Identification of Lipid Mediators in Peripheral Human Tissues Using an Integrative In Vivo Microdialysis Approach
Received: 09-Feb-2016 / Accepted Date: 03-Mar-2016 / Published Date: 10-Mar-2016 DOI: 10.4172/2155-9872.1000306
Abstract
Endocannabinoids and related N-acylethanolamines (NAEs) are lipid mediators involved in a number of physiological and pathological mechanisms in peripheral tissues. Microdialysis (MD) technique allows continues sampling of endogenous substances in the interstitial fluids of the tissues. The main limitation of MD sampling of lipophilic compounds is low recovery due to adsorption to the MD system and particularly to the catheter membranes. In this in vivo study microdialysate samples were collected from human trapezius muscle and forearm skin. The levels of arachidonoylethanolamide (AEA), 2-arachidonoylglycerol (2-AG), oleoylethanolamide (OEA), palmitoylethanolamide (PEA), and stearoylethanolamide (SEA) were analyzed in both microdialysate and in catheter membrane samples using liquid chromatography tandem mass spectrometry. OEA, PEA and SEA were identified in all microdialysate and catheter membrane samples from trapezius and skin. 2-AG was found in all catheter membrane samples from both tissues but not in the actual microdialysate. In conclusion sampling of OEA, PEA and SEA was achievable from trapezius and skin with the presented MD set-up. 2-AG is present in both trapezius muscle and skin tissue but adsorbs to the membranes in a higher extent than the NAEs. Furthermore, consideration of data conserved in the membrane during an MD experiment could be a relevant and more broadly applicable extension of MD sampling methodology which could fill an “information gap” and enhance an adequate interpretation of microdialysate data outcomes.
Keywords: Microdialysis; Endocannabinoids; 2-arachidonoylglycerol; N-acylethanolamines; Trapezius muscle; Skin tissue; Catheter membrane
303424Introduction
Endocannabinoids (ECs) and related N-acylethanolamines (NAEs) are lipid mediators involved in a number of physiological processes. Arachidonoyl ethanolamide (AEA) and 2-arachidonoyl glycerol (2- AG) are the two most well-studied endogenous cannabis receptor (CB1, CB2) agonists. They are involved in appetite regulation, inflammation, neuroprotection and pain modulation [1-3]. AEA is also a member of the NAE family and exerts its effects on the transient receptor potential vanilloid (TRPV1) receptors [4].
Other NAEs, such as oleoyl ethanolamide (OEA), palmitoyl ethanolamide (PEA), and stearoyl ethanolamide (SEA) lack significant affinity for CB receptors but possess relevant affinity for other receptors. PEA modulates pain and inflammation via peroxisome proliferatoractivated receptor type-α (PPAR-α) activation [5,6], and has been suggested to be “a modulator of immune-neural homeostasis” [7]. OEA has anorexic properties [7] and is involved in the regulation of feeding and body weight through activation of PPAR-α [8]. OEA is also associated with both analgesic properties which has been suggested to occur independently of PPAR-α activation [9], and induction of visceral pain via TRPV1 activation [10]. SEA has a cannabimimetic activity partly similar to AEA [11]. The in energy homeostasis role of the endocannabinoid system in adipose tissue, liver and skeletal muscle has been reviewed [12]. It has also been suggested that 2-AG controls cellular differentiation in skeletal muscle [13]. Furthermore, ECs and endogenous PPAR-α activating NAEs are proposed to be key players as regulators of nociceptive information transmitting from peripheral sites of injury and inflammation to CNS [14,15].
The microdialysis (MD) technique is a well-established method for sampling of unbound endogenous compounds in peripheral tissues [16]. Sampling of microdialysate involves perfusion of a MD membrane with an aqueous solution (perfusate). The catheter contains a semipermeable membrane and mimics a capillary blood vessel [17] allowing substances to pass by diffusion across the membrane. The absence of a target molecule in the dialysate can be due to actual absence of the agent in the tissue but can also be explained by methodological issues such as inadequate analytical sensitivity or binding of the target molecule to the membrane. Many experimental conditions, including probe membrane composition and surface area, perfusate flow rate, temperature, nature of the dialyzed tissue and physicochemical properties of the target molecules need to be considered in the experimental design [18].
MD sampling of ECs and NAEs have mainly been performed in the brain tissue of rodents [19-22], although AEA, OEA and PEA have been measured in MD samples from human brain immediately after an ischemic stroke [23]. There are a limited number of MD studies investigating the peripheral levels of ECs in humans. Zoerner et al. have analyzed levels in abdominal adipose tissues in healthy males [24]. We have previously investigated levels of PEA and SEA from the interstitial fluid of trapezius muscle in women with chronic pain conditions compared to healthy controls [25,26].
Even though MD is a promising technique for peripheral sampling of ECs and NAEs, the lipophilic properties of these compounds makes measurement challenging. Low recovery due to interfering adsorption effects to the tubes and catheter membranes of the MD system has been pointed out to be a major obstacle.
We have previously investigated the relative recovery of radiolabeled AEA, PEA and 2-AG in an in vitro study (PEA data published) [26] (AEA and 2-AG unpublished data). In that study liquid scintillation was used for measurements of the lipids. The relative recovery for the different compounds was 25-35%. The adsorption of the lipids to the catheter membrane and the tube was also investigated by liquid scintillation. The adsorption was mainly to the catheter membrane (tube membrane ratio 1:80).
The aims of the present in vivo study were to investigate the trapezius muscle and the forearm skin as potential tissues suitable for MD sampling of targeted endogenous lipid mediators. The focus of the study was to identify NAEs and ECs and examine to what extent those adsorb to the catheter membrane at certain time points. The integrative approach of analyzing both the microdialysate samples and the catheter membranes for the targeted compounds provide information on the feasibility to find these compounds in the tissues in general and an estimation of the degree of adsorption to the catheter membranes in vivo.
Materials and Methods
Microdialysis system
The commercially available MD catheter CMA 63 with polyarylethersulfone (PAES) membrane and 20 kDa cut-off, and the specially designed microvials for sample collection from M Dialysis (Stockholm, Sweden) were used. The perfusion fluid consisted of Ringer-acetate solution from Baxter Medical (Kista, Sweden) containing 3mM glucose, 0.5mM lactate. The CMA 107 MD pump was used at a flow rate of 5 μL/min [25,26].
Microdialysis in trapezius muscle
Sampling of dialysate from trapezius muscle was accomplished on a 39 year old healthy male as previously described [26]. The subject was instructed not to drink any beverages with caffeine, not to smoke on the day of MD and to avoid NSAID medication the week before the experiment. Ultrasonographic measurements were conducted on trapezius muscle and used as a tool for guiding the placement of the catheters in the muscle. The catheters were inserted parallel to the muscle fibers into the pars descendens of the trapezius muscle at half the distance between the processus spinosus of the seventh cervical spine and the lateral end of the acromion. The skin and the subcutaneous tissue, where the catheter entered the trapezius muscle was anaesthetized with a local injection of 0.5 ml Xylocaine (20 mg/ml) without adrenalin. Care was taken not to anaesthetize the underlying muscle. Four catheter membranes denoted A, B, C and D were inserted, two in the right side trapezius muscle and two in the left side. Dialysate were collected in 20 minutes intervals. In order to study the adsorption of the compounds over time the catheters were removed at different time points. Catheter A was withdrawn after 20 min, and thus only one sample of muscle dialysate was collected. B was removed from the muscle after 100 min, and consequently five dialysate samples were collected. C was withdrawn after 140 min, resulting in seven dialysate samples. Catheter membrane D was removed after 220 min resulting in eleven fractions. The vials used for sampling of the dialysate were weighed before and after collection in order to confirm that sampling proceeding had occurred according to the set perfusion rate. All samples were kept on ice during the experiment and after the experiment the dialysate was stored in aliquots in Eppendorf tubes (Costar, micro centrifuge tube, 0.65 ml, Sigma-Aldrich) at -70°C until analysis.
Microdialysis in forearm skin
MD sampling from skin was performed on a 33 year old healthy female volunteer following the same instructions as described above for trapezius muscle MD. Three catheters (denoted A, B, and C) were inserted into dermis in the left dorsal forearm. The insertion of the catheters followed the technique previously described in Ref. [27]. The point of insertion of each catheter was anaesthetized with 0.2 ml Xylocaine (10 mg/ml). A splitable introducer (SI-2) was inserted to dermis and used to guide the catheter insertion. Samples were collected every 20 minutes. Catheter A was withdrawn after 20 min. Catheter B was withdrawn after 100 min giving five dialysate fractions and catheter C was removed after 140 min resulting in seven fractions. Each vial was weighed before and after collection in order to confirm that sampling was achieved according to the set perfusion rate. All samples were kept on ice during the experiment and after the experiment the dialysate was stored in aliquots at -70°C until analysis.
Ethical approval
All procedures for sampling of microdialysate from trapezius muscle and forearm skin were approved by Linköping University Ethics Committee Dnr: M233-09 and 2010/164-32 and Dnr: 03250 respectively. The participants gave their informed written consent before the experiments.
EC and NAEs analysis
High pressure liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) was used. A selected reaction monitoring (SRM) method was applied for simultaneously identification and quantification of AEA, OEA, PEA, SEA and 2-AG. Similar SRM transitions as described by other authors [28,29] were monitored.
Chemicals
AEA, OEA, PEA, SEA, (purities of ≥ 98%) 1-AG (purity ≥ 95%, with a mix of 1-AG: 2-AG, 9:1) and 2-AG (purity ≥ 95% with a mix of 9:1 2-AG: 1-AG) were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Methanol and Acetonitrile (HPLC-grade) were from Sigma-Aldrich (Steinheim, Germany). Formic acid (reagent-grade) was obtained from Scharlau Chemie (Barcelona, Spain) and Ammonium acetate from Merck (Damstadt, Germany). Milli-Q water (Milli-Q plus unit, Millipore, Molsheim, France) was used in all analyses.
Preparation of standards
AEA, OEA, PEA and SEA stock solutions were prepared in ethanol. 2-AG was prepared in acetonitrile which compared to ethanol is known to reduce the isomerization of 2-AG to 1-AG [30]. All stock solutions were stored at -70°C. A mixture of standard solution containing 5 μM 2-AG and 1 μM AEA, OEA, PEA and SEA was prepared, aliquoted and stored in -70°C. Further dilutions were carried out in liquid chromatography mobile phase and were used on the same day as prepared.
MD sample preparations
On the day of analysis, 50 μl of microdialysate and perfusate were dried by SpeedVacc vacuum concentration system (Savant, Farmingdale, NY, USA) and dissolved in 100 μl methanol, vortexed and centrifuged [25]. The supernatants were transferred to new tubes (0.65 ml) and dried. The residues were dissolved in 20 μl LC mobile phase, vortexed and transferred to glass insert vials for LC-MS/MS.
Catheter membrane samples were prepared as follows; after withdrawal of the catheter from the tissue the membranes were separated from the tubing, cut into two equal halves and placed in Eppendorf tubes (Micro tube, 1.5 ml, Sarstedt), which were kept on ice during the experiment and stored in-70°C until analysis. On the day of analysis, methanol (1 ml) was added to the catheter membrane and vortexed for 15 sec, the catheter membrane was removed from the tube. The extraction solution was dried by SpeedVacc and the residue was dissolved in 100 μl methanol, vortexed and centrifuged. The supernatant was transferred to a new tube and dried. The residue was dissolved in 20 μl LC mobile phase, vortexed and transferred to glass insert vial for LC-MS/MS. The sample preparation procedure was also performed on an unused catheter membrane in order to detect any background signal.
High pressure liquid chromatography-tandem mass spectrometry
An HPLC-MS/MS system containing a Thermo Scientific Accela AS auto sampler and Accela 1250 pump coupled to a Thermo Scientific TSQ Quantum Access max triple quadrupole mass spectrometer with an HESI II probe as ionization source was used. Liquid chromatography was performed using isocratic elution on a Xbridge C8 guard column (column dimensions 2.1 mm × 10 mm) coupled to a Xbridge C8 analytical column (column dimensions 2.1 mm × 150 mm) both with the particle size 2.5 μm obtained from Waters (Dublin, Ireland). The mobile phase consisted of methanol-acetonitrile-Milli-Q water 60:25:15 (v/v/v), 0.1% (v/v) formic acid and 1 g/L ammonium acetate. The sample injection volume was 10 μl and the LC flow-rate was 300 μl/min. The electrospray interface was operated in positive ion mode and the spray voltage was set to 4.5 kV. The capillary temperature was set to 350°C and sheath gas pressure to 40 arb units. The ion sweep gas pressure was set to 0.4 arb units. The SRM (m/z) transitions were: 348.3/62.4, 326.3/62.4, 300.3/62.4, 328.3/62.4, and 379.3/287.3 for AEA, OEA, PEA, SEA and 2-AG respectively. The linearity of the measuring ranges were assessed with standard curves ranging from 0.1-20 nM for OEA and SEA, 0.5-20 nM for AEA and PEA, and 25-1000 nM for 2-AG in human muscle dialysate, with R2: 0.96-0.99 (SD ≤ 0.02) for the different compounds. The limit of detection (LOD) of the compounds (defined as the concentration at which a signal-to-noise ratio of greater than 3:1 was achieved following direct analyses from stock solutions) was ~ 0.1 fmol for OEA, PEA and SEA, ~ 0.5 fmol for AEA and ~ 50 fmol for 2-AG. Limit of quantification (defined as the concentration at which a signal-to-noise ratio of 10:1 or greater was achieved) was ~ 1 fmol for OEA and SEA, ~ 5 fmol for PEA, 10 fmol for AEA and ~ 250 fmol for 2-AG.
Quantification was performed using external standards curves, linear regression and equal weighting. Xcalibur® (version 2.1, Thermo Scientific) software was used for peak integration and quantification.
Software for statistics and graphics
The IBM SPSS version 22.0 (IBM Corporation, Route 100 Somers, New York, USA) was used for statistics. GraphPad Prism computer programme (GraphPad Software Inc, San Diego, CA, USA) and Matlab and Simulink version R2013b (The Math Work, Inc) were used for graphical illustrations.
Results
Technical aspects
The trapezius muscle MD set-up resulted in 23 samples with sufficient volume for the analysis. (The 60 min time point from catheter B couldn’t be collected, probably due to some failure with the MD pump.) Sample volumes from time point 20 from A, B and C catheters and time point 80 from B catheters deviated more than 20% from the actual set flow. In the skin MD set up, sampling from the catheter B failed at three time points (20, 80 and 100 min). 10 samples had sufficient volume for analysis. Of these samples, deviation more than 20% from the set volume occurred at three time points (A, 20 min and B, 40 and 60 min).
Concentrations of ECs and NAEs from trapezius muscle
OEA, PEA and SEA could be measured in all MD samples (n=23) and from all the catheter membranes (n=4). In microdialysate samples the min to max concentrations (nM) were; OEA (0.5-11.3), PEA (0.5- 5.1) and for SEA (0.1-1.8), and corresponding concentrations from the membranes were; OEA (8.0-16.9), PEA (14.3-19.9), SEA (5.0-6.9).
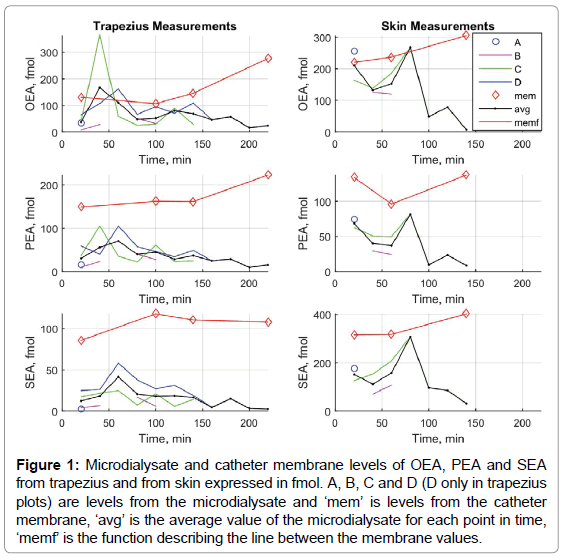
Levels of AEA could be measured in two microdialysate samples (1.2, 1.5) and detected in three of the four membranes. 2-AG could be measured in all four catheter membranes (241.9-1533.4) and detected in three out of 23 microdialysate samples, (one 2-AG value are extrapolated since it was above the highest standard point). In order to make an adequate comparison of the NAE levels in dialysate with the levels extracted from the catheter membranes, the number of moles was calculated. Individual microdialysate and catheter membrane levels of OEA, PEA and SEA expressed in femto moles (fmol) are presented in Table 1 and are illustrated in Figure 1.
| TRAPEZIUS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 200 | 220 | Dtot | CM | CM/Dtot |
| OEA | ||||||||||||||
| A | 35.4 | 35.4 | 130.8 | 3.7 | ||||||||||
| B | 7.9 | 28.8 | DM | 50.8 | 32.1 | 119.6 | 106.9 | 0.9 | ||||||
| C | 40.1 | 363.2 | 59.5 | 25.8 | 29.9 | 90 | 29.1 | 637.5 | 146 | 0.2 | ||||
| D | 65.3 | 107.7 | 162.3 | 66.5 | 95.2 | 70.8 | 109.3 | 46.4 | 57.6 | 16.6 | 24.4 | 822 | 277 | 0.3 |
| PEA | ||||||||||||||
| A | 14.8 | 14.8 | 149 | 10.1 | ||||||||||
| B | 10 | 23 | DM | 39.8 | 27.8 | 100.7 | 162.1 | 1.6 | ||||||
| C | 36.6 | 104.7 | 35.2 | 22.4 | 60.5 | 23.1 | 24.6 | 307 | 161.3 | 0.5 | ||||
| D | 59.1 | 40 | 105.2 | 57.4 | 46.3 | 34.1 | 49 | 24.9 | 29 | 9.5 | 15.3 | 469.8 | 222.6 | 0.5 |
| SEA | ||||||||||||||
| A | 2.8 | 2.8 | 85.6 | 31.1 | ||||||||||
| B | 4.1 | 6.6 | DM | 16.7 | 6 | 33.4 | 117.8 | 3.5 | ||||||
| C | 17.3 | 21.6 | 24.8 | 7.3 | 20.8 | 5.9 | 14.1 | 111.8 | 110.3 | 1 | ||||
| D | 24.9 | 26.2 | 58 | 38 | 27 | 31.2 | 19 | 4.6 | 15 | 3.4 | 2.5 | 249.6 | 107.8 | 0.4 |
| 2AG | ||||||||||||||
| A | _ | 3066.9 | ||||||||||||
| B | _ | _ | _ | DET | _ | 1374.5 | ||||||||
| C | _ | _ | _ | _ | _ | DET | _ | 483.7 | ||||||
| D | _ | _ | DET | _ | _ | _ | _ | _ | _ | _ | _ | 1961.8 | ||
| AEA | ||||||||||||||
| A | _ | _ | ||||||||||||
| B | _ | _ | _ | _ | _ | DET | ||||||||
| C | _ | 47.6 | _ | _ | _ | _ | _ | DET | ||||||
| D | _ | _ | _ | 36.2 | _ | _ | _ | _ | _ | _ | _ | DET | ||
| SKIN | ||||||||||||||
| OEA | ||||||||||||||
| A | 256.3 | 256.3 | 220.5 | 0.9 | ||||||||||
| B | DM | 126 | 120.1 | DM | DM | 246 | 236.7 | 1 | ||||||
| C | 164 | 138.6 | 185.5 | 267.6 | 49.3 | 78.3 | 7.5 | 890.8 | 304.7 | 0.3 | ||||
| PEA | ||||||||||||||
| A | 74.7 | 74.7 | 133.9 | 1.8 | ||||||||||
| B | DM | 29.4 | 24 | DM | DM | 53.4 | 96 | 1.8 | ||||||
| C | 62.5 | 50.5 | 49.7 | 81.8 | 9.8 | 23.5 | 8.5 | 286.2 | 137.2 | 0.5 | ||||
| SEA | ||||||||||||||
| A | 176.9 | 176.9 | 315.2 | 1.8 | ||||||||||
| B | DM | 70.5 | 107.4 | DM | DM | 177.9 | 317 | 1.8 | ||||||
| C | 126.3 | 153.2 | 208.1 | 307.5 | 96.2 | 85.5 | 31.1 | 1007.8 | 402.4 | 0.4 | ||||
| 2AG | ||||||||||||||
| A | _ | 845.1 | ||||||||||||
| B | _ | _ | _ | _ | _ | 70.8 | ||||||||
| C | _ | _ | _ | _ | _ | _ | _ | 134.1 | ||||||
Table 1: OEA, PEA and SEA levels in fmol from each time point from human trapezius muscle and forearm skin. A, B, C and D represent the different catheters. Dtot is the sum of moles in dialysate collected from each catheter. CM is the catheter membrane levels. CM/Dtot is a quota and presents the dimension of the relative adsorption. DM equals dialysate missing, or dialysate volume too small for measurement, and could be due to problems with the MD pump or to a failure in the membrane throughput. DET denotes detected but not quantifiable levels.
Figure 1: Microdialysate and catheter membrane levels of OEA, PEA and SEA from trapezius and from skin expressed in fmol. A, B, C and D (D only in trapezius plots) are levels from the microdialysate and ‘mem’ is levels from the catheter membrane, ‘avg’ is the average value of the microdialysate for each point in time, ‘memf’ is the function describing the line between the membrane values.
Concentrations of ECs and NAEs from forearm skin
OEA, PEA and SEA levels were measured in all microdialysate samples (n=10) and from the catheter membranes (n=3). In microdialysate samples the min to max concentrations (nM) were; OEA (0.3-10.5), PEA (0.5-4.6), SEA (2.8-26.1) and corresponding concentrations from the membranes were; OEA (16.5-22.7), PEA (10.2- 14.6), SEA (50.8-64.9) (some values of SEA and OEA are extrapolated since the concentrations were above the highest standard). 2-AG was measured in all membranes (n=3), (35.4-951.7) but was absent in the dialysate. AEA could not be detected in either the dialysate or the membranes. Individual microdialysate and catheter membrane levels of OEA, PEA and SEA expressed in fmol are presented in Table 1 and Figure 1.
Discussion
Major findings of the present study were:
• OEA, PEA and SEA could be measured in all dialysate and membrane samples collected from the human trapezius muscle and from the forearm skin tissue.
• The presence of 2-AG in peripheral tissue in human was demonstrated by detection in the membrane samples.
• The main interfering adsorption effects of NAEs to the catheter membranes appear to occur during the first 20 min of sampling.
These findings taken together suggest that sampling of the lipid mediators PEA and OEA together with SEA are achievable from both trapezius muscle and forearm skin tissue with the presented MD setup. The experimental procedure of analyzing both the dialysate and the catheter membrane over time provides information about the relative adsorption of these ligands to membranes in vivo. Rather than having a static accumulation rate during the time period it seems that the membranes are ‘charged’ in a more dynamic fashion primary during the first 20 min after catheter insertion (Figure 1). In addition the descending CM/Dtot ratios shown in Table 1 are indicating that the relative adsorption decreases towards zero over time. One obvious trend for all compounds seen in Figure 1 is that the average levels decreases over time. This could be explained by a tissue depletion of the targeted analytes over time. Moreover, a “saturation” of the membranes of the targeted analytes and other substances e.g., proteins might also influence the ability of the compounds to cross the membrane over time.
Even if AEA was quantified in two dialysate samples and detected in three membrane samples from trapezius, it was not present in the same order as the other NAEs or 2-AG (2-AG from membrane). These results indicate a relatively low abundance of AEA in the tissues compared to 2-AG and other NAEs. This is in line with previously described levels of AEA compared to 2-AG measured from various biological systems [28,31-33].
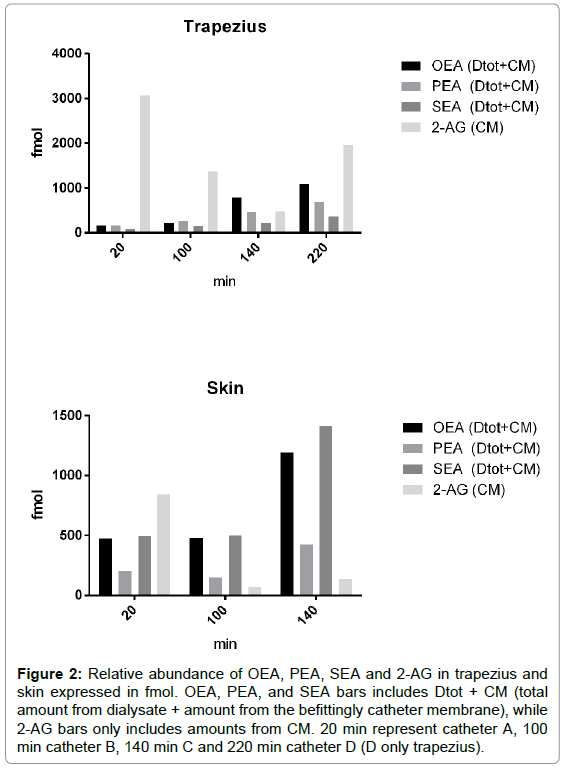
2-AG was identified in the catheter membranes used in both the muscle and skin MD sampling. This indicates that 2-AG is present in the tissues but fails to cross over the catheter membrane, which in turn indicates membrane unsuitability. Even though there is evidence supporting the multiple bioactive role of 2-AG in peripheral tissues [12,14], its actual concentration in skeletal muscle and skin tissue is not known. The presented study identifies 2-AG (and 1-AG) in the tissues and gives a rough estimate of its relative abundance compared to NAEs in trapezius muscle and in the forearm skin. In Figure 2 the total amount of OEA, SEA, PEA and 2-AG (fmol) from each catheter (A, B, C, (D)) from both tissues are shown. 20 min represent the total amount from catheter A. 100 min from B and 140 from catheter C and 220 min from D (D only trapezius).
Figure 2: Relative abundance of OEA, PEA, SEA and 2-AG in trapezius and skin expressed in fmol. OEA, PEA, and SEA bars includes Dtot + CM (total amount from dialysate + amount from the befittingly catheter membrane), while 2-AG bars only includes amounts from CM. 20 min represent catheter A, 100 min catheter B, 140 min C and 220 min catheter D (D only trapezius).
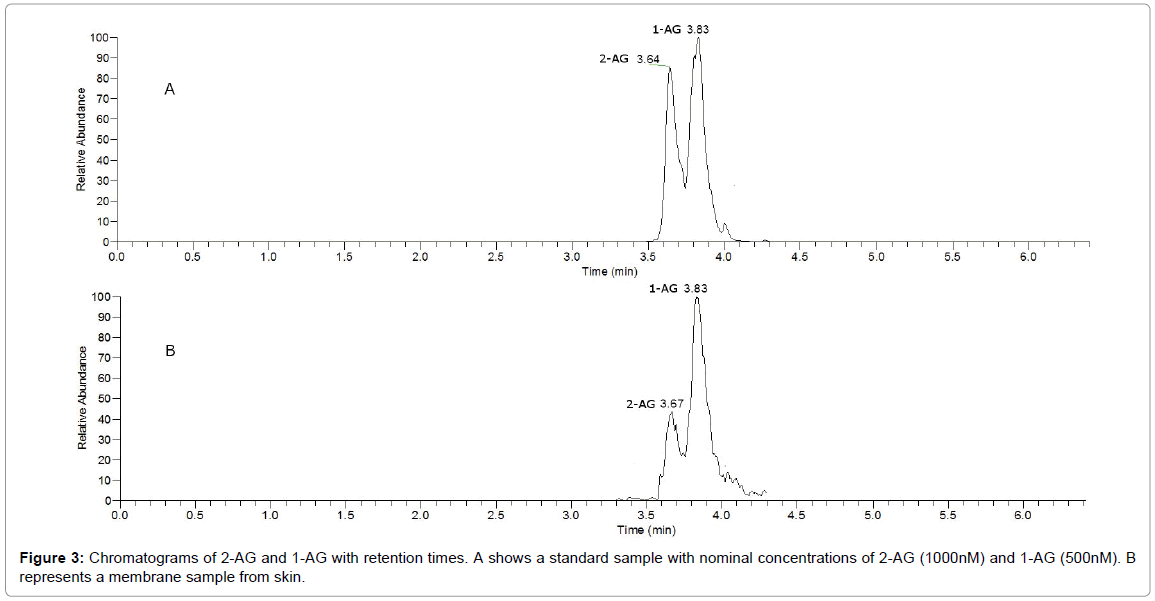
2-AG exists as a mixture of 1-AG and 2-AG isomers and are chromatographically resolved (Figure 3). In this study as in previously reports [28,29,34] we report the total amount of the two isomers as 2-AG. 2-AG undergoes spontaneous isomerization to 1-AG [35], and in addition, methanol as extraction solvent for sample preparation have been reported to further accelerate the isomerization [33], which could be an experimental cause of the distribution between 2-AG and 1-AG (Figure 3).
Inclusion of hydroxypropyl-β-cyclodextrine (HPCD) in the perfusate was reported to increase the recovery of AEA in vitro [20], and HPCD have been a common perfusion fluid additive in rat brain MD studies of ECs and NAEs [19,29]. Although HPCD was shown to improve the recovery of AEA and 2-AG in vitro in a proof-of-concept study, in the subsequent in vivo sampling from adipose tissue in the same study, only AEA was measured in dialysate samples [24]. 2-AG levels have been reported to be ~150 fold higher than AEA in adipose tissue [33], this together indicates that HPCD additives have limited ability to facilitate MD sampling and improve recovery for 2-AG when sampled from adipose tissue.
Investigation of data conserved in the catheter membrane during an MD experiment could be an applicable extension of MD sampling methodology even for other compounds e.g., proteins. Previously, ‘end point biopsy’ and MD have been combined in an attempt to relate levels of cytokines in microdialysate to actual presence of cytokines in the tissue [27]. Whilst “end point biopsy” is achievable, membranes are available in all MD experimental settings and can thus more conveniently fill an “information gap” than the more cumbersome performance of tissue biopsy.
Besides the problems of adsorption of these lipids to plastic surfaces, the problem of contamination of some of them from solvents, glass bottles and plastic tubes also has been raised. Contamination of PEA and SEA from chloroform have been reported [36], and polypropylene have been shown to release additional amounts of PEA, and OEA where dichloromethane and chloroform were used during evaporation [31]. We observed low background levels of SEA, PEA and OEA in the LC mobile phase that we used and also in some processed perfusate blank samples. Baseline blank levels (mobile phase and processed perfusate blanks) were not corrected for in the two measurement series in the present paper, due to that only relatively low levels were found. In the trapezius measurements, baseline blank peaks over LOD were found only for SEA, and the highest blank level represented ˂4% of the mean sample level. In the skin measurements a high blank found was for PEA representing ˂3% of the sample mean. Neither 2-AG nor AEA were detected in the mobile phase, processed perfusate solution and unused catheter membrane samples. Thus, without speculation about the origin of these contaminants (PEA, SEA, OEA), we confirm their presence which needs to be considered when similar sample preparation and LC-MS/MS set-ups are conducted as in this study.
Conclusion
Sampling of the lipid mediators OEA, PEA and SEA from the trapezius muscle and from skin tissue was successfully achieved with the presented MD set-up. Adsorption of NAEs to catheter membranes did occur and was most pronounced during the first 20 min after catheter insertion. To the best of our knowledge this is the first study to report the presence of the endocannabinoid 2-AG in human trapezius muscle and forearm skin.
Further, we conclude that consideration of data conserved in the membrane during an MD experiment could be a more broadly applicable extension of MD sampling methodology which could enhance an adequate interpretation of microdialysate data outcomes and be relevant in situations such as choice of membranes.
Acknowledgements
The authors would like to thank Eva-Britt Lind for her technical expertise in conducting MD procedures, PhD Per Leandersson for his technical support when setting up the LC-MS/MS method used in this study and professor Christopher Fowler for his advice and comments. This study was funded by the Swedish Research Council (K2011-69X-21874-01-6 and K2015-99x-21874-05-4), Swedish Council for Working Life and Social Research (2010-0913) and the County Council of Östergötland. The authors declare no conflicts of interest.
References
- De Petrocellis L, Melck D, Bisogno T, Di Marzo V (2000) Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem Phys Lipids 108: 191-209.
- Fowler CJ, Holt S, Nilsson O, Jonsson KO, Tiger G, et al. (2005) The endocannabinoid signaling system: pharmacological and therapeutic aspects. Pharmacology, biochemistry, and behavior 81: 248-262.
- Pacher P, Bátkai S, Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58: 389-462.
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, et al. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452-457.
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, et al. (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Molecular pharmacology 67: 15-19.
- Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, et al. (2009) Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proceedings of the National Academy of Sciences of the United States of America 106: 20966-20971.
- de Fonseca FR, Navarro M, Gomez R, Escuredo L, Nava F, et al. (2001) An anorexic lipid mediator regulated by feeding. Nature 414: 209-212.
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, et al. (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425: 90-93.
- SuardÃaz M, Estivill-Torrús G, Goicoechea C, Bilbao A, RodrÃguez de Fonseca F (2007) Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 133: 99-110.
- Wang X, Miyares RL, Ahern GP (2005) Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. The Journal of physiology 564: 541-547.
- Maccarrone M, Cartoni A, Parolaro D, Margonelli A, Massi P, et al. (2002) Cannabimimetic activity, binding, and degradation of stearoylethanolamide within the mouse central nervous system. Mol Cell Neurosci 21: 126-140.
- Silvestri C, Ligresti A, Di Marzo V (2011) Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocr Metab Disord 12: 153-162.
- Iannotti FA, Silvestri C, Mazzarella E, Martella A, Calvigioni D, et al. (2014) The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proceedings of the National Academy of Sciences of the United States of America 111: E2472-E2481.
- Piomelli D, Sasso O (2014) Peripheral gating of pain signals by endogenous lipid mediators. Nature neuroscience 17: 164-174.
- Piomelli D, Hohmann AG, Seybold V, Hammock BD (2014) A lipid gate for the peripheral control of pain. J Neurosci 34: 15184-15191.
- de la Peña A, Liu P, Derendorf H (2000) Microdialysis in peripheral tissues. Adv Drug Deliv Rev 45: 189-216.
- Ungerstedt U (1991) Microdialysis--principles and applications for studies in animals and man. J Intern Med 230: 365-373.
- Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, et al. (2007) AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharmaceutical research 24: 1014-1025.
- Bequet F, Uzabiaga F, Desbazeille M, Ludwiczak P, Maftouh M, et al. (2007) CB1 receptor-mediated control of the release of endocannabinoids (as assessed by microdialysis coupled with LC/MS) in the rat hypothalamus. The European journal of neuroscience 26: 3458-3464.
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC (1999) Pain modulation by release of the endogenous cannabinoid anandamide. Proceedings of the National Academy of Sciences of the United States of America 96: 12198-12203.
- Plaza-Zabala A, Berrendero F, Suarez J, Bermudez-Silva FJ, Fernandez-Espejo E, et al. (2010) Effects of the endogenous PPAR-alpha agonist, oleoylethanolamide on MDMA-induced cognitive deficits in mice. Synapse 64: 379-389.
- Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, et al. (2012) Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS chemical neuroscience 3: 407-417.
- Schäbitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, et al. (2002) Release of fatty acid amides in a patient with hemispheric stroke: a microdialysis study. Stroke 33: 2112-2114.
- Zoerner AA, Rakers C, Engeli S, Batkai S, May M, et al. (2012) Peripheral endocannabinoid microdialysis: in vitro characterization and proof-of-concept in human subjects. Analytical and bioanalytical chemistry 402: 2727-2735.
- Ghafouri N, Ghafouri B, Larsson B, Turkina MV, Karlsson L, et al. (2011) High levels of N-palmitoylethanolamide and N-stearoylethanolamide in microdialysate samples from myalgic trapezius muscle in women. PLoS One 6: e27257.
- Ghafouri N, Ghafouri B, Larsson B, Stensson N, Fowler CJ, et al. (2013) Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain 154: 1649-1658.
- Sjogren F, Anderson CD (2010) Are cutaneous microdialysis cytokine findings supported by end point biopsy immunohistochemistry findings? AAPS J 12: 741-749.
- Balvers MG, Verhoeckx KC, Witkamp RF (2009) Development and validation of a quantitative method for the determination of 12 endocannabinoids and related compounds in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877: 1583-1590.
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA (2007) Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Analytical Biochemistry 360: 216-226.
- Zoerner AA, Batkai S, Suchy MT, Gutzki FM, Engeli S, et al. (2012) Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J Chromatogr B Analyt Technol Biomed Life Sci 883-884: 161-171.
- Fanelli F, Di Lallo VD, Belluomo I, De Iasio R, Baccini M, et al. (2012) Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. Journal of Lipid Research 53: 481-493.
- Matias I, Gatta-Cherifi B, Tabarin A, Clark S, Leste-Lasserre T, et al. (2012) Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One 7: e42399.
- Zoerner AA, Gutzki FM, Batkai S, May M, Rakers C, et al. (2011) Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochim Biophys Acta 1811: 706-723.
- Gachet MS, Rhyn P, Bosch OG, Quednow BB, Gertsch J (2015) A quantitiative LC-MS/MS method for the measurement of arachidonic acid, prostanoids, endocannabinoids, N-acylethanolamines and steroids in human plasma. J Chromatogr B Analyt Technol Biomed Life 976-977: 6-18.
- Vogeser M, Schelling G (2007) Pitfalls in measuring the endocannabinoid 2-arachidonoyl glycerol in biological samples. Clin Chem Lab Med 45: 1023-1025.
- Skonberg C, Artmann A, Cornett C, Hansen SH, Hansen HS (2010) Pitfalls in the sample preparation and analysis of N-acylethanolamines. J Lipid Res 51: 3062-3073.
Citation: Stensson N, Ghafouri N, Träff H, Anderson CD, Gerdle B (2016) Identification of Lipid Mediators in Peripheral Human Tissues Using an Integrative In Vivo Microdialysis Approach. J Anal Bioanal Tech 7:306. DOI: 10.4172/2155-9872.1000306
Copyright: © 2016 Stensson N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12184
- [From(publication date): 4-2016 - Aug 18, 2025]
- Breakdown by view type
- HTML page views: 11223
- PDF downloads: 961