Hepatoprotective Activity of Phoenix Dactylifera Fruits Aqueous Extract against Ethanol Induced Hepatotoxicity in Albino Rats
Received: 02-Jun-2022 / Manuscript No. jham-22-68016 / Editor assigned: 04-Jun-2022 / PreQC No. jham-22-68016 (PQ), / Reviewed: 18-Jun-2022 / QC No. jham-22-68016 / Revised: 24-Jun-2022 / Manuscript No. jham-22-68016 (R) / Accepted Date: 24-Jun-2022 / Published Date: 30-Jun-2022 DOI: 10.4172/2573-4555.1000329
Abstract
In rats, the ameliorative effect of aqueous extracts of the mesocarp (flesh) of dates (Phoenix dactylifera L.) was studied using ethanol-induced hepatotoxicity. The rats were divided into six groups and among them; three groups received the mesocarp extract of Phoenix dactylifera (10mg, 20mg, and 40 mg/kg) and ethanol 20% (3.76 gm/kg/day) orally. Two groups were considered controls and one group received the ethanol intervention while another received distilled water and the last group was treated with the Standard drug Silymarin (100 mg/kg). In both treated and untreated groups, the change in the biochemical markers like SGPT (Serum glutamic pyruvic transaminase) and SGOT (Serum oxaloacetic transaminase) were determined to assess the hepatic injury. The group which received the ethanol treatment exhibited enhanced levels of SGPT and SGOT. The intervention with the fruit extract in a dose-dependent way has restored the altered levels of the biomarkers to near normal levels which were evident from the marked reduction in serum enzymes, SGOT and, SGPT. Hence, it was concluded that the extract from the mesocarp of Phoenix dactylifera exhibits hepatoprotective activity against ethanol-induced hepatotoxicity in the rat model. Plasma concentration of the enzyme activities was estimated to assess the liver damage. The treatment with the aqueous extract of date fruits (Phoenix dactylifera L.) reduced the ethanol-induced elevated plasma enzyme concentration and ameliorated morphological and histological liver damage significantly. This study proposes that ethanol-induced liver damage can be ameliorated by administering P. dactylifera flesh extract.
Keywords: Phoenix dactylifera; Silymarin; SGOT; SGPT
Keywords
Phoenix dactylifera; Silymarin; SGOT; SGPT
Introduction
The key organ for regulating body homeostasis is the liver. It is involved with almost all the biochemical pathways related to growth, fight against disease, nutrient supply, energy production, and reproduction1. The liver is intended to protect against the dangers of toxic medications and substances in addition to performing physiological duties. Despite significant scientific progress in the field of hematology in recent years, liver disease is on the rise. Hepatitis and jaundice are two major liver illnesses with a significant mortality rate.
Modern medicine is still grappling with how to treat liver illness. Presently only a few hepatoprotective drugs and that too from natural sources (there is not a single effective allopathic medication), are available for the treatment of the liver disorder. In an assessment by the WHO in 2005, 4% of the burden of disease and 3.2% of all deaths globally were attributable to alcohol. ALD is the foremost health risk in developing countries and ranks third in developed countries [1]. Per-capita alcohol consumption has declined in the US and Europe, except in some northern European countries such as UK and Finland. However, episodic alcohol abuse has increased. This is especially true in Latin America and Asia. In the US, about two-thirds of the population is estimated to consume alcohol, of which 14 million fit arbitrary criteria of alcohol abuse. ALD prevalence in the US is estimated at >2 million people. About 5% to 10% of male and 3% to 5% female drinkers are alcohol dependent in the US. In England in 2005, just fewer than 15,000 deaths were attributable to alcohol, which was around 3% of all the deaths. Men were more affected than women, with 4.4% of all male deaths caused by alcohol and 2.0% of female deaths.
Alcoholic Liver Cirrhosis mortality has trended downward but will continue to decline only if alcohol consumption continuous to decline only if alcohol consumption continues to decline further [2]. ALD development is more rapid and occurs at a lower dose of alcohol in women than in men. The mortality related to liver cirrhosis increases with age. Data obtained in autopsy studies shows that 10% to 15% of alcoholic people have cirrhosis at the time of death. About 20% of alcoholic people and heavy drinkers develop fatty liver or steatosis. If heavy drinking continues, roughly 40% of cases of alcoholic hepatitis will develop into cirrhosis. The presence of Hepatitis C infection increases the risk of cirrhosis in alcoholic patients [3], and the prevalence of Hepatitis C liver Cirrhosis continues to rise. Thus, the prevalence of this form of alcoholic cirrhosis will possibly increase.
Pathophysiology
Alcohol is metabolized primarily in the Liver and to a lesser extent in the gastrointestinal tract. There are two main pathways of alcohol metabolism within the liver: alcohol dehydrogenase and Cytochrome P450 (CYP) 2E1. Alcohol dehydrogenase, hepatocyte cytosolic enzyme, converts alcohol to acetaldehyde. Acetaldehyde dehydrogenase, [4] a mitochondrial enzyme subsequently metabolizes acetaldehyde to acetate. CYP 2E1 also converts alcohol to acetaldehyde.
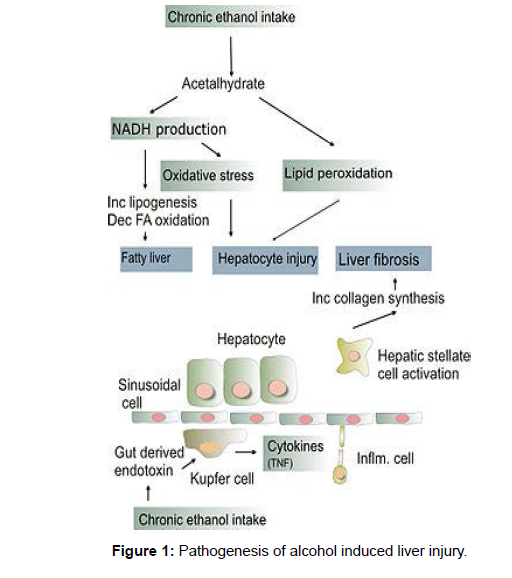
Several interrelated pathways are involved in liver damage.Nicotinamide adenine dinucleotide (NAD) was reduced to NADH (reduced form of NAD) by alcohol dehydrogenase and acetaldehyde dehydrogenase [5]. The altered ratio of NAD/NADH causes the inhibition of gluconeogenesis and fatty acid oxidation resulting in fatty liver. CYP 2E1, which is upregulated in chronic alcohol use, oxidizes Nicotinamide adenine dinucleotide phosphate (NADPH) to NADP18 generating free radicals. Chronic alcohol exposure also activates hepatic macrophages to produce tumor necrosis factor α (TNF- α) 19 to increase the production of reactive oxygen species. This oxidative stress promotes hepatocyte necrosis and apoptosis, which is huge in alcoholics who are deficient in antioxidants like glutathione and Vitamin E. Inflammation and fibrosis, is caused due to lipid peroxidation initiated by free radicals [6]. Inflammation is also induced by acetaldehyde that, when bound covalently to cellular proteins, forms adducts that are antigenic (Figure 1).
Causes
There are many causes of Fatty Liver Disease, some of them are:
• Drinking too much alcohol is termed Alcoholic Liver Disease (ALD).
• Type 2 Diabetes
• The rise in cholesterol level and triglyceride fats in the blood
• Overweight and obesity: this factor is one of the most common causes of fatty liver as many people are overweight and obese
• Some kinds of drugs “if used for a long time” like amiodarone,tamoxifen, and methotrexate.
• Metabolic abnormalities
• Galactosemia
• Glycogen Storage Diseases
• Homocystinuria
• Tyrosinemia
• Over nutrition, Malnutrition, Total Parenteral Nutrition-TPN and starvation diet
• Diseases like celiac sprue and Wilson Disease
• Genetic factors.
Symptoms
Symptoms of alcohol-induced liver disease depend on how much and how long a person has been consuming alcohol. The most common symptoms of alcohol-induced liver disease are given below. However, these symptoms may be experienced differently in each individual.
Symptoms may include:
• Enlarged liver
• Jaundice- yellowing of the skin and eyes
• Increased white blood cell count
• Spider-like veins in the skin
• Portal hypertension
• Enlarged spleen
• Ascites-fluid build-up in the abdominal cavity
• Kidney failure
• Confusion
Hepatotoxicity
A toxic liver injury brought about by drugs and chemicals like alcohol, CCL4, galactosamine, paracetamol, isoniazid, rifampicin, etc., may virtually mimic any form of naturally occurring liver disease [7]. Hepatoprotective effects were studied against chemicals and drugsinduced (Table 1).
| Category of agent | Mechanism | Histological lesion | Examples |
|---|---|---|---|
| 1.Intrinsic toxicity | |||
| a.Direct | Membrane injury destruction of the structural basis of cell metabolism | Necrosis (zonal) and/ or steatosis | CCl4,CHCl3,Phosphorus |
| b.Indirect Cytotoxic | Interference with the specific metabolic pathway leads to structural injury | Steatosis or Necrosis | Ethionine,Thioacetamide,Paracetamol, Ethanol |
| b.Cholestatic | Interference with hepatic excretory pathway leads to cholestasis | Bile duct injury | Rifampicin, Steroids |
| 2.Host idiosyncrasy | |||
| a.Hypersensitivity | Drug allergy | Necrosis or Cholestasis | Sulfonamides, Halothane |
| b.Metabolic abnormality | Production of hepatotoxic metabolites | Necrosis or Cholestasis | Isoniazid |
Table 1: Classification of hepatotoxins
Treatment
The primary treatment of alcohol-induced liver disease is ceasing from alcohol consumption. This is the only way to reverse liver injury or to prevent the liver damage from becoming worse. Without treatment, patients with alcohol-induced liver damage are likely to develop liver cirrhosis. Other treatments for alcoholic hepatitis include:
Nutrition
Doctors recommend a calorie-rich diet to aid the liver in its regeneration process. As fat interferes with alcohol metabolism, dietary fat should be reduced [8]. Instead, the diet is usually supplemented with vitamins and minerals (including calcium and iron)Many nutritionists recommend a high protein diet, with frequent small meals eaten during the day, about 5-6 instead of the usual 3. Nutritionally, the diet which supplements the nutrients enhances liver function and, supports the liver is recommended. These include carnitine, which will help to reverse fatty livers, and Vitamin C, which is an antioxidant [9], that aids in collagen synthesis and increases neurotransmitter production such as norepinephrine and serotonin, as well as providing the nutrients that have been expended due to alcohol consumption. Eliminating any food that may be showing intolerance and alkalizing the body is crucial. There are few supplements like choline, glutamine, and Vitamin C, which are recommended to help reduce cravings for alcohol. As studies prove that glucose increases the toxicity of centrilobular hepatotoxicants by inhibiting cell division and repair, it is suggested fatty acids are utilized by the liver instead of glucose as a source of energy to aid in repair; thus, it is recommended that the patient intakes a diet rich in protein and essential fatty acids, e.g. Omega-3 [10]. Liver damage due to alcohol stress can be minimized by ceasing alcohol consumption,cigarette smoking, and increasing exercise.
Stress
Withdrawals from alcohol intake and nutrition modification form the backbone in the management of ALD. Symptom treatment can include corticosteroids for severe cases, anti-cytokines (infliximab and pentoxifylline), Propylthiouracils to modify metabolism, and colchicine to inhibit hepatic fibrosis.
Antioxidants
It is widely believed that alcohol-induced liver damage occurs via the generation of oxidants. Thus, natural antioxidant supplements like milk thistle are routinely recommended by alternative health care practitioners. Unfortunately, there is no valid clinical data to show the effect of milk thistle [11]. “Milk thistle for alcoholic and/ or hepatitis B or C liver diseases—a systematic Cochrane hepato-biliary group review with meta-analyses of randomized clinical trials”.
Transplant
When everything fails and the liver is damaged beyond repair, the only alternative is liver transplantation. While this is a valid option, liver transplant donors are scarce. One of the criteria to become eligible for liver transplantation is to cease alcohol consumption for a minimum of six months.
Plant introduction
Phoenix dactylifera L (Date palm) vernacularly known as ‘Nakhla’ and the ‘Tree of Life’ by the Arabs, is considered as one of the oldest cultivated fruit trees. It is believed to be indigenous to the Arabian Gulf countries [12]. Many Middle Easterners believe that consuming date fruits, especially in the morning on an empty stomach, can reverse the actions of any toxic material that the subject may have been exposed to. Different parts of this plant are traditionally claimed to be used for the treatment of a broad spectrum of ailments including memory disturbances, fever, and loss of consciousness, inflammation, paralysis, and nervous disorders. The fruits of Phoenix dactilyfera are used as an astringent in intestinal troubles, treatment for sore throat, colds, bronchial asthma, to relieve fever, cystitis, gonorrhea, edema, liver, and abdominal troubles, and to counteract alcohol intoxication.
The date palm (Phoenix dactylifera) is a dioeciously, medium-sized tree with pinnate leaves and small yellowish flowers which develop into fruits called dates (Figure 2).
Scientific classification
Kingdom: Plantae
Division: Magnoliolphyta
Class: Liliopsida
Order: Arecales
Family: Arecaceae
Genus: Phoenix
Species: dactylifera
Binomial name: Phoenix dactylifera
Assessment of the Hepatoprotective Activity
A total of 36 animals were equally divided into 6 groups (n=6), All the test drugs were administered orally (Table 2)
| Sl. No | Group | Treatment |
|---|---|---|
| 1 | Normal Control | Received vehicle for 18 days (Distilled water p.o) |
| 2 | Disease Control | Received only 20% Ethanol (3.76g/kg/day p.o) for 18days |
| 3 | Standard | Received only 20% Ethanol (3.76g/kg/day p.o) for 18days + Reference Drug Silymarin (100mg/kg p.o) from Day 8 |
| 4 | Test 1 | Received only 20% Ethanol (3.76g/kg/day p.o) for 18days + aqueous extract of Phoenix dactylifera fruits (10mg/kg) from day 8 |
| 5 | Test 2 | Received only 20% Ethanol (3.76g/kg/day p.o) for 18days + aqueous extract of Phoenix dactylifera fruits (20mg/kg) from day 8 |
| 6 | Test 3 | Received only 20% Ethanol (3.76g/kg/day p.o) for 18days + aqueous extract of Phoenix dactylifera fruits (40mg/kg) from day 8 |
Table 2: Assessment of th Hepatoprotective Activity
Biochemical analysis
Assay of serum GOT and GPT activities
On the 18th day rats were treated with ether and blood samples were collected by retro-orbital puncture in sterilized centrifuge tubes. Blood was allowed to coagulate at 37℃ for 30 min and the serum was used for the assay of marker enzymes i.e. serum glutamic oxaloacetate transaminases (SGOT), serum glutamic pyruvic transaminase (SGPT), which reflected the functional state of the liver analyzed according to the method of Reitman and Frankel (1957).
Histopathological study
Each rat was laprotomized to obtain the liver immediately after collecting blood under ether anesthesia. Small fragments of the liver were fixed in 10% formalin solution [13], dehydrated with ethanol solution from 50% to 100% embedded in paraffin, and cut into 5μm thick sections which were stained using hematoxylin eosin dye for photo microscopic observation including necrosis, steatosis and fatty change of hepatic cells.
Statistical analysis
All the data was expressed as Mean ± S.D and analyzed using one way analysis of variance (ANNOVA) followed by Dunnet test and compared with respective control group. A value of P<0.05 was considered significant.
Results and discussion
The Phytochemical screening revealed the presence of Alkaloids, Carbohydrates, Steroids, Tannins, Saponins, Flavonoids, and Glycosides in Phoenix dactylifera. The Hepatoprotective studies of Phoenix dactylifera extract did not show any significant decrease in the AST and ALT levels in concentrations 10mg/kg and 20mg/kg and reduced the elevated levels of AST and ALT at 40mg/kg as shown in the (Table 3).
| Group | AST (IU/L) | ALT (IU/L) |
|---|---|---|
| Normal | 96.83 ± 6.34 | 32.50 ± 1.52 |
| Ethanol treated | 162.17 ± 2.56 | 78.33 ± 1.63 |
| Silymarin (100mg/kg) + Ethanol | 112.33 ± 2025 | 29.67 ± 2.34 |
| P. dactylifera fruits aqueous extract (10mg/kg) + Ethanol | 152.17 ± 1.17 | 71.00 ± 1.26 |
| P. dactylifera fruits aqueous extract (20mg/kg) + Ethanol | 145.33 ± 4.46 | 61.17 ± 1.17 |
| P. dactylifera fruits aqueous extract (40mg/kg) + Ethanol | 128.00 ± 5.10 | 50.17 ± 2.79 |
Table 3: significant decrease in the AST and ALT levels in concentrations
All the values were expressed as Mean ± SD
Oral administration of ethanol at a dose of 3.76 g/kg/day caused significant (P<0.0001) rise in level of serum marker enzymes such as AST and ALT [14], compared with the control group Silymarin (100mg/kg) significantly (P<0.0001) reduced AST and ALT levels near to normal. A significant (P<0.0001) decrease was observed in the AST and ALT of animals treated with different doses (10 mg/kg, 20 mg/ kg, 40 mg/kg) of P. dactylifera fruits aqueous extract and showed dose dependent activity. At the dose of 40mg/kg P. dactylifera fruits aqueous extract showed comparable activity with standard drug silymarin.
In Control animals, the liver sections showed normal hepatic cells with well-preserved cytoplasm, prominent nucleus, and normal liver parenchymal cells. Ethanol (3.76 g/kg/day, p.o) induced hepatic injury produced liver cell necrosis. Aqueous extract of P. dactylifera treated liver showed diffuse areas of liver cells necrosis and isolated liver cells and focal areas of liver cells showed evidence of regeneration. Silymarin treated liver histopathology also showed diffuse areas of liver cells necrosis and focal areas of liver cells regeneration.
Liver damage induced by ethanol is perhaps the best studied model of liver cirrhosis. The reduction of ethanol induced elevated plasma levels of AST and ALT when treated with the aqueous extract of P. dactylifera shows their ability to restore the normal functional status of poison liver and also to protect against subsequent ethanol toxicity [15]. The mechanism by which P. dactylifera induces its hepatoprotective activity is not certain. However, it is possible that β-sitosterol, a constituent of P. dactylifera, is at least partly responsible for the protective activity against ethanol induced hepatotoxicity. Flavonoids in P. dactylifera could be a factor in contributing to its hepato protective ability through inhibiting the Cytochrome P 450 aromatase.
Conclusion
This study clearly demonstrates that aqueous extract of Phoenix dactylifera (40mg/kg) significantly decreased SGOT and SGOT in the animals treated with ethanol. Comparative studies were obtained with standard drug Silymarin.
The data suggest that the daily oral consumption of an aqueous extract of the flesh of P. dactylifera as a part of the daily diet was prophylactic to ethanol poisoning.
References
- Stewart S, Jones D, Day CP (2003) Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med 7:408-413.
- Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, et al.( 2003 ) A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-α production. Am J Pathol 163:1137-1146.
- Tamayo C, Diamond S (2007) Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn). Integr Cancer Ther 6:146-157.
- Mulrow C, Lawrence V, Jacobs B, Dennehy C, Sapp J, et al. (2000) Milk thistle: effects on liver disease and cirrhosis and clinical adverse effects: summary. InAHRQ Evidence Report Summaries 2000. Agency for Healthcare Research and Quality (US).
- Angulo P, Patel T, Jorgensen RA, Therneau TM, Lindor KD(2000) Silymarin in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 32:897-900.
- Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM(2003) Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol 37:336-339.
- Szilard S, Szentgyörgyi D, Demeter I (1988) Jan Protective effect of Legalon in workers exposed to organic solvents. Acta Medica Hun 45:249-256.
- Palasciano G, Portincasa P, Palmieri V, Ciani D, Vendemiale G, et al. (1994) the effect of silymarin on plasma levels of malon-dialdehyde in patients receiving long-term treatment with psychotropic drugs. Curr Ther Res 55:537-545.
- Floersheim GL, Weber O, Tschumi P, Ulbrich M ( 1982) Clinical death-cap (Amanita phalloides) poisoning: prognostic factors and therapeutic measures. Analysis of 205 cases. Schweiz Med Wochenschr 112:1164-77.
- Hruby K, Fuhrmann M, Csomos G, Thaler H(1983 ) Pharmacotherapy of Amanita phalloides poisoning using silybin. Wiener Klinische Wochenschrift. 95:225-231.
- Biglari F, AlKarkhi AF, Easa AM (2008) Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food chem 107:1636-1641.
- Ziouti A, El Modafar C, Fleuriet A, El Boustani S, Macheix JJ(1996)Phenolic compounds in date palm cultivars sensitive and resistant to Fusarium oxysporum. Bio Plan 38:451-457.
- Hong YJ, Tomas-Barberan FA, Kader AA, Mitchell AE (2006) The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera). J Agri Food Chem 54:2405-2411.
- Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F(2005) Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem 53:7592-7599.
- Boudries H, Kefalas P, Hornero-Méndez D (2007) Carotenoid composition of Algerian date varieties (Phoenix dactylifera) at different edible maturation stages. Food Chem 101:1372-1377.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Tiwari VK, Akhil KV, Varshini BS (2022) Hepatoprotective Activity of Phoenix Dactylifera Fruits Aqueous Extract against Ethanol Induced Hepatotoxicity in Albino Rats. J Tradit Med Clin Natur, 11: 329. DOI: 10.4172/2573-4555.1000329
Copyright: © 2022 Tiwari VK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3164
- [From(publication date): 0-2022 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 2677
- PDF downloads: 487