Groundnut (Arachis hypogea L.) Storage Methods and Associated Mycotoxin in Ethiopia
Received: 03-Apr-2022 / Manuscript No. acst-22-52865 / Editor assigned: 06-Apr-2022 / PreQC No. acst-22-52865(PQ) / Reviewed: 11-Apr-2022 / QC No. acst-22-52865 / Revised: 17-Apr-2022 / Manuscript No. acst-22-52865(R) / Published Date: 25-Apr-2022 DOI: 10.4172/2329-8863.1000507
Abstract
The assessment was conducted in major groundnut producing areas of Ethiopia. The objective of the study was to detect important mycotoxins in stored groundnut and to estimate post-harvest loss due to associated mycotoxigenic fungi and mycotoxins in stored groundnut. The collected samples were detected by using culture media and HPLC with the standard laboratory methods of SOP/7.2-c-23. The collected data was analyzed using SPSS (version: 26.0) and the mean separated by LSD. The collected samples were composited into five, OR-ELW mixed, EIH Babile mixed, Werer mixed, Limu Shayi mixed and Limu Kosa mixed for the aflatoxin detection. Higher Levels of AFB1 386.10 and 360.96 μg/kg were recorded in the sample collected from Limu Kosa and Limu Shayi areas with the total aflatoxin of 542.25 μg/kg. From the study it was observed that at the initial storage periods the amount of aflatoxin in the sample was low. Therefore, aflatoxins content in the sample shows an increasing trend as the storage periods increased due to the farmers stored immature, drying on the ground with soil contamination, using improper storage methods and laying sacks one over the other which favors the increment of moisture content and temperature and support the development of moulds. Therefore, storage periods are the single most important factor if there are favorable environmental conditions for the development of the toxins. Extension workers interventions are more advisable especially during storage of the crop for the control of the impact of aflatoxins and for exporting safe products and reducing health risks.
Keywords
Aflatoxin; Chromatogram; Food losses; Mycotoxin; Oil seed; Storage
Introduction
Groundnut (Arachis hypogea L.), which is also known as peanut, earthnut, monkey nut and goobers, is an annual legume. It ranks the 13th most important food crop and 4th most important oilseed crop of the world being cultivated in more than 100 countries in six continents. Despite of its significant the crop is affected by mycotoxins such as aflatoxins (AFs), fumonisins (FUMs), deoxynivalenol (DON), ochratoxin A (OTA), and zearalenone (ZEN) are agriculturally important. Different studies showed that 18 different aflatoxins were identified, with aflatoxin B1, B2, G1, G2, M1, and M2 being the most common. Among these, Aflatoxin B1 and G1 arise frequently (Mishra and Das 2003). Reports the amount of aflatoxin in peanut were 5 to 250 μg/kg. Also, Eshetu (2010) stated the aflatoxin level was reached up to 447 μg/kg in groundnut seed from his assessment in eastern Ethiopia. Additionally, the finding of showed the aflatoxin B1 level was up to 2,526 and 158 μg/kg, in groundnut seed and groundnut cake from the local “Halawa”, respectively, of eastern Ethiopia. Aflatoxins are highly harmful, immunosuppressive, mutagenic, teratogenic and carcinogenic chemicals increase the liver disease and carcinogenicity. Moreover, reports about 40% of liver cancer incidences in Africa have been allocated to dietary due to aflatoxin exposure. Mycotoxin’s infection has strictly affected Africa consequential in huge economic loss; for example, AFs contamination of crops alone has been reported to cause an annual loss of more than USD 750 million [1]. The economic losses imposing the rejection of the country’s export products in response to dangerous levels of mycotoxins. Numerous factors of storage facilities the degree to which fungal growth and aflatoxin contaminations. Some of these factors are relative humidity and temperature within the storage and the produce. Diener and Cole (1982) observed that when the seed moisture exceeds 9% at the equilibrium humidity of 80% and 30°C temperature, the chances of invasion by Aspergillus flavus increase drastically. The development of mycotoxigenic fungi associated to stored groundnut in Ethiopia and its correlations to the country’s vast agro ecological disparity, farmers’ poor storage practices, and its impact have not been documented. Therefore, the objective of this research is: - To detect and quantify important mycotoxins. To estimate PHL due to associated mycotoxigenic fungi and mycotoxins in stored groundnut [2].
Materials and Methods
Survey areas and sample collection
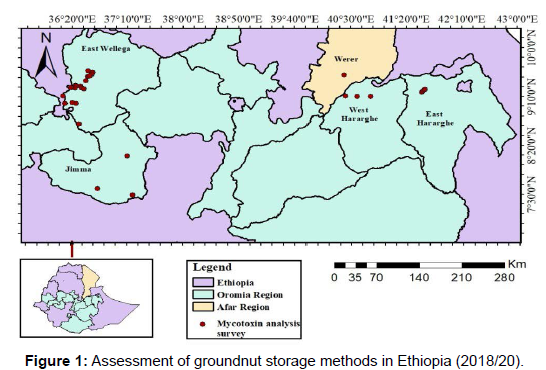
The samples were collected from east Wollega, Werer agricultural research center, Limu Shayi and Limu Kosa woredas of Jimma zone, Bable, Fedis, Chalanko and Miesso woredas of Hararghe zone. Storage fungi contaminating groundnut were assessed and sampled from farmer’s wholesaler’s and retailer’s storage. The samples were taken from the bottom, sides, middle and top of the sack storage. 250 to 500 gram of groundnut was sampled from each sampling stop. Data such as (GPS, temperature, RH, grain moisture content, storage duration, storage method and pest conditions) was taken (Figure 1).
Isolation and Identification of Fungi Using Agar Plate Method
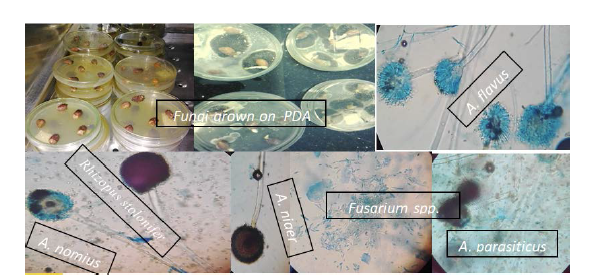
Two methods: culture media and HPLC were used for identification and detection of mycotoxin associated to groundnut. Groundnut associated mycotoxin was isolated and identified through culture media from the samples. Mold, rotted and decayed grains were collected and identified on agar media in the laboratory (Figure 2). Associated fungal organisms were grown in the laboratory using their respective media and identified through the use of microscope [3].
Detection and Quantification of Mycotoxins Using HPLC Method
50.14gm of disodium phosphate (DSP) was dissolved with 700ml of distill water in 1000ml flask. 42.50gm of Sodium phosphate mono basic was dissolved in 350ml of distill water. The two dissolved solution was mixed to adjust it to 7.4 PH. 200ml of buffer were filled into 1000ml of graduating cylinder. Take 230ml of buffer from the prepared and add 20ml polytene 2020. 20gm of samples were weighed and 2gm of NaCl was added into conical flask and Shaked by using mechanical shaker, and then filtered by vacuum pump. The two layers was separated and the bottom layers was used for the analysis. Take 7ml of samples and 43ml of buffer (Figure 3). Elute 50ml of solution in ingenuity affinity column (Afla CLEAN) and wash by distill water. Then add 2ml methanol to degrade the proteins and wait for 5 minutes and elute. Finally use the preserve glass and take into vial and inject and the analysis undergone. 200 gm of samples were weighed and placed in labeled paper bags before they were sent to the Bless Agri food laboratories services PLC (ISO/IEC 17025:2017 Accredited) which was established by the joint venture of Ethiopia and French investors. The total Aflatoxin content analysis in the samples were performed using HPLC protocols comprising of two chromatographic pumps, sampling system, and fluorescence detector (HPLC-FLD). Methods described (2002) and (1991) were followed. AFB1, AFB2, AFG1, AFG2 and total aflatoxin was considered for data analysis [4].
Data Analysis
Descriptive statistics such as frequency distribution and percentages analysis were used. All the collected data were computed using Microsoft excel 2010 and SPSS statistical software (Version 26) for the differences among aflatoxin percentage microgram per kilogram (Figure 4).
Results and Discussions
Hand and mechanical shelling
Most of the interviewed farmers 86% (n= 77) in all areas shelling their groundnut pods by their hands and stored in sacks which is labour intensive but it is effective for small scale farmers especially for selection of planting seed for the following season, reducing the contaminant as well mould development. Few of the interviewed farmers 22% (n = 77) especially in Sasiga areas of east Wollega zone used mechanical rotary types shellers that is important for shelling a number of sacks at a time in a continuous operation (rather than shelling in batches) and new designs produce very little wastage in terms of damaged seed [5].
Fungi isolated and identified from the groundnut samples
The samples were collected from 4 zones, 11woredas and a total of 77 samples were taken for the laboratory analysis which was done through agar plate methods. The result indicated that five fugal species, Aspergillus, Fusarium, Penicillium, Rhizopus & Trichoderma were identified. Among these, Aspergillus spp. was the most dominant 75% incidence in all the collected samples (Figure 5). Fusarium, Penicillium, Rhizopus and Trichoderma spp. were occurred with low incidence in the samples collected from storage. Ihejirikal (2005) reported that Aspergillus niger was occurred with the highest incidence 60% followed by Aspergillus versicolor 25% whereas, Aspergillus fumigatus occurred with the lowest incidence of 15%. Likewise, Aliyu and Kutama (2007) were identified six fungal species, Aspergillus Rhizopus, Penicillium Curvularia, Fusarium and Mucor. However, Vikas and Mishra (2010) identified nine species of fungi from the seeds of groundnut stored for one year. The fungal development was highly obtained as the storage period increased because of the metabolic activity of the produce, inappropriate storage condition and moisture increment due to microbial activities [6].
Aflatoxin detected from the groundnut samples by HPLC
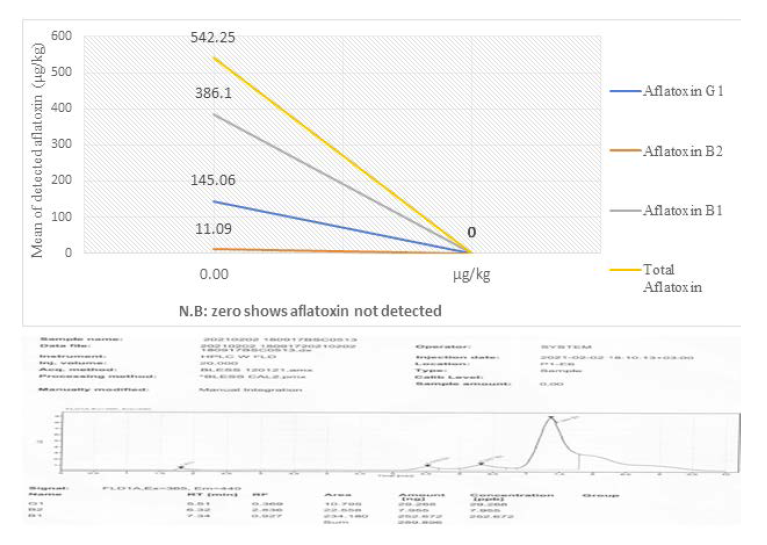
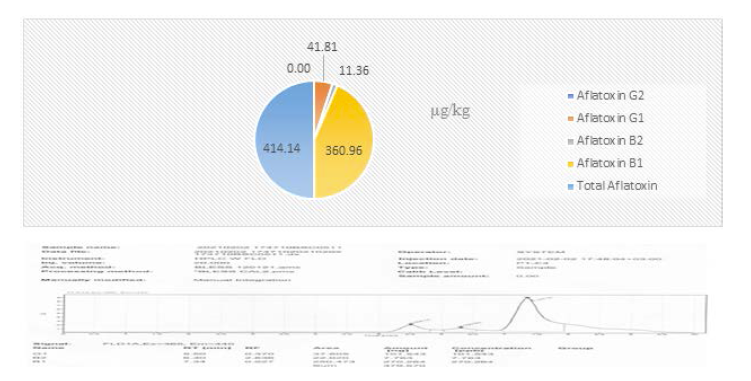
Four aflatoxin types were detected from the collected samples. The samples were composited into five samples code, BSC0509, BSC05010, BSC0511, BSC0512 and BSC0513. However, Mishra and Das (2003), 18 different types of aflatoxins have been identified, with aflatoxin B1, B2, G1, G2, M1, and M2 being the most common. Among the composited samples aflatoxin was not detected in the sample’s code of BSC0512. This is due to the samples were collected at the initial storage periods. AFG2 was only detected in the samples collected from Babile woredas of east Hararghe. Aflatoxin B1 was obtained with high concentration level 386.10 and 360.96μg/kg in the samples of BSC0513 and BSC0511 of Jima zone (Figures 6 and 7).
The concentration of aflatoxins shows increasing trends as the storage periods increases. This indicated that storage periods were the major factors for the toxin’s development if there is favorable condition and improper storage of the produce. While, indicated that the total aflatoxin in groundnut extended from 5 to 250 μg/kg. Furthermore,stated the concentrations aflatoxin B1 were 2,526 and 158 μg/kg, in groundnut seed and cake from Eastern Ethiopia. Moreover, reports the aflatoxin levels of 12,000 μg/kg in groundnut seed sampled from Babile district in Eastern. Bisrat and Gebre (1981) reports the mean levels of aflatoxin B1 was 34.7 and 105 μg/kg in samples of groundnut and peanut butter, respectively, in Ethiopia. Another, studies of (1981) showed aflatoxin levels of 5-250 μg/kg in groundnut seed from eastern Ethiopia. Recently, (2012) reported that total aflatoxin levels ranged between 15 and 11865 μg/kg in groundnut seed. Minimum levels of AFB1 19.18 and 163.92 μg/kg was recorded in the samples analyzed from BSC0510 and BSC0509, respectively [7].
This is due to in Werer the groundnut was sampled from agricultural research center which the storage was appropriately kept with recommended moisture content, well aerated and clean. In Hararghe the farmers dry on the well stoned ground no moisture absorbance of the produce [8-10].
References
- Mumtaz A, Sadaqat HA, Mubarik MK, Saif-ul-Malook MAN (2015) A review on genetics of seed yield and quality related traits in Brassica group. Biolog Sci 2:1-6.
- Nduwumuremyi A, Tongoona P, Habimana S (2013) Mating designs: helpful tool for quantitative plant breeding analysis. Int J plants breed gen 1:117-129.
- Panhwar SA, Baloch MJ, Jatoi WA, Veesar NF, Majeedano MS (2008) Combining ability estimates from line x tester mating design in upland cotton. Proc Pakistan acad sci 45: 69-74.
- Rashid M, Cheema AA, Ashra M (2007) Line x tester analysis in Basmati rice. Pak J Bot 39: 2035-2042.
- Saif-ul-malook MA, Ali Q, Mumtaz A (2014) Inheritance of yield related traits in maize (Zea mays) under normal and drought conditions. Nat Sci 12: 36-49.
- Saladaga FA (1989) The theoretical basis and practice of polycross as used in sweet potato. Res Develop for Small Far: 83-98.
- Shattuck VI, B Christie, C Corso (1993) A principle of Griffing’s combining ability analysis. Genetica 90: 73- 79.
- Singh RJ, Kollipara KP, Hymowitz T (1993) Backcross (BC2‐BC4). Derived Fertile Plants from Glycine max and G. tomentella Intersubgeneric Hybrids. Crop Sci 33: 1002-1007.
- Sleper DA, Poehlman JM (2006) Breeding field crops.
- Stuber CW (2004) Breeding: mating designs. Encyclo plant cro Sci 1:225-228.
Google Scholar, Crossref, Indexed at
Google Scholar, Cross Ref , Indexed at
Google Scholar, Crossref, Indexed at
Citation: Fufa N, Zelek T (2022) Groundnut (Arachis hypogea L.) Storage Methods and Associated Mycotoxin in Ethiopia. Adv Crop Sci Tech 10: 507. DOI: 10.4172/2329-8863.1000507
Copyright: © 2022 Fufa N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2213
- [From(publication date): 0-2022 - Dec 17, 2025]
- Breakdown by view type
- HTML page views: 1640
- PDF downloads: 573