Research Article Open Access
Immunohistochemical and Fluorescence In Situ Hybridization Analysis of Angiosarcoma of Soft Tissue and Bone Including those Occurring in Unusual Backgrounds
Bibianna Purgina1,3*, Michael Nalesnik1, Kathleen Cieply1, Kimberly Fuhrer1, Mark Goodman2, Richard McGough2 and Uma N.M. Rao11Department of Pathology, Presbyterian-Shadyside Hospital, University of Pittsburgh Medical Center, USA
2Department of Orthopaedic Surgery, University of Pittsburgh Medical Center, USA
3Department of Anatomical Pathology, University of Ottawa, Canada
- *Corresponding Author:
- Bibianna Purgina
Pathologist and Assistant Professor
Division of Anatomical Pathology
Department of Pathology and Laboratory Medicine
The Ottawa Hospital/University of Ottawa, 501 Smyth Rd.
4th Floor CCW, Room 4223, Ottawa, ON, K1H 8L6, Canada
Tel: 613-737-8899
Fax: 613-737-8461
E-mail: bpurgina@ottawahospital.on.ca
Received date: February 24, 2014; Accepted date: April 19, 2014; Published date: April 21, 2014
Citation: Purgina B, Nalesnik M, Cieply k, Fuhrer K, Goodman M, McGough R and Rao UNM (2014) Immunohistochemical and Fluorescence In Situ Hybridization Analysis of Angiosarcoma of Soft Tissue and Bone Including those Occurring in Unusual Backgrounds. J Clin Exp Pathol 4:171. doi:10.4172/2161-0681.1000171
Copyright: © 2014 Purgina B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Background: Angiosarcomas (AS) are high-grade sarcomas that comprise less than 1% of soft tissue sarcomas. We compared clinical, pathological and molecular features of primary and recurrent conventional AS, including cases occurring in unusual backgrounds, such as organ transplants, at a site of metal orthopedic implant, and at a site of prior trauma.
Materials and Methods: Paraffin blocks from 15 patients with AS were retrieved from our archives. Ten patients had recurrent AS, 4 patients had metastases. AS were morphologically categorized them into epithelioid and conventional types. Immunohistochemical stains included three vascular markers, cytokeratins, Akt, Ki67, HHV8 and EBV in paired samples of primary and recurrent/metastatic AS. Fluorescence In Situ Hybridization (FISH) analysis for Epidermal Growth Factor Receptor (EGFR) and Hepatocyte Growth Factor Receptor (MET) was performed on all AS samples.
Results: Conventional AS were positive for more than two vascular markers and negative for cytokeratins. Epithelioid AS demonstrated variable positivity for vascular markers and cytokeratins. All tumors were negative for HHV8 and EBV. All AS displayed cytoplasmic immunopositivity for the phosphorylated forms of Akt.
Conclusion: AS with epithelioid features had fewer positive vascular markers, possibly reflecting a lack of differentiation. There were no differences in pattern of immunoreactivity between post-transplant and conventional AS and primary and metastatic AS. No amplification of either EGFR or MET was found but a majority of the cases demonstrated hyperploidy and three cases demonstrated monosomy for EGFR in greater than 50% of cells. Both MET and EGFR are located on chromosome 7 and the detected hyperploidy and monosomy of these two genes are likely related to copy number alterations of chromosome 7. It is unlikely that these two genes play a role in biology of AS. AS with epithelioid features and metal associated AS, which are extremely rare, had an aggressive clinical course.
Keywords
Angiosarcoma; Metal-associated; EGFR; MET; Epithelioid; FISH
Introduction
Angiosarcoma (AS) is considered to be a rare, high-grade sarcoma displaying endothelial differentiation and comprising less than 1% of all soft tissue sarcomas. Anatomical distribution of primary AS is wide but it commonly arises in the skin and subcutaneous tissue in 3 clinical scenarios: (i) in the face and scalp of elderly patients; (ii) in extremities of patients with chronic lymphedema, (iii) in sites of previous radiation therapy, and (iv) exposure to chemical carcinogens [1]. Primary AS of the bone are extremely rare, comprising less than 1% of malignant primary bone tumors, and those associated with long- standing metal prosthesis are even more rare [2].
AS displays a disorganized proliferation of atypical endothelial cells, and forms open anastomosing vascular channels or blood-filled slit-like spaces. Epithelioid AS is a histologic variant composed of highly atypical “epithelioid-appearing” endothelial cells that are large, round and have an abundance of cytoplasm. AS may express a variety of endothelial/vascular markers such as CD34, CD31, Ulex europaeus, Factor VIII and D2-40, and these markers demonstrate variable specificities and sensitivities. For instance, CD31 may also highlight histiocytes. CD34 demonstrates immunoreactivity in many non-vascular neoplasms such as solitary fibrous tumor, dermatofibrosarcoma protuberans, spindle cell lipoma and epithelioid sarcoma. In addition, epithelioid AS typically expresses cytokeratins, which may lead to a misdiagnosis of carcinoma, particularly in small biopsy material. It is best to use several of vascular markers when considering a vascular neoplasm. Based on current recommendations, AS is not graded. Morphologically low grade and epithelioid areas may be described, but the overall diagnosis should reflect a high-grade sarcoma [1,3].
AS is one of the few sarcomas that metastasize to regional lymph nodes and distant metastases to lung, bones and even brain have been reported [1]. Due to its rarity, there have been few cytogenetic and molecular studies, none of which have shown a specific translocation or specific marker chromosome in AS [4]. There exist only a few published studies that include over 100 patients [5-7]. Since rare malignancies affect small patient populations, these entities pose a challenge for both clinicians and pathologists to diagnose and manage and for investigators to discover new diagnostic tools, prognostic markers, and treatment options. Over the past several decades, no major changes in the management of AS have evolved and new therapeutic strategies are needed. The Akt/mammalian target of Rapamycin (mTOR) pathway, among others, has been identified as a therapeutic target in some epithelial and mesenchymal malignancies [8]. We examined AS occurring in 15 patients, including those occurring in unusual backgrounds in order to determine the role of EGFR, MET and phosphorylated forms of Akt in this tumor.
Materials & Methods
Case material
Following Internal Review Board approval a total of 15 adult patients with primary, recurrent and/or metastatic AS were retrieved from the surgical pathology archives of UPMC, Pittsburgh, PA for the period 2003 to 2011 (total of 20 samples). Of the 15 patients, material from the primary lesion was available for all 15 patients. Matched recurrences were available for 4 patients (Table 2 cases 6, 8, 9 and 12). Case 2 (Table 2) presented with tibial AS and during the clinical work-up of this mass a second AS of the penis of similar morphology was identified, with one of these lesions representing a metastasis. Follow-up clinical data from the tumor registry was available for all patients and linked to the de-identified samples through an honest broker per protocol. All sections, including immunohistochemical stains, for all patients were reviewed and the diagnoses confirmed, according to the current World Health Organization Soft Tissue Tumor classification by a UPMC pathologist (U. Rao) familiar with soft tissue and bone lesions. All cases had sufficient material for further ancillary studies.
Immunohistochemical studies
A diagnostic immunohistochemical was performed at the time of initial diagnosis for each of the 15 patients and included at least 3 vascular markers, wide spectrum cytokeratins and low molecular weight cytokeratins, Ki67, and in older and/or immunocompromised patients, HHV8 and EBV. Immunohistochemical analysis using antibodies against the phosphorylated forms of Akt was performed for all 15 patients (total 19 samples).
Immunohistochemical analysis was performed on 4-μm sections of formalin-fixed, paraffin-embedded tissue using previously described methods. The antibodies, manufacturers, clones and dilutions are listed in Table 1. Immunohistochemistry slides were graded as positive (overall strong and diffuse staining), focally positive (defined areas of strong to moderate staining), or negative (lacking any staining). The labeling intensity for Akt was independently scored by two soft tissue pathologists as none, weak, moderate, strong and the amount of positive tumor cells were recorded as focal or diffuse.
| Antibody | Company | Clone | Host | Dilution |
|---|---|---|---|---|
| Vascular Markers | ||||
| Ulexeuropaeus | Abcam, Cambridge, MA | n/a | Polyclonal Rabbit | 0.111111 |
| Factor VIII | Dako, Carpenteria, CA | n/a | Polyclonal Rabbit | 0.388889 |
| D2-40 | Signet, Princeton, NJ | D2-40 | Monoclonal Mouse | 1:25 |
| CD34 | Ventana, Tucson, AZ | QBEnd/10 | Monoclonal Mouse | Predilute |
| CD31 | Dako, Carpenteria, CA | JC70A | Monoclonal Mouse | 1:40 |
| Epithelial Markers | ||||
| PanKeratin | Abcam, Cambridge, MA | 80 | Monoclonal Mouse | 0.111111 |
| Cytokeratin AE1/AE3 | Dako, Carpenteria, CA | AE1/AE3 | Monoclonal Mouse | 0.111111 |
| Cam5.2 | BD (Becton Dickinson), Franklin Lakes, NJ | Cam 5.2 | Monoclonal Mouse | 1:10 |
| Viral Pathogen Markers | ||||
| HHV8 | Vector, Burlingame, CA | 13B10 | Monoclonal Mouse | 1:50 |
| EBV | Dako, Carpenteria, CA | CS 1-4 | Monoclonal Mouse | 0.111111 |
| Proliferation Index Marker | ||||
| Ki67 | Dako, Carpenteria, CA | MIB-1 | Monoclonal Mouse | 0.111111 |
| Receptor Tyrosine Kinase Marker | ||||
| CD117 (c-kit) | Dako, Carpenteria, CA | n/a | Polyclonal Rabbit | 0.111111 |
| Protein Kinase Marker | ||||
| AKT | Cell Signaling Technology, Danvers, MA | n/a | Monoclonal Rabbit | 1:50 |
Table 1: Antibodies used for immunohistochemistry
Fluorescence in situ hybridization
Samples of primary, recurrent and metastatic AS in all 15 patients (total 19 samples) were selected for FISH analysis for EGFR and MET amplification. Due to prior decalcification FISH was not performed on the material from the tibial mass in case 2. Formalin-fixed paraffin-embedded sections, were mounted, and serially sectioned at 5-mm intervals. An H&E section was used by the Pathologist to determine the area of the tissue to be targeted for analysis. FISH slides were deparaffinized in xylene twice for 10 minutes, dehydrated twice with 100% ethanol and then pretreated for 30 minutes in 0.2N HCl (Sigma). Slides were then digested for 28 minutes in protease solution (0.5 mg/ml) at 37°C.
FISH for EGFR was performed using the LSI EGFR/CEP7 dual-color probe (Abbott Molecular, Inc., Des Plaines, IL) and for MET using the C-MET 7q31/CEP7 probe (red/green) Amplification (Rainbow Scientific Inc, Windor, CT). The target slide and probe were co-denatured at 90°C for 12 minutes and incubated overnight at 37°C in a humidified chamber. Post-hybridization washes were performed using 2XSSC/0.3% Igepal (Sigma) at 72°C for 2 minutes. Slides were air-dried in the dark and counterstained with DAPI I (Abbott Molecular). Analysis was performed using an Applied Imaging Workstation equipped with Chroma Technology filters containing band excitors for SpectrumOrange, FITC, DAPI. Only individual and well-delineated cells were scored. Overlapping cells were excluded from the analysis. At least 60 cells were analyzed in the targeted region.
Each tumor specimen was assessed by the average number of copies of the EGFR and MET gene per cell, the average ratio of the EGFR and MET gene to chromosome 7 copy numbers, and ploidy. Amplification was defined as a ratio of EGFR or MET signals to chromosome 7 centromere signals of ≥ 1.30. Hyperploidy was defined as a mean of > 2 chromosome 7 signals per cell.
Results
Population characteristics
The mean age of the patients with AS was 67.8 years of age (range 40-94). The male to female ratio was almost equal (7M: 8F). Most (73.3%) were over 60 years old and only one was younger than 50. The most common anatomic locations were subcutaneous/deep soft tissue and scalp region.
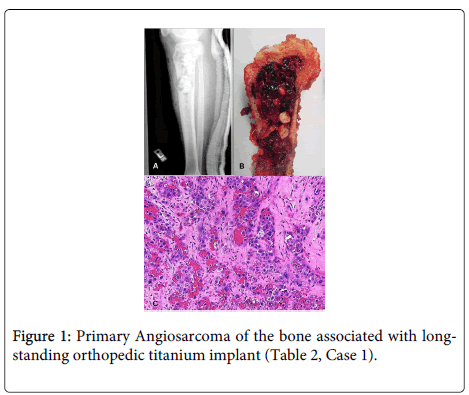
The cases are detailed in Table 2. Our series also included five uncommon cases of AS requiring special mention. One case was a primary bone AS associated in a 68-year-old man who presented with a four month history of increasing pain in his left tibia which had a long-standing metal orthopedic implant for a bicondylar and tibial plateau fracture (Table 2, Case 1 and Figure 1).
| Case | Age, Sex | Past Medical History | Site | Epithelioid or Conventional | Treatment | Outcome (months) |
|---|---|---|---|---|---|---|
| 1 | 68M | Remote MVA with left tibia plateau fracture; ORIF with titanium implants. | Bone (1°). Mets to soft tissue and lungs. | Epithelioid | Surgery | DOD (4) |
| 2 | 72M | Prostate carcinoma | Penis | Epithelioid | Surgery | DOD (1.5) |
| R tibia | ||||||
| 3 | 50F | NF1, multivisceral transplant for NF1 (stomach, liver, small bowel, pancreas). Widespread metastatic AS. | AbdosubQ (widespread mets) | Epithelioid | Supportive care | DOD (1) |
| 4 | 58F | Renal transplant for cystic malformation. | Native Kidney | Conventional | Surgery | ANED (43) |
| 5 | 57M | MVA 20 years ago with hematoma and myositis ossificans | LE deep soft tissue | Epithelioid | Surgery | AWD (6) |
| 6 | 80F | CRC, R DVT, L ankle fracture. | AbdosubQ (1°); | Conventional | Surgery + palliative XRt | DOD (37) |
| LE (recur) | ||||||
| 7 | 76F | Breast carcinoma | Scalp subQ | Conventional | Surgery | DOD (7) |
| 8 | 77F | Nil significant | Scalp (1°); | Epithelioid | Surgery | DOD (19) |
| Scalp (recur) | Conventional | |||||
| 9 | 40M | Lung carcinoma | LE subQ (1°); | Epithelioid | Surgery + XRt | ANED (45) |
| LE subQ (recur) | ||||||
| 10 | 63M | Paraplegia, bilateral AKA for PVD. Widespread metastatic AS. | LE subQ (1°) (widespread mets) | Epithelioid | Surgery | DOD (1.5) |
| 11 | 74F | Breast ca treated with lumpectomy and XRt | Breast | Conventional | Surgery + Chemo | AWD (53) |
| 12 | 62M | 1° AS of scalp with multiple (9) recurrences | Scalp (1°) | Conventional | Surgery + XRt | AWD (48) |
| Scalp (recur) | ||||||
| 13 | 94F | Nil significant | Scalp (1°) | Conventional | Surgery +XRt/Supportive care | DOD (44) |
| 14 | 84M | SCC of mandibular gingiva | Cheek mucosa (1°) | Conventional | Surgery | AWD (9) |
| 15 | 62F | 1° AS of leg subQ with multiple recurrences | LE subQ (1°) | Epithelioid | Surgery | DOD (13) |
| M=male; F=female, MVA=motor vehicle accident; ORIF = open reduction and internal fixation; R=right; L=left; mets=metastases; 1°=primary; subQ=subcutaneous tissue; XRt=radiation therapy; AWD=alive with disease; ANED=alive no evidence of disease; DOD=dead of disease; 1°=primary; Recur=recurrence; LE= lower extremity; SubQ=subcutaneous tissue; SCC=squamous cell carcinoma; CRC=colorectal carcinoma; ca=carcinoma; AKA=above knee amputation; PVD=peripheral vascular disease; NF=neurofibromatosis type 1 | ||||||
Table 2: Clinical Features in 15 patients with Angiosarccoma.
A. X-ray of the left proximal tibia, following hardware removal, reveals a large, ill-defined lesion extending from the epiphysis into the diaphysis. B. Gross image from the proximal tibia reveals a hemorrhagic fleshy mass, measuring approximately 16.0 cm, in the region of the recently removed titanium rods. C. Medium Power view (H&E stain, 200x magnification) reveals a highly atypical vascular lesion composed of large, irregular anastomosing vessels lined by atypical epithelioid-appearing endothelial cells with prominent nucleoli.
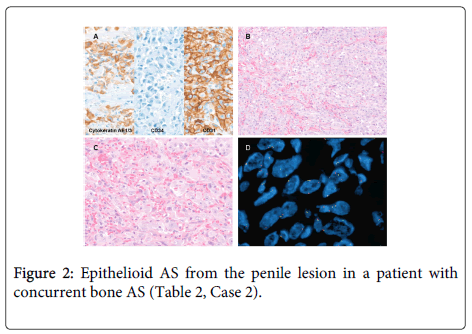
A. The lesion is immunoreactive for cytokeratin AE1/3 and CD31, and negative for CD34. Medium power (B, H&E stain, 200x) and high power views (C, H&E stain, 400x) demonstrate irregular vascular channels lined by highly atypical endothelial cells with epithelioid features. D. FISH for EGFR demonstrates hyperploidy in 1.6% of cells. No EGFR amplification is seen.
Following radiologic studies and biopsy, which identified a large, 16.0 cm ill-defined AS associated with the orthopedic implant and involving the epiphysis and diaphysis of the left proximal tibia. He subsequently underwent a left above knee amputation. The second AS in bone occurred in the tibia of a 72-year-old man. During the work-up of this mass, a concurrent penile AS of similar morphology was identified. He had a history of radiation and hormone-treated prostate carcinoma (Table 2, Case 2 and Figure 2). Ancillary studies were performed on the penile lesion. The patient rapidly deteriorated and died of metastatic disease within 6 weeks of diagnosis. The possibility of radiation-induced AS with widespread metastases was considered likely in this patient.
There were two cases of AS which arose in patients who had received organ transplantation. One of these patients had a history of neurofibromatosis type 1 and multivisceral organ transplantation (Table 2, Case 3). She developed multifocal and metastatic epithelioid AS of subcutaneous and deep soft tissue. She rapidly deteriorated and died within a month of diagnosis. The second patient with a history of a kidney transplant had a complex cystic mass of her native kidney and numerous mucinous cysts in the head of the pancreas and vascular malformation of the portal system that resulted in portal hypertension (Table 2, Case 4). Her native kidney was removed and revealed a 2.3 cm conventional AS with angiolymphatic invasion. She received no adjuvant radiation or chemotherapy and she is alive with no evidence of AS (43 months).
The fifth patient is a 57-year-old man who developed an epithelioid AS adjacent a vehicular injury related myositis ossificans that had remained stable on X-rays for 20 years and recently developed discomfort and swelling (Table 2, Case 5). The lesion was surgically resected revealed and revealed a 6.0 cm focus of epithelioid AS, adjacent to a large area of heterotopic ossification, skeletal muscle atrophy and fibrosis with hemosiderin deposition.
Immunohistochemistry Features
A. The lesion is immunoreactive for CD31 and negative for cytokeratin AE1/3 (with internal control). The Ki67 is positive in > 50% of cells. Medium power (B, H&E stain, 200x) and high power views (C, H&E stain, 400x) demonstrate irregular vascular channels lined by atypical endothelial cells. D. FISH for EGFR demonstrates hyperploidy in 30.3% of cells. No EGFR amplification is seen.
All conventional AS without epithelioid features were positive for two or more vascular markers that included CD31, CD34 and Factor VIII and all were negative for cytokeratins (Figure 3). Epithelioid AS were mostly focally positive for CD31, variable for the other vascular markers and the five of eight cases were positive with cytokeratins (Figure 2). All epithelioid AS were negative for S100, melan A and tyrosinase, excluding malignant melanoma. Conventional cytogenetics and karyotyping was performed in only one case (case 1). Conventional karyotype following short term culture revealed 47, XY, +8[2]/46, XY [26].
| Case | Site | Epi or Conv |
FISH for EGFR | FISH for MET | AKT | ckit | Vascular Markers | Cyto- | Ki67 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | %Hyper | %Mono | Ratio | %Hyper | %Mono | keratins | ||||||||
| 1 | Soft tissue met | Epi | 1 | 0 | 1.01 | 16.60% | M,D + | Neg | CD31 F+; CD34 F+; Ulex- | panCK-; CAM5.2- | 10-50% | |||
| 2 | Penis | Epi | 1.03 | 1.60% | 1.03 | 0 | S,D + | Neg | CD31 +; CD34 -; Ulex+; FVIII+ | CAM5.2 focal +; panCK focal + | 10-50% | |||
| Tibia | Decalcified specimen –FISH not done | Decalcified specimen – FISH not done | >50% | |||||||||||
| 3 | AbdosubQ | Epi | 1.71 | 1.60% | 52.50% | 1.13 | 16.60% | S,D + | Neg | CD31 F+ CD34 F+; D2-40 F+ | panCK- | 10-50% | ||
| 4 | Native Kidney | Conv | 1.01 | 13.30% | 1.03 | 10% | W,F+ | Neg | CD31+; CD34+; FVIII | panCK- | <10% | |||
| 5 | LE | Epi | 1 | 4.60% | 1.18 | 3% | M,D + | Neg | CD31+; CD34-; D2-40-; FVIII- | AE1/3+ | <10% | |||
| 6 | AbdosubQ (1°); | Conv | 0.99 | 24.60% | Not done | W,D + | Neg | CD31+; CD34+; D2-40-; FVIII+ | AE1/3- | 10-50% | ||||
| LE (recur) | 1.07 | 30.30% | 0.96 | 10% | M,D + | Neg | Not done | 10-50% | ||||||
| 7 | Scalp | Conv | 1.09 | 25.40% | 1.02 | 59.30% | M,D + | Neg | CD31+; CD34-; FVIII+ | panCK- | >50% | |||
| 8 | Scalp (1°); | Epi | 0.96 | 5.00% | 1.16 | 16.60% | Not done | Neg | CD31 F+; CD34 F+; FVIII+; Ulex+ | panCK- | >50% | |||
| Scalp (recur) | Conv | 1 | 0 | Not done | M,D + | Neg | CD31+, CD34+, FVIII+ | Not done | >50% | |||||
| 9 | LE (1°); | Epi | 1.06 | 3.30% | 50.80% | 0.93 | 0 | S,D + | Neg | CD31 F+; CD34 F+; FVIII+ | AE1/3+ | 10-50% | ||
| LE (recur) | 1.02 | 0 | Not done | M, D + | Neg | |||||||||
| 10 | LE (1°) | Epi | 1.02 | 3.30% | 1 | 0 | S,D + | Neg | CD31+; CD34 F+; Ulex+; | panCK- | <10% | |||
| 11 | Breast | Conv | 1.03 | 3.30% | 0.98 | 6% | M,D + | Neg | CD31+; CD34+; Ulex+ | AE1/3- | >50% | |||
| 12 | Scalp (1°) | Conv | 1.04 | 36.10% | 0.93 | 0 | S,D + | Neg | CD31+; CD34+; FVIII+ | panCK- | <10% | |||
| Scalp (recur) | 1.01 | 0 | Not done | M,D + | Neg | CD31+; CD34+; FVIII+ | panCK- | <10% | ||||||
| 13 | Scalp (1°) | Conv | 1.02 | 0 | 50.00% | 0.93 | 0 | M,F + | Neg | CD31+; CD34+; FVIII+; D2-40+ | panCK- | 10-50% | ||
| 14 | Cheek mucosa (1°) | Conv | 1.08 | 11.90% | 0.95 | 0 | W,F + | Neg | CD31+, CD34+; FVIII+; D2-40+ | panCK- | <10% | |||
| 15 | LE (1°) | Epi | 1.02 | 45.00% | 0.98 | 16.60% | W,D + | Neg | CD31+; CD34-; FVIII focal + | panCK-; AE1/3- | 10-50% | |||
| Epi=Epithelioid; Conv=Conventional; IHC=immunohistochemistry results; + = positive; - = negative; W = weak; M = moderate; S = strong; F = focal, D = diffuse; neg – negative; FVIII – Factor VIII; panCK = pancytokeratin; AE1/3 = cytokeratin AE1/3; 1° – primary; subQ – subcutaneous tissue; recur – recurrence; LE – lower extremity; UE – upper extremity; met – metastases; Abdo - abdomen | ||||||||||||||
Table 3: Immunohistochemistry Results and FISH for EGFR and MET in 15 patients with Angiosarcoma.
Ki67 positivity was variable in both conventional and epithelioid AS (Table 3). HHV8, EBV and ckit were negative. The 4 cases of metastatic AS demonstrated epithelioid features (Table 2, Cases 1, 2, 3 and 10). There were 4 cases with recurrences (Table 2, Cases 6, 8, 9, and 12), some with up to nine recurrences, which had either conventional or epithelioid features. There was no difference in the pattern of immunoreactivity in post-transplant AS. All 15 cases, including recurrences (Table 2, Cases 6, 8, 9 and 12) and metastatic lesions (Table 2, Cases 2), a total of 19 samples, demonstrated cytoplasmic immunoreactivity with Akt (Table 2). Majority of the cases demonstrated at least moderate staining (15/19) and a diffuse staining pattern (15/19). Focal nuclear staining was also noted, as was weaker staining in the adjacent morphologically normal appearing blood vessels
Fluorescence in situ hybridization results
None of the 15 patients’ demonstrated EGFR or MET amplification (Table 2) but 14 of the 19 samples demonstrated EGFR hyperploidy in the range of 1.6-45% (Table 2, Figures 2 and 3) and EGFR monosomy in at least 50% of the cells in 3 of the 19 samples. Low levels of MET hyperploidy were present in some tumors that were not congruent to EGFR ploidy.
EGFR Hyperploidy was present in the primary lesions of Case 6, 8, and 12 and EGFR monosomy was present in the primary lesion of Case 9, but neither hyperploidy nor monosomy was present in the matched recurrence.
Discussion
We compared the clinical-pathological features of AS of soft tissue and bone, and those occurring in unusual backgrounds and also compared them with matched recurrences when available. Previous reports on AS occurring in bone at the site of a metal prosthesis is extremely rare [2], as are those occurring in organ transplant patients [9,10]. However, despite varied backgrounds, all AS had similar and overlapping morphologic features and immunophenotypes. There was no difference in the pattern of immunoreactivity among our matched primary and recurrent/metastatic cases. In our series, the majority of patients had an aggressive clinical course that in part was determined by individual clinical stage at presentation and was comparable to those reported in literature [5-8,11]. Nine of our fifteen patients have succumbed to their disease and the clinical outcome of the patients in our study is thus comparable to several larger studies of patients with AS treated at tertiary centers [6,7,11]. Numerous studies have shown that patients initially presenting with metastatic AS have significantly worse outcomes [4,5,8,11-14]. Four of our cases presented with metastatic disease and succumbed to their disease within 2 months (range 1.0-4.0 months). Patients with completely resected AS do better and this reinforces surgery as the main treatment for AS [5].
Our epithelioid subset of AS patients had a much more aggressive course with multiple recurrences, metastatic disease and shorter survival (6.7 months versus 29.3 months) and proved fatal in six patients including the patient with metal-associated AS. Currently four patients are alive with local recurrence of tumor and two patients are alive with no evidence of disease (Table 2).
Risk factors for AS include radiation therapy and lymphedema. AS has been described developing in association with foreign bodies, AV fistulas and an increased risk has been described in patients with neurofibromatosis [1]. Metal alloys and other implants are commonly used in orthopedic practice for fractures and degenerative joint disease, but the incidence of AS arising in such cases is rare [2]. AS arising in association with non-functioning AV fistula in post-renal transplant patients have been reported [1,9,10] however, to the best of our knowledge there is no reported association between organ transplantation and AS and there have been no reported cases of AS arising in a native non-functioning kidney in a renal transplant patient.
For AS, grading has not been considered of prognostic value [1,3,15,16]. Although we did not grade any of our cases, those we designated as epithelioid were associated with an adverse prognosis, which is in concert with prior studies [7,16]. A fair number of cases especially those with epithelioid features were cytokeratin positive, which emphasizes the need of additional immunohistochemical markers not only to distinguish them epithelial malignancies but also from sarcomas which express cytokeratin especially epithelioid sarcoma. CD31 is useful in an obvious case of AS, but its known aberrant expression in histiocytes should be kept in mind and we recommend using additional vascular markers in a difficult case [4]. Our study supports this observation that, regardless of anatomic location, AS demonstrating epithelioid features had a much more aggressive course with multiple recurrences, metastatic disease and shorter survivals, and is immunoreactive for fewer vascular markers than conventional AS, possibly reflecting a lack of differentiation.
There are very few genetic studies of AS and most are limited to isolated cases and small studies. The most commonly reported alterations include trisomy 5, gains at 1q, 7q, 8q, gains and losses at 7p, loss of Y and various abnormalities on chromosome 8, 20 and 22 [1,17,18]. In our series, only one patient had conventional cytogenetic analysis and showed clonal gains of chromosome 8 but not chromosome 7. Flow cytometry has demonstrated diploid, tetraploid and aneuploidy patterns, none of which correlated with clinical outcome and histology [19]. However, of interest, alterations in TP53/MDM2 pathway with increased expression of both TP53 and MDM2 have been shown in 60% of AS [19]. Other studies have demonstrated that secondary AS (those arising in the setting of chronic lymphedema or at sites of previous radiation therapy) demonstrates MYC gene amplification and thus is genetically distinct form of AS [20-22]. They also determined that there was no correlation between MYC amplification and epithelioid morphology or increased cell turnover. This finding may have future implications in diagnosis and treatment of AS.
MTOR pathway in these tumors has been explored rarely because of potential targeted therapy with tyrosine kinase inhibitors, rapamycin [7]. EGFR, KRAS and BRAF have been extensively investigated in epithelial malignancies however except for a rare instance of synovial sarcoma, these do not appear to be major players in mesenchymal neoplasms [23,24]. There is increasing evidence that regional gains or high-level amplifications on chromosome 7q are found in various bone and soft tissue sarcomas, however target genes are unknown. MET and its ligand Hepatocyte Growth Factor (HGF) map to 7q (7q31 and 7q21 respectively) have shown to have a role in tumor progression in sarcomas [25,26]. A recent integrative Comparative Genomic Hybridization array revealed increased copy number with gene amplification in 56% of myxofibrosarcomas and MET protein expression correlated with deep location, higher tumor grade and advanced clinical stage [25]. MET overexpression was independently predictive of poor metastases free and overall survival in 67% of cases. We therefore sought to define the role of EGFR and MET, two genes found on chromosome 7, in AS using FISH, and although no amplification was found, some cases showed hyperploidy. The presence of EGFR and MET hyperploidy in both arms of chromosome 7 is interesting. This may merely suggest chromosome copy number gains, however not all of the cases overlap. This implies chromosome specific changes in both arms of chromosome 7 and it’s possible that chromosome 7 harbors areas of interest other than EGFR and MET important in tumorigenesis and progression.
The role of kinase inhibitors are being explored in AS, particularly those with a potential role in vascular biology and in AS [7,27-36]. The most commonly studied markers to date include the vascular endothelial growth factors (VEGFs), EGFR, ckit and representatives of the Akt/mTOR pathway. Most studies indicate that EGFR overexpression is associated with poor prognosis [37-40]. EGFR, an important growth factor, is a cell membrane receptor with intrinsic protein kinase activity, and is of great clinical interest due to its involvement in several cancers and its potential as a therapeutic target [41].
The success in treating certain sarcomas with KIT-tyrosine-kinase inhibitors have led to studies investigating c-kit expression in AS. Recent studies have shown c-kit overexpression in 47 to 58% of AS however, no activating mutations in exons 11 or 17 were identified [7,33,41,44]. None of our cases tested for c-kit demonstrated overexpression, likely the result of small sample size, however we realize that immunoexpression of ckit need not correlate with gene mutation. Further study of the potential role of KIT-tyrosine kinase inhibitors in AS is required.
All cases were studied for EGFR amplification by FISH (Table 3). None of the cases demonstrated EGFR amplification but 14 of the 19 samples demonstrated hyperploidy in the range of 1.6-45% (Figures 2 and 3) and 3 cases demonstrated monosomy in more than 50% of the tumor cells (Table 2). Interestingly, hyperploidy was present in the primary lesion of case 6, 8, 12 and monosomy was noted in case 9, but neither hyperploidy or monosomy was seen in the clinical recurrence (Table 2). This likely represents the selection of a separate clone. There does not appear to be a correlation between EGFR hyperploidy/monosomy and the presence of epithelioid features however, our sample size is small and further studied is required to determine if such an association exists.
The significance of hyperploidy and monosomy in AS is uncertain. Studies attempting to correlate EGFR expression with hyperploidy in other malignancies shows conflicting results [39,45-47]. Thus far, there is little data on the clinical significance of hyperploidy in terms of survival, response to EGFR-tyrosine kinase inhibitors or standard chemotherapy in malignancies associated with EGFR overexpression. The presence of hyperploidy and monosomy is likely a reflection of the high-grade nature of AS with probable losses and gains in many other chromosomes.
The Akt/mTOR pathway has been extensively studied in various malignancies. mTOR can induce cell proliferation, enhance cell survival and inhibit apoptosis. It is activated by insulin and various other growth factors. Several studies have demonstrated Akt/mTOR pathway deregulation in pathologic endothelial process [7,28-30,34-36,48]. Elevated levels of PI3-kinase and activation (phosphorylation) of Akt has been demonstrated in AS arising in chickens and overexpression of active Akt results in vascular malformations in mice [29,36]. Deletion of FoxO transcription factors, which are downstream targets in the Akt/mTOR pathway, important tumor suppressors and regulate vascular homeostasis, leads to vascular lesions in mice [34]. Lahat et al., looked at 3 markers of the Akt/mTOR pathways and found that 85% of their AS samples demonstrated activated Akt [7]. As with our study, they noted weak focal staining of the adjacent normal appearing vessels. They also found increased expression intensity in metastatic AS. All of our cases of AS demonstrated Akt immunoreactivity. This is in keeping with previous studies that have demonstrated Akt activation in both benign and malignant vascular lesions. A recent study assessing the activation of the Akt/mTOR pathway in sarcomas was largely independent of activation of epidermal growth factor receptor (EGFR) [23]. There was no correlation between the amount and intensity of staining among the epithelioid versus conventional histology and clinical outcome in our series.
Although our study is limited by small sample size, we did not demonstrate any correlation between EGFR and MET hyperploidy with clinical or morphological parameters. The results suggest that these two genes do not have a role in AS genesis and progression but do suggest involvement of chromosome 7. AS is a rare tumor with an aggressive and rapidly lethal course. The lack of effective treatment warrants continued investigation in order to identify new therapeutic targets. Larger series exploring other molecular targets may lead to the discovery of new potential treatment strategies in AS.
References
- Fletcher CDM, Bridge JA, Hogendoorn, PCW, Mertens F (2013) World Health Organization, International Academy of Pathology. Pathology and genetics of tumours of soft tissue and bone. Lyon; Oxford: IARC Press; Oxford University Press distributor.
- McDonald DJ, Enneking WF, Sundaram M (2002) Metal-associated angiosarcoma of bone: report of two cases and review of the literature. ClinOrthopRelat Res : 206-214.
- Rubin BP, Fletcher CD, Inwards C, Montag AG, Peabody T, et al. (2006) Protocol for the examination of specimens from patients with soft tissue tumors of intermediate malignant potential, malignant soft tissue tumors, and benign/locally aggressive and malignant bone tumors. Arch Pathol Lab Med 130: 1616-1629.
- Miettinen M editor (2010) Modern Soft Tissue Pathology. (1stedn). New York, NY, USA: Cambridge University Press.
- Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG (2005) A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 11: 241-247.
- Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, et al. (2007) Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol 18: 2030-2036.
- Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, et al. (2010) Angiosarcoma: clinical and molecular insights. Ann Surg 251: 1098-1106.
- Blay JY (2011) Updating progress in sarcoma therapy with mTOR inhibitors. Ann Oncol 22: 280-287.
- Qureshi YA, Strauss DC, Thway K, Fisher C, Thomas JM (2010) Angiosarcoma developing in a non-functioning arteriovenous fistula post-renal transplant. J SurgOncol 101: 520-523.
- Webster P, Wujanto L, Fisher C, Walker M, Ramakrishnan R, et al. (2011) Malignancies confined to disused arteriovenous fistulae in renal transplant patients: an important differential diagnosis. Am J Nephrol 34: 42-48.
- Fata F, O'Reilly E, Ilson D, Pfister D, Leffel D, et al. (1999) Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 86: 2034-2037.
- Espat NJ, Lewis JJ, Woodruff JM, Antonescu C, Xia J, et al. (2000) Confirmed angiosarcoma: prognostic factors and outcome in 50 prospectively followed patients. Sarcoma 4: 173-177.
- Lahat G, Dhuka AR, Lahat S, Smith KD, Pollock RE, et al. (2009) Outcome of locally recurrent and metastatic angiosarcoma. Ann SurgOncol 16: 2502-2509.
- Penel N, Mallet Y, Robin YM, Fournier C, Grosjean J, et al. (2008) Prognostic factors for adult sarcomas of head and neck. Int J Oral MaxillofacSurg 37: 428-432.
- Nascimento AF, Raut CP, Fletcher CD (2008) Primary angiosarcoma of the breast: clinicopathologic analysis of 49 cases, suggesting that grade is not prognostic. Am J SurgPathol 32: 1896-1904.
- Deyrup AT, McKenney JK, Tighiouart M, Folpe AL, Weiss SW (2008) Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J SurgPathol 32: 72-77.
- Wong KF, So CC, Wong N, Siu LL, Kwong YL, et al. (2001) Sinonasalangiosarcoma with marrow involvement at presentation mimicking malignant lymphoma: cytogenetic analysis using multiple techniques. Cancer Genet Cytogenet 129: 64-68.
- Baumhoer D, Gunawan B, Becker H, Füzesi L (2005) Comparative genomic hybridization in four angiosarcomas of the female breast. GynecolOncol 97: 348-352.
- Fletcher CD (1996) Vascular tumors: an update with emphasis on the diagnosis of angiosarcoma and borderline vascular neoplasms. MonogrPathol 38: 181-206.
- Mentzel T, Schildhaus HU, Palmedo G, Büttner R, Kutzner H (2012) Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol 25: 75-85.
- Guo T, Zhang L, Chang NE, Singer S, Maki RG, et al. (2011) Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer 50:25-33.
- Manner J, Radlwimmer B, Hohenberger P, Mössinger K, Küffer S, et al. (2010) MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 176: 34-39.
- Dobashi Y, Suzuki S, Sato E, Hamada Y, Yanagawa T, et al. (2009) EGFR-dependent and independent activation of Akt/mTOR cascade in bone and soft tissue tumors. Mod Pathol 22: 1328-1340.
- Teng HW, Wang HW, Chen WM, Chao TC, Hsieh YY, et al. (2011) Prevalence and prognostic influence of genomic changes of EGFR pathway markers in synovial sarcoma. J SurgOncol 103: 773-781.
- Lee JC, Li CF, Fang FM, Wang JW, Jeng YM, et al. (2010) Prognostic implication of MET overexpression in myxofibrosarcomas: an integrative array comparative genomic hybridization, real-time quantitative PCR, immunoblotting, and immunohistochemical analysis. Mod Pathol 23: 1379-1392.
- Lahat G, Zhang P, Zhu QS, Torres K, Ghadimi M, et al. (2011) The expression of c-Met pathway components in unclassified pleomorphic sarcoma/malignant fibrous histiocytoma (UPS/MFH): a tissue microarray study. Histopathology 59:556-561.
- Antonescu CR, Yoshida A, Guo T, Chang NE, Zhang L, et al. (2009) KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res 69: 7175-7179.
- Bundscherer A, Vogt T, Köhl G, Landthaler M, Hafner C (2010) Antiproliferative effects of rapamycin and celecoxib in angiosarcoma cell lines. Anticancer Res 30: 4017-4023.
- Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, et al. (1997) Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 276: 1848-1850.
- Dickerson EB, Thomas R, Fosmire SP, Lamerato-Kozicki AR, Bianco SR, et al. (2005) Mutations of phosphatase and tensin homolog deleted from chromosome 10 in canine hemangiosarcoma. Vet Pathol 42: 618-632.
- Kuwai T, Nakamura T, Sasaki T, Kim SJ, Fan D, et al. (2008) Phosphorylated epidermal growth factor receptor on tumor-associated endothelial cells is a primary target for therapy with tyrosine kinase inhibitors. Neoplasia 10:489-500.
- Kiesel H, Müller AM, Schmitt-Graeff A, Veelken H (2009) Dramatic and durable efficacy of imatinib in an advanced angiosarcoma without detectable KIT and PDGFRA mutations. Cancer BiolTher 8: 319-321.
- Miettinen M, Sarlomo-Rikala M, Lasota J (2000) KIT expression in angiosarcomas and fetal endothelial cells: lack of mutations of exon 11 and exon 17 of C-kit. Mod Pathol 13: 536-541.
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, et al. (2007) FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128: 309-323.
- Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, et al. (2006) Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 10: 159-170.
- Perry B, Banyard J, McLaughlin ER, Watnick R, Sohn A, et al. (2007) AKT1 overexpression in endothelial cells leads to the development of cutaneous vascular malformations in vivo. Arch Dermatol 143: 504-506.
- Meert AP, Martin B, Delmotte P, Berghmans T, Lafitte JJ, et al. (2002) The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J 20: 975-981.
- Kopp R, Ruge M, Rothbauer E, Cramer C, Kraemling HJ, et al. (2002) Impact of epidermal growth factor (EGF) radioreceptor analysis on long-term survival of gastric cancer patients. Anticancer Res 22: 1161-1167.
- Cohen EE, Haraf DJ, Kunnavakkam R, Stenson KM, Blair EA, et al. (2010) Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer. J ClinOncol 28: 3336-3343.
- Kim YT, Park SW, Kim JW (2002) Correlation between expression of EGFR and the prognosis of patients with cervical carcinoma. GynecolOncol 87: 84-89.
- Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5: 341-354.
- Abraham JA, Hornicek FJ, Kaufman AM, Harmon DC, Springfield DS, et al. (2007) Treatment and outcome of 82 patients with angiosarcoma. Ann SurgOncol 14: 1953-1967.
- Pawlik TM, Paulino AF, McGinn CJ, Baker LH, Cohen DS, et al. (2003) Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer 98: 1716-1726.
- Komdeur R, Hoekstra HJ, Molenaar WM, Van Den Berg E, Zwart N, et al. (2003) Clinicopathologic assessment of postradiation sarcomas: KIT as a potential treatment target. Clin Cancer Res 9: 2926-2932.
- Ionescu DN, Sasatomi E, Cieply K, Nola M, Dacic S (2005) Protein expression and gene amplification of epidermal growth factor receptor in thymomas. Cancer 103: 630-636.
- Lopez-Gines C, Gil-Benso R, Ferrer-Luna R, Benito R, Serna E, et al. (2010) New pattern of EGFR amplification in glioblastoma and the relationship of gene copy number with gene expression profile. Mod Pathol 23: 856-865.
- Martín-Ezquerra G, Salgado R, Toll A, Gilaberte M, Baró T, et al. (2010) Multiple genetic copy number alterations in oral squamous cell carcinoma: study of MYC, TP53, CCDN1, EGFR and ERBB2 status in primary and metastatic tumours. Br J Dermatol 163: 1028-1035.
- Abraham RT (2002) Identification of TOR signaling complexes: more TORC for the cell growth engine. Cell 111: 9-12.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15781
- [From(publication date):
June-2014 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11092
- PDF downloads : 4689