2015) Granulocyte-macrophage colony-stimulating factor primes interleukin-13 production by macrophages via protease-activated receptor-2. Blood Cells Mol Dis 54: 353-359.
- Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, NK, and p38 protein kinases. Science 298: 1911-1912.
- Banfi C, Brioschi M, Barbieri SS (2009) Mitochondrial reactive oxygen species: a common athway for PAR1- and PAR2-mediated tissue factor induction in human endothelial cells. J Thromb Haemost 7: 206-216.
- Rharass T, Lemcke H, Lantow M (2014) Ca2+-mediated mitochondrial reactive oxygen species metabolism augments Wnt/β-catenin pathway activation to facilitate cell differentiation. J Biol Chem 289: 27937-27951.
- Yamaguchi R, Yamamoto T, Sakamoto A (2015) Mechanism of interleukin-13 production by granulocyte-macrophage colony-stimulating factor-dependent macrophages via protease-activated receptor-2. Blood Cells Mol Dis 55: 21-26.
- Wills-Karp M (2004) Interleukin-13 in asthma pathogenesis. Immunol Rev 202: 175-190.
- Fuss IJ, Strober W (2008) The role of IL-13 and NK T cells in experimental and human ulcerative colitis. Mucosal Immunol 1 Suppl 1: S31-33.
- Lee CG, Homer RJ, Zhu Z (2001) Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 194: 809-821.
- Novovic S, Andersen AM, Nord M (2013) Activity of neutrophil elastase reflects the progression of acute pancreatitis. Scand J Clin Lab Invest 73: 485-493.
- Yadav D, O'Connell M, Papachristou GI (2012) Natural history following the first attack of acute pancreatitis. Am J Gastroenterol 107: 1096-1103.
- ItoT (2007) Can measurement of chemokines become useful biological and functional markers of early-stage chronic pancreatitis? J Gastroenterol. 42 Suppl 17: 72-77.
- Shinozaki S, Mashima H, Ohnishi H (2010) IL-13 promotes the proliferation of  rat pancreatic stellate cells through the suppression of NF-kappaB/TGF-beta1 pathway. Biochem Biophys Res Commun 393: 61-65.
- Shinde AV, Humeres C, Frangogiannis NG (2017) The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta Mol Basis Dis 1863: 298-309.
- Xue J, Sharma V, Hsieh MH (2015)Alternatively activated macrophages promote  pancreatic fibrosis in chronic pancreatitis. Nat Commun 6: 7158.
- Nhu QM, Shirey K, Teijaro JR (2010) Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol 3: 29-39.
- Yamaguchi R, Yamamoto T, Sakamoto A (2016) Neutrophil elastase enhances IL-12p40 production by lipopolysaccharide-stimulated macrophages via transactivation of the PAR-2/EGFR/TLR4 signaling pathway. Blood Cells Mol Dis 59: 1-7.
- Chattopadhyay S, Veleeparambil M, Poddar D (2015) EGFR kinase activity is required for TLR4 signaling and the septic shock response. EMBO Rep 16: 1535-1547.
- De S, Zhou H, DeSantis D (2015) Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc Natl Acad Sci U S A 112: 9680-9685.
- Wu Y, Lu J, Antony S (2013) Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-γ and lipopolysaccharide in human pancreatic cancer cell lines. J Immunold 190: 1859-1872.
- Nadeem A, Alharbi NO, Vliagoftis H (2015) Proteinase activated receptor-2-mediated dual xidase-2 up-regulation is involved in enhanced airway reactivity and inflammation in a mouse model of allergic asthma. Immunology 145: 391-403.
- Krausgruber T, Blazek K, Smallie T (2011) IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 12: 231-238.
- Yamaguchi R, Yamamoto T, Sakamoto A (2016) A protease-activated receptor 2 agonist (AC-264613) suppresses interferon regulatory factor 5 and decreases interleukin-12p40 production by lipopolysaccharide-stimulated macrophages: Role of p53. Cell Biol Int 40: 629-641.
- Martin HJ, Lee JM, Walls D (2007) Manipulation of the toll-like receptor 7 signaling pathway by Epstein-Barr virus. J Virol 81: 9748-9758.
- Qin J, Qian Y, Yao J (2005) SIGIRR inhibits interleukin-1 receptor- and toll-like  receptor 4-mediated signaling through different mechanisms. J Biol Chem  280: 25233-25241.
- Gulen MF, Kang Z, Bulek K (2010) The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity 32: 54-66.
- Xu D, Meyer F, Ehlers E (2011) Interferon regulatory factor 4 (IRF-4) targets IRF-5 to regulate Epstein-Barr virus transformation. J Biol Chem  286: 18261-18267.
- Eguchi J, Kong X, Tenta M (2013) Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes 62: 3394-3403.
- Adib-Conquy M, Adrie C, Fitting C (2006) Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients. Crit Care Med 34: 2377-2385.
- O'Connor TM, O'Connell J, O'Brien DI (2004) The role of substance P in inflammatory disease. J Cell Physiol 201: 167-180.
- May A, Goadsby PJ (2001) Substance P receptor antagonists in the therapy of migraine. Expert Opin Investig Drugs 10: 673-678.
- Beinborn M, Blum A, Hang L (2010) TGF-beta regulates T-cell neurokinin-1 receptor internalization and function. Proc Natl Acad Sci U S A 107: 4293-4298.
- Palma C, Nardelli F, Manzini S (1999) Substance P activates responses correlated with tumour growth in human glioma cell lines bearing tachykinin NK1 receptors. Br J Cancer 79: 236-243.
- Yamaguchi R, Sakamoto A, Yamaguchi R (2018) Transcription factor specificity protein 1 modulates TGFβ1/Smad signaling to negatively regulate SIGIRR expression by human M1 macrophages stimulated with substance P. Cytokine 108: 24-36.
- Cao J, Wang M, Wang T (2017) CCAAT enhancer binding protein β has a crucial role in regulating breast cancer cell growth via activating the TGF-β-Smad3 signaling pathway. Exp Ther Med 14: 1554-1560.
- Rooney JW, Calame KL (2001) TIF1beta functions as a coactivator for /EBPbeta and is required for induced differentiation in the myelomonocytic cell line U937. Genes Dev 15: 3023-3038.
- Koinuma D, Tsutsumi S, Kamimura N (2009) Chromatin immunoprecipitation on microarray analysis of Smad2/3 binding sites reveals roles of ETS1 and TFAP2A in transforming growth factor beta signaling. Mol Cell Biol 29: 172-186.
- Schmidlin F, Dery O, DeFea KO (2001) Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J Biol Chem  276: 25427-25437.
- Morlot S, Roux A (2013) Mechanics of dynamin-mediated membrane fission. Annu Rev Biophys 42: 629-649.
- McConalogue K, Déry O, Lovett M (1999) Substance P-induced trafficking of  beta-arrestins. The role of beta-arrestins in endocytosis of the neurokinin-1 receptor. J Biol Chem 274: 16257-16268.
- Fong G, Backman LJ, Alfredson H (2017) The effects of substance P and acetylcholine on human tenocyte proliferation converge mechanistically via TGF-β1.PLoS One 12: e0174101.
- Douglas SD, Leeman SE (2011) Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci 1217: 83-95.
- Abraham S, Sweet T, Khalili K (2009) Evidence for activation of the TGF-beta1 promoter by C/EBPbeta and its modulation by Smads. J Interferon Cytokine Res 29:1-7.
- Sakamoto A, Yamaguchi R (2018) Cross-talk between the transcription factor Sp1 and C/EBPβ modulates TGFβ1 production to negatively regulate the expression of chemokine RANTES. Heliyon 4: e00679.
- Ho WZ, Lai JP, Zhu XH (1997) Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 159: 5654-5660.
- Yamaguchi R, Yamamoto T, Sakamoto A (2016) Substance P enhances tissue factor release from granulocyte-macrophage colony-stimulating factor-dependent  macrophages via the p22phox/β-arrestin 2/Rho A signaling pathway. Blood Cell Mol Dis 57: 85-90.
- Louvet A, Teixeira-Clerc F, Chobert MN (2011) Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 54: 1217-1226.
- Liu H, Ning H, Men H (2012) Regulation of CCL5 expression in smooth muscle  cells following arterial injury. PLoS One 7: e30873.
- Chakraborty S, Nepiyushchikh Z, Davis MJ (2011) Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation 18: 24-35.
- Roux SL, Borbely G, SÅ‚oniecka M (2016) Transforming Growth Factor Beta 1 Modulates the Functional Expression of the Neurokinin-1 Receptor in Human Keratocytes. Curr Eye Res 41: 1035-1043.
- Presser LD, McRae S, Waris G (2013) Activation of TGF-β1 promoter by hepatitis C virus-induced AP-1 and Sp1: role of TGF-β1 in hepatic stellate cell activation and invasion. PLoS One 8: e56367.
- Sleiman SF, Langley BC, Basso M (2011) Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J Neurosci 31: 6858-6870.
- Choi ES, Nam JS, Jung JY (2014) Modulation of specificity protein 1 by  mithramycin A as a novel therapeutic strategy for cervical cancer. Sci Rep 4: 7162.
- Ambrosino C, Iwata T, Scafoglio C (2006) TEF-1 and C/EBPbeta are major  p38alpha MAPK-regulated transcription factors in proliferating cardiomyocytes. Biochem J 396: 163-172.
- Foka P, Kousteni S, Ramji DP (2001) Molecular characterization of the Xenopus CCAAT-enhancer binding protein beta gene promoter. Biochem Biophys Res Commun 285: 430-436.
- Niehof M, Streetz K, Rakemann T (2001) Interleukin-6-induced tethering of  STAT3 to the LAP/C/EBPbeta promoter suggests a new mechanism of transcriptional  regulation by STAT3. J Biol Chem 276: 9016-9027.
- Berrier A, Siu G, Calame K (1998) Transcription of a minimal promoter from the NF-IL6 gene is regulated by CREB/ATF and SP1 proteins in U937 promonocytic cells. J Immunol 161: 2267-2275.
- Lee WY, Weber DA, Laur O (2010) The role of cis dimerization of signal regulatory protein alpha (SIRPalpha) in binding to CD47. J Biol Chem  285: 37953-37963.
- Latour S, Tanaka H, Demeure C (2001) Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol 167: 2547-2554.
- Fournier B, Andargachew R, Robin AZ (2012) Surfactant protein D (Sp-D) binds to membrane-proximal domain (D3) of signal regulatory protein α (SIRPα), a site distant from binding domain of CD47, while also binding to analogous region on signal regulatory protein β (SIRPβ). J Biol Chem 287: 19386-19398.
- Barclay AN, Van den Berg TK (2014) The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 32: 25-50.
- Burgess AW, Camakaris J, Metcalf D (1977) Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem 252: 1998-2003.
- Kopf M, Schneider C, Nobs SP (2015) The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 16: 36-44.
- Hackett TL, Shaheen F, Zhou S (2014) Fibroblast signal transducer and activator of transcription 4 drives cigarette smoke-induced airway fibrosis. Am J Rspir Cell Mol Biol 51: 830-839.
- Wu C, Wang X, Gadina M (2000) IL-12 receptor beta 2 (IL-12R beta 2)-deficient  mice are defective in IL-12-mediated signaling despite the presence of high affinity  IL-12 binding sites. J Immunol 165: 6221-6228.
- Tsoutsou PG, Gourgoulianis KI, Petinaki E (2006) Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Respir Med 100: 938-945.
- Janssen WJ, McPhillips KA, Dickinson MG (2008) Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med 178: 158-167.
- Agazie YM, Hayman MJ (2003) Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol 23: 7875-7886.
- Li XJ, Goodwin CB, Nabinger SC (2015) Protein-tyrosine phosphatase Shp2 positively regulates macrophage oxidative burst. J Biol Chem 290: 3894-3909.
- Yu MC, Su LL, Zou L (2008) An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol 9: 898-907.
- Feng GJ, Goodridge HS, Harnett MM (1999) Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol 163: 6403-6412.
- Cheng MK, Doumad AB, Jiang H (2004) Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol 141: 441-448.
- Maeng YS, Min JK, Kim JH (2006) ERK is an anti-inflammatory signal that suppresses expression of NF-kappaB-dependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell Signal 18: 994-1005.
- Liu A, Gong P, Hyun SW (2012) TRAF6 protein couples Toll-like receptor 4 signaling to rc family kinase activation and opening of paracellular pathway in human lung microvascular endothelia. J Biol Chem 287: 16132-16145.
- Robinson RT, Khader SA, Locksley RM (2008) Yersinia pestis evades TLR4-dependent induction of IL-12(p40)2 by dendritic cells and subsequent cell migration. J Immunol 181: 5560-5567.
- Devaney JM, Greene CM, Taggart CC (2003) Neutrophil elastase up-regulates  interleukin-8 via toll-like receptor 4. FEBS Lett 544: 129-132.
- Benabid R, Wartelle J, Malleret L (2012) Neutrophil elastase modulates cytokine expression: contribution to host defense against Pseudomonas aeruginosa-induced pneumonia. J Biol Chem 287: 34883-34894.
- Walsh DE, Greene CM, Carroll TP (2001) Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J Biol Chem 276: 35494-35499.
- Taniguchi T, Ogasawara K, Takaoka A (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19: 623-655.
- Saigusa R, Asano Y, Taniguchi T (2015) Multifaceted contribution of the TLR4-activated IRF5 transcription factor in systemic sclerosis. Proc Natl Acad Sci 112: 15136-151341.
- Lawrence T, Natoli G (2011) Transcriptional regulation of macrophage polarization:enabling diversity with identity. Nat Rev Immunol 11: 750-761.
- Ahmad S, Ahmad A, Rancourt RC (2013) Tissue factor signals airway epithelial basal cell survival via coagulation and protease-activated receptor isoforms 1 and 2. Am J Respir Cell Mol Biol 48: 94-104.
- Wang P, Gao H, Ni Y (2003) Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem 278: 6363-6370.
- Cui YX, Kerby A, McDuff FK (2009) NPM-ALK inhibits the p53 tumor suppressor pathway in an MDM2 and JNK-dependent manner. Blood 113: 5217-5227.
- Mori T, Anazawa Y, Iiizumi M (2002). Identification of the interferon regulatory factor 5 gene (IRF-5) as a direct target for p53. Oncogene 21: 2914-2918.
- Zhang X, Li CF, Zhang L (2016) TRAF6 Restricts p53 Mitochondrial Translocation, Apoptosis, and Tumor Suppression. Mol Cell 64: 803-814.
- Montana G, Lampiasi N (2016) Substance P Induces HO-1 Expression in RAW 264.7 Cells Promoting Switch towards M2-Like Macrophages. PLoS One 11: e0167420.
- Lim JE, Chung E, Son Y (2017) A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci Rep 7: 9417.
Citation: Yamaguchi R, Sakamoto A, Yamaguchi R, Haraguchi M, Narahara S, et al. (2019) Essential Role of GM-CSF-Dependent Macrophages in Human Autoimmune and Inflammatory Responses. J Cytokine Biol 4: 128.
Copyright: © 2019 Yamaguchi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3649
- [From(publication date): 0-2019 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 2689
- PDF downloads: 960