Impact of DAA Therapy in HCV on Thrombocytopenia and Fibrosis: A Prospective Study in Tertiary Care Centre
Received: 31-Dec-2021 / Manuscript No. JIDT-22-50972 / Editor assigned: 03-Jan-2022 / PreQC No. JIDT-22-50972 (PQ) / Reviewed: 14-Jan-2022 / QC No. JIDT -22-50972 / Revised: 14-Jan-2022 / Manuscript No. JIDT-22-50972 (R) / Accepted Date: 17-Jan-2022 / Published Date: 24-Jan-2022 DOI: 10.4172/2332-0877.1000485
Abstract
Aim: This study evaluated the impact of Direct Acting Antivirals (DAA) on Thrombocytopenia and Fibrosis.
Background: Majority of transmission of Hepatitis C viral infections occur through transfusion of blood and blood products. Successful treatment of HCV infection with Pegylated IFN free DAA therapy is not having any impact on platelet improvement and fibrosis.
Primary objective: To assess DAA treatment response in progression of fibrosis in HCV patients and respective Genotypes 1,2,3,4,5 and 6.
Secondary objective: To assess treatment response of DAA through Sustainable Virological Response (SVR) in HCV patients.
Methods: A Prospective longitudinal study was conducted in Institute of hepato-biliary sciences, Madras Medical College, Chennai. This study included patients with Chronic Liver Disease (CLD) due to chronic HCV infection and treated successfully with DAA. Total of 302 patients were treated during this 2016-2018. Of these 135 patients diagnosed with cirrhosis were excluded from the study. A total of 167 patients with F1 to F3 (fibrosis) were included in the study. All the patients on DAA were followed up for a period of 2 years whether they achieved SVR or not. Patients were monitored with complete blood count, liver function tests, USG Abdomen and liver stiffness.
Results: 463 (57.3%) patients were male and 344 (42.6%) were female. Average age of male was 42.41 (± 16.69) and female was 44.22 (± 16.01). 261 (32.3%) patients had history of surgery and 232 (28.7%) patients had a history of blood transfusion. 111(13.75%) patients had hemodialysis for chronic kidney disease. 111 (13.75%) patients had comorbidity of Diabetic Mellitus and 112 (13.8%) had systemic hypertension. Among 807 serological positive patients 302 patients were enrolled for the treatment. Among them 161 patients with genotype 1, 5 patients with genotype 2, 117 patients with genotype 3, 18 patients with genotype 4, 1 patient with genotype 6 and all of them were followed up to End of the Treatment (ETR) and SVR. Genotype 1, 2, 4 and 6 achieved 100% SVR for DAA. Genotype 3 achieved only 97.95% of SVR for DAA Sofosbuvir (NS5B Polymerase inhibitor) and Daclatasvir (NS5A polymerase inhibitor).
Conclusion: Among the patients treated with DAA, SVR was not associated with considerable regression of fibrosis, also no significant improvement in platelet count in F1-F3 fibrosis even though patients achieved SVR. Genotype 3 patients with compensated cirrhosis did not achieve SVR with DAA therapy.
Keywords: Direct acting antivirals; Sustainable virological response; Hepatitis C virus; Thrombocytopenia
Abbreviations
DAA: Direct Acting Antivirals; SVR: Sustainable Virological Response; ETR: End of the Treatment; CLD: Chronic Liver Disease; HVPG: Hepatic Venous Pressure Gradient; ALT: Serum Alanine Amino Transferase; ASt: Aspartate Amino Transferase; CHC: Chronic Hepatitis C; HCV: Hepatitis C Virus; RBV: Ribavirin; GT: Genotype; HCC: Hepato Cellular Carcinoma; USG: Ultrasonogram
Introduction
There is an estimated 71 million people with chronic Hepatitis C Virus (HCV) infection worldwide, representing ~1% of the population [1]. HCV is a small enveloped RNA virus belonging to the family Flaviviridae and genus hepacivirus [2]. HCV genomic RNA is a single- stranded with positive polarity, which is packaged by core protein and enveloped by a lipid bilayer containing two viral glycoproteins (E1 and E2) to form the virion [3]. DAAs have changed HCV treatment paradigms [4]. Several studies have shown that successful treatment of HCV infection reduces the risk to develop HCC; still there is approximately 1% risk remains even after the achievement of SVR [5]. Current findings argue strongly in favour of early treatment before the development of cirrhosis and in implementing or continuing HCC surveillance among patients with cirrhosis even after achieving SVR [6]. Platelets contain proteins required for hemostasis, as well as many growth factors required for organ development, tissue regeneration and repair. Thrombocytopenia, which is frequently observed in 64%- 84% of patients with Chronic Liver Disease (CLD) and cirrhosis, can manifest from decreased thrombopoietin production and accelerated platelet destruction caused by hypersplenism [7]. There is experimental evidence of platelets improving liver fibrosis and accelerating liver regeneration [8]. Thrombocytopenia significantly correlates with increased Hepatic Venous Pressure Gradient (HVPG) [9]. HCV genotype 1 is the most prevalent worldwide, comprising 83.4 million cases (46.2% of all HCV cases), approximately one-third of which are in East Asia. Genotype 3 is the next most prevalent genotype globally (54.3 million, 30.1%); genotypes 2, 4, and 6 are responsible for a total of 22.8% of all cases; genotype 5 comprises the remaining <1% [10].
Patients with HCV infection are commonly asymptomatic and incidentally diagnosed while undergoing blood investigations for some other procedure. Symptomatic patients presented with nausea, abdominal pain, flu like symptom and mild transaminitis. In most individuals, HCV RNA is usually detectable within two weeks and anti HCV antibodies within 3 to 12 weeks of exposure of HCV [11]. Serum Alanine Amino Transferase (ALT) levels usually rise within 8–10 weeks, with a peak ALT of 10–20 times the upper limit of normal. Serum HCV RNA levels may fluctuate widely during the acute phase and even become negative transiently, only to reappear again. This finding is only seen in the acute phase and may be a clinical clue to the diagnosis of acute HCV infection. Spontaneous resolution occurs in 15–25% of subjects and may be up to 45% in persons who present with jaundice, children, and young women. Higher rates of spontaneous clearance were also observed in persons with certain polymorphisms (the rs12979860-C, rs8099917-T and the ss469415590 TT) near to the IL-28B gene (interferon lambda) [12]. HLA class II alleles may play a role in spontaneous clearance. Less genetic diversity of the viral E1 and E2 envelope genes were observed in subjects with spontaneous recovery compared to those who progressed to chronic infection [13]. The previous therapeutic history of HCV by the Interferon-based regimens, and later with addition of ribavirin (RBV), was the standard of care for many years for patients with Chronic Hepatitis C treatment (CHC). However, treatment end results diverse between genotypes, with particularly poor cure rates of 40% being reported in GT1 and GT4 cases [14]. Many Directly Acting Antivirals (DAAs) have been approved for use as part of CHCV combination therapies since 2011, and patient outcomes have significantly improved [15].
Materials and Methods
A Prospective longitudinal study was conducted in Institute of hepato-biliary sciences, Madras Medical College, Chennai. This study included patients with Chronic Liver Disease (CLD) due to chronic HCV infection and treated successfully with DAA. Total of 302 patients were treated during this 2016-2018. Of these 135 patients diagnosed with cirrhosis were excluded from the study. A total of 167 patients with F1 to F3 (fibrosis) were included in the study. All the patients on DAA were followed up for a period of 2 years whether they achieved SVR or not. Patients were monitored with complete blood count, liver function tests, USG Abdomen and liver stiffness.
Study design
In this prospective longitudinal study, HCV patients who were treated with DAA for a period between January 2016 and December 2018 at Institute of hepato-biliary sciences, Madras Medical College, Chennai, Tamil Nadu, India were included.
Treatment duration was determined by the cirrhosis and non- cirrhosis condition of the patients. No patients received ribavirin along with DAA. All patients who were treated with DAA were followed up for a period of 36 months. The study ended on the 31st December 2018.
Study population
Study population included 807 patients attended above outpatient clinic with chronic HCV infection. Sample size was determined using online tool https://openepi.com›Sample Size. Patients who had Hbn, HSV, CMV, Wilsons Disease and HIV Co-infections were excluded from this study. Patients with fibrosis stages (F1-F3) were taken in to the study.
Data collection
All patients attended the outpatient clinic at Institute of Hepato- Biliary Sciences, Madras Medical College, Chennai were screened for anti-HCV, HBsAg through ELISA method. Among 807 patients who tested anti-HCV positive, 302 patients started on DAA were followed up for a period of 2 years whether they achieved SVR or not. Patients were monitored with complete blood count, liver function tests, USG Abdomen and liver stiffness. Informed consent was got from the patients before starting the treatment. All aspects of the treatment and adverse events were explained to the patients.
Inclusion criteria
Patients with fibrosis (F1, F2 and F3) due to Chronic HCV Infection diagnosed by Fibroscan alone were included in the study.
Exclusion criteria
Patients with chronic Hbn and HCV co-infection, Chronic HCV and HIV co-infection, HSV, CMV, Non-cirrhotic HCV patients, compensated cirrhosis due to HCV, DCLD due to HCV and ALD were excluded from the study.
Sample collection and analysis
3 ml of blood sample was collected from the patients who attended outpatient clinic. Screening of anti HCV and HBsAg was performed through by ELISA Method (Transasia Bio Medicals Ltd test kit). Sensitivity and Specificity of HCV ELISA were 97.6% and 92.6%. Circulating blood cells, include red blood cells, white blood cells are counted and sized electronically by Sysmex XN 1000 fully automated 5 part hematology analyser and LFT was performed in Erba-EM360 fully automated analyser [16,17]. HCV RNA quantitative analysis is performed by RT PCR method by SRL Diagnostics, Chennai.
End points
The Primary efficacy end point was Sustainable Virological Response (SVR), defined as an undetectable HCV RNA viral Load 12 weeks after treatment completion [18]. Patients with detectable HCV RNA at the SVR time point were considered to have viral relapse. Secondary endpoints included efficacy of DAA in the improvement of the fibrosis status [19].
Data analysis
With 302 patients, the 95% CI for SVR was expected no more than 2.4% in both directions on the basis of a hypothesized 90% SVR. The primary analysis of efficacy was the proportion of overall patients who achieved SVR with a 2 sided 95% CI. All the data collected were entered in MS Excel Sheet. Analysis done by SPSS 20.0. Quantitative data expressed in mean and standard deviation. Qualitative data was analysed through univariate and multivariate logistic regression analysis with 95% CI and statistically significant was assessed by P<0.05. Independent variables were analysed through student t test.
Treatment
Study patients were treated with Sofosbuvir–NS5B polymerase inhibitor, Daclatasvir–NS5A polymerase inhibitor, Ledipasvir-NS5A polymerase inhibitor, Velpatasvir-NS5A polymerase inhibitor which was provided by the tertiary care centre [8,20,21].
Genotype 1 and 4 treated with Sofosbuvir 400 mg and Ledipasvir 90 mg. Genotype 2 and 3 with Sofosbuvir 400 mg and Daclatasvir 60 mg. Sofosbuvir 400 mg and Velpatasvir 100 mg as pangenotype antiviral treatment irrespective of genotype and monitoring the haematological,
biochemical parameters. The primary efficacy end point was SVR, defined as an undetectable HCV RNA viral load 12 weeks after treatment completion. Patients with detectable HCV RNA at the SVR time point were considered to have viral relapse. Secondary endpoints included efficacy by subgroups: Genotypes, fibrosis status and cirrhosis. Cirrhosis was determined by USG abdomen and Fibroscan.
Intervention and follow up
All the 302 Patients who were treated with Direct Acting Antivirals (DAA) for HCV followed up for 36 months.
Study oversight
This study was intramurally of Hepato- funded by Institute biliary sciences, Madras Medical College, Chennai; and conducted in compliance with the provisions and declarations of AASLD practise guidelines and local regulatory requirements.
Results
Among total 807 patients 463 (57.3%) patients were male and 344 (42.6%) were female. Average age of male was 42.41 (± 16.69) and female was 44.22 (± 16.01). 261 (32.3%) patients had history of surgery and 232 (28.7%) patients had a history of blood transfusion. 111 (13.75%) patients had hemodialysis for Chronic kidney disease. 111 (13.75%) patients had Diabetes Mellitus. 112 (13.8%) patients had systemic hypertension (Table 1). The percentage of fibrosis progression 26.1% is increased than fibrosis regression (13.9%), 44.7% patient remained in F4 fibrosis stage and 15.2% patients remained in F1 to F3 fibrosis (Table 2).
| Type | Male | Female | Total |
|---|---|---|---|
| 463(%) | 344 (%) | 807 | |
| Age | 42.41 SD (± 16.69) | 44.22 SD (± 16.01) | |
| Blood transfusion | 114 (24.6) | 121 (35.1) | 235 (29.1) |
| Surgery | 123 (26.5) | 147 (42.7) | 270 (33.4) |
| Haemodialysis | 64 (13.82) | 47 (13.66) | 111 (13.75) |
| SHT | 62 (13.3) | 50 (14.4) | 112 (13.8) |
| Diabetes Mellitus | 61 (13.1) | 50 (14.4) | 111 (13.75) |
Table 1: Patient’s past history of blood transfusion, surgery, haemodialysis, SHT and diabetic mellitus.
| Fibricon stages | No. of patients (%) |
|---|---|
| F1-F2 | 48 (39.66) |
| F1-F3 | 5 (4.13) |
| F1-F4 | 5 (4.13) |
| F3-F4 | 10 (8.2) |
| F2-F3 | 10 (8.2) |
| F2-F4 | 1 (0.82) |
| F4-F2 | 9 (7.4) |
| F4-F3 | 17 (14.04) |
| F2-F1 | 2 (1.52) |
| F3-F1 | 4 (3.3) |
| F4-F1 | 0 |
| F3-F2 | 10 (8.2) |
| Total | 121 |
Table 2: The percentage of fibrosis progression 79 (26.1) is increased than fibrosis regression 42 (13.9) 135 (44.7) patients remained in F4 fibrosis without any regression and 46 (15.2) remains stable in their respective stages (F1-F3) fibrosis.
Treatment results of DAA
Among 807 serological positive patients, 302 patients were enrolled for the treatment. Among them one hundred and sixty one (161) patients with genotype 1, five (5) patients with genotype 2, one hundred and seventeen (117) patients with genotype 3, eighteen patients with genotype 4, and 1 patient with genotype 6 [18]. All of them were followed up to End Treatment Response (ETR) and SVR. Genotype 1, 2, 4 and 6 achieved 100% SVR for DAA. Genotype 3 achieved only 97.95% of SVR for DAA Sofosbuvir (NS5B Polymerase inhibitor) and Daclatasvir (NS5A polymerase inhibitor) (Figure 1). The four patients of Genotype 3 with compensated cirrhosis treated with DAA for 24 weeks achieved ETR but didn’t achieve SVR 12. Out of 302 patients, 135 patients were excluded due to F4 fibrosis. Remaining 167 patients with fibrosis stages (F1-F3) were followed up to 3 years after SVR (Table 3).
| Type | Patients n/N | SVR (95% CI)% |
|---|---|---|
| Over all | 259/302 | 85.76 (83.0-88.7) |
| Completed Treatment | 259/263 | 98.47 (90.3-99.4) |
| Cirrhosis | 64/68 | 94.11 (93.5-97.8) |
| Non Cirrhosis | 195/195 | 100 (92.3-100.0) |
| Fibrosis Stage | ||
| 0 | 36/36 | 100 (92.5-100.0) |
| 1 | 69/69 | 100 (91.5-100.0) |
| 2 | 43/43 | 100 (95.5-100.0) |
| 3 | 47/48 | 97.91 (86.2-97.1) |
| 4 | 64/67 | 95.52 (86.5-96.8) |
| Treatment Duration | ||
| 12 weeks | 195/195 | 100 (92.5-100.0) |
| 24 weeks | 64/68 | 94.11 (92.6-97.8) |
| Genotypes | ||
| 1 | 143/143 | 100 (97.8-100.0) |
| 2 | 05-May | 100 (98.6-100.0) |
| 3 | 94/98 | 95.9 (86.4-98.5) |
| 4 | 16/16 | 100(97.3-100.0) |
| 6 | 01-Jan | 100 (92.5-100.0) |
Table 3: Only four patients were treated for 24 weeks. All these four patients weren’t achieve SVR 12 belonged to genotype 3 (one patient in F3 and 3 patients in F4).
There was no change in the level of haemoglobin before and after the treatment with DAA in both male (p value 0.152) and female (p value 0.491) patients. P-values of both before and after the treatment were calculated by paired t test. The level of ASt and ALT was decreased significantly after the treatment with DAA for both male (p value 0.017) and female (p value 0.048). The level of platelet was decreased and not improved even after the treatment with DAA for both male (p value 0.024) and female (p value 0.01658) (Table 4).
| Treatment | Stats | HB | Bilirubin Total | Ast | Alt | Plt | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P value | Before | After | P Value | Before | After | P Value | Before | After | P Value | Before | After | P Value | ||
| Male | Average | 11.03 | 9.8 | 0.152 | 1.14 | 0.49 | 0.025 | 58.4 | 28 | 0.017 | 61.6 | 28 | 0.023 | 2.04 | 1.7 | 0.025 |
| Std. dev | 1.97 | 1.89 | 2.3 | 0.62 | 89.5 | 17 | 101.88 | 22 | 1.12 | 0.8 | ||||||
| Female | Mean | 11.03 | 10.12 | 0.491 | 0.7 | 0.9 | 0.046 | 89.71 | 28 | 0.048 | 83.8 | 28 | 0.025 | 1.8 | 1.72 | 0.017 |
| Std. dev | 1.69 | 1.47 | 1.5 | 1.8 | 169.5 | 16 | 159.42 | 21 | 0.9 | 1 | ||||||
Table 4: Variables observed before and after the treatment-variables Hb, bilirubin total, AST, ALT and PLT.
Liver stiffness
Liver stiffness measurement was performed using Fibroscan, (Echosens, Paris France) with patients in supine position and maximal abduction of right arm and transducer probe placed in the right intercostal space in the midaxillaryline. Transducer probe produces low frequency (50 Hz) elastic shear waves which traverses the liver tissue. Concordance between the liver stiffness (kPa) and fibrosis stage according to METAVIR Score was recorded and compared with the standard values of HCV [22]. There was no fibrosis regression observed among the patients undergone DAA therapy (Table 3). SWE reference kPa values for fibroscan for HCV are given as F0-F1 6.3-7.6, F2-7.7- 10.0, F3-10.1-15.6 and F4->15.6.25. Cirrhosis patients has less SVR percentage (94.11%) when compared to the non-cirrhotic patients who undergone the treatment. Assessment of cirrhosis is determined by the LFT, USG Abdomen and Fibroscan. 99.37% of the patients with F1-F3 achieved SVR.
Genotype
Antiviral therapy and treatment duration 12/24 weeks was mentioned in each patient according to the viral genotype and subtype and severity of liver disease. Among all the genotypes, Genotype 3 had less achievement in SVR. HCV RNA quantification was assessed by real time PCR, with a limit of detection of 15 IU/mL.
Patients were followed up monthly with clinical and laboratory evaluation during antiviral treatment. Virological response was assessed by the quantification of HCV RNA. Virological failures and early discontinuations of therapy due to adverse events were also registered.
Discussion
Direct acting antivirals have become widely used for patients with Chronic Hepatitis C virus infection [23]. Consistent with other studies, the majority of patients successfully achieved viral clearance by post-treatment week 12 and 24 [24]. Hemoglobin, platelet count, liver function test, USG abdomen and fibroscan were taken before the treatment and same were compared at the end of the treatment, SVR 12 and during follow up. When compared to base line values, patients had no significant improvement in platelet, fibrosis after the completion of treatment and follow up. Meanwhile there was an improvement in alanine transmainase and aspartate transaminase enzymes (Table 4). One patient from F3 Fibrosis and three patients in F4 fibrosis did not achieve SVR in genotype 3 were observed. This might be due to the NS5A gene mutation. We noted excellent virological response rates in patients infected with genotype 1 HCV regardless of choice of NS5A inhibitor.
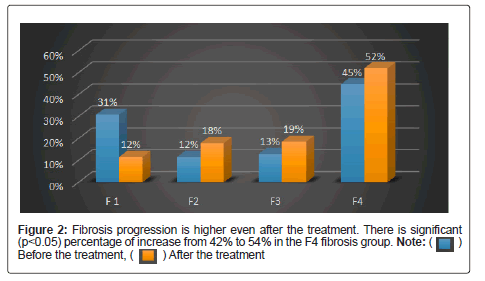
It is still controversial, whether and to what amount fibrosis and portal hypertension are reversible in patients with Hepatitis C Virus (HCV)-associated fibrosis and Sustained Virologic Response (SVR) after interferon-free antiviral therapy (Table 5) [25]. In the current study, we investigated dynamics of liver stiffness as surrogate marker of fibrosis in patients with chronic HCV infection. There is no significant regression of fibrosis after the treatment with DAA (p<0.05) (Table 6). This is confirmed by liver stiffness assessment with Fibroscan before and after treatment with DAA (Figure 2). 167 patients with F1 to F3 fibrosis were treated with DAA and undergone SVR, fibroscan, liver profiles, hematological investigations. There is no improvement in thrombocytopenia and fibrosis. As there is no regression in fibrosis even after attaining SVR these patients should be kept under surveillance for the development of cirrhosis and HCC (Table 7 and Figure 3).
| Fibroscan | Before the treatment N=302 (%) |
After the Treatment N=302 (%) |
P value |
|---|---|---|---|
| F1 | 93 (30.79) | 35 (11.58) | 0.3996 |
| F2 | 35 (11.59) | 54 (17.88) | 0.2565 |
| F3 | 39 (12.9) | 56 (18.54) | 0.28671 |
| F4 | 135 (44.7) | 157 (51.98) | 0.000596 |
| Fibroscan (Total) | 302 | 302 | 0.003 |
Table 5: Fibroscan stages of 302 patients during the follow up to SVR 12.
| Fibroscan | Before the treatment N=302 (%) |
After the Treatment N= 302 (%) |
P value |
|---|---|---|---|
| F 1 | 93 (30.79) | 35 (11.58) | 0.3996 |
| F2 | 35 (11.59) | 54 (17.88) | 0.2565 |
| F3 | 39 (12.9) | 56 (18.54) | 0.28671 |
| F4 | 135 (44.7) | 157 (51.98) | 0.000596 |
| Fibroscan (Total) | 302 | 302 | 0.003 |
Note: Progression of Fibrosis observed in 58 (48%) patients. Patients progressed from F1 stage to F2, F3 and F4.
Progression observed in 11 (9%) patients and regression in 2 (2%) patients. Patients progressed and regressed from Stage F2 to F3 and F4 and F2 to F1.
Regression observed in 14 (12%) patients and progression observed in 10 (8%) patients. Patients Regressed and Progressed from F3 to F1, F2 and F3 to F4.
Regression observed in 26 (21%) patients and Progression observed in f4 remains stable even after the treatment.
Progression rate (65%) is higher than the regression rate (35%)
Table 6: Correlation of fibrosis regression/progression before and after the treatment with DAA.
| Property | Before the treatment | After the treatment |
|---|---|---|
| Mean | 14.9509934 | 16.060596 |
| Variance | 76.5039027 | 50.4197378 |
| Observations | 302 | 302 |
| Pearson correlation | 0.68291646 | |
| Hypothesized mean difference | 0 | |
| df | 301 | |
| t Stat | -2.9720276 | |
| P(T<=t) one-tail | 0.00159856 | |
| t Critical one-tail | 1.64993169 | |
| P(T<=t) two-tail | 0.00319713 | |
| t Critical two-tail | 1.96787653 |
Table 7: The difference of the properties before and after the treatment.
Conclusion
Among the patients treated with DAA, SVR was not associated with a considerable reduction in the progression of fibrosis. There is no improvement in the platelet count even after the treatment with DAA. 35.1% of HCV male patients had a past history of blood transfusion and 45.7% of female patients had history of surgery. The introduction of DAA improved the effective treatment module among the HCV patients with or without cirrhosis. This study focused on the improvement of liver fibrosis before and after the treatment of DAA on HCV patients. Patients received the DAA treatment for 12 weeks and 24 weeks. 12 weeks prescribed for non-cirrhotic condition and 24 weeks for cirrhosis, decompensated cirrhosis and relapsed patients. The incidence of liver cancer would also decrease with result of repeal of chronic inflammation due to virus. Eventhough patients had achieved SVR 12 after DAA therapy as there is no significant regression (p<0.003) in fibrosis and thrombocytopenia (P<0.05). Hence all patients who achieved SVR12 need HCC surveillance with LFT, alpha fetoprotein and USG abdomen lifelong. There are other factors for liver disease such as non-alcoholic fatty liver disease, alcoholic liver disease, metabolic disorders which cannot be ruled out in a patient with HCV even after achieving SVR 12.
Acknowledgement
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published
Compliance with Ethical Guidelines
All procedures performed were in accordance with the ethical standards of the institutional Ethical Committee after approval from the Committee members. Informed consent was obtained from all individual participants included in the study.
References
- Marshall AD, Pawlotsky JM, Lazarus JV, Aghemo A, Dore GJ, et al. (2018) The removal of DAA restrictions in Europe – One step closer to eliminating HCV as a major public health threat. J Hepatol 69: 1188-1196.
[Crossref] [Google scholar] [PubMed]
- Chevaliez S, Pawlotsky JM, Tan SL (2006) HCV genome and life cycle. 1st ed. Norfolk, Horizon Bioscience, UK.
- Lavie M, Goffard A, Dubuisson J (2006) HCV glycoproteins: Assembly of a functional E1-E2 Heterodimer. 1st ed. Norfolk, Horizon Bioscience, UK.
- Torres HA, Shigle TL, Hammoudi N, Link JT, Samaniego F, et al. (2017) The oncologic burden of hepatitis C virus infection: A clinical perspective: Hepatitis C and cancer. CA Cancer J Clin 67: 411-431.
[Crossref] [Google scholar] [PubMed]
- Hedenstierna M, Nangarhari A, Weiland O, Aleman S (2016) Diabetes and cirrhosis are risk factors for hepatocellular carcinoma after successful treatment of chronic hepatitis C. Clin Infect Dis 63: 723-729.
[Crossref] [Google scholar] [PubMed]
- El-Serag HB, Kanwal F, Richardson P, Kramer J (2016) Risk of hepatocellular carcinoma after sustained virological response in veterans with hepatitis C virus infection. Hepatology 64: 130-137.
[Crossref] [Google scholar] [PubMed]
- Mitchell O, Feldman DM, Diakow M, Sigal SH (2016) The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med 8: 39-50.
[Crossref] [Google scholar] [PubMed]
- Kurokawa T, Ohkohchi N (2017) Platelets in liver disease, cancer and regeneration. World J Gastroenterol 23: 3228-3239.
[Crossref] [Google scholar] [PubMed]
- Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, et al. (2005) Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 353: 2254-2261.
[Crossref] [Google scholar] [PubMed]
- Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, et al. (2015) Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 61: 77-87.
[Crossref] [Google scholar] [PubMed]
- How long does it take to test positive?-Viral hepatitis and liver disease, U.S. Department of Veterans, 2021.
- Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, et al. (2009) Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461: 399-401.
[Crossref] [Google scholar] [PubMed]
- Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, et al. (2000) The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288: 339-344.
[Crossref] [Google scholar] [PubMed]
- Burstow NJ, Mohamed Z, Gomaa AI, Sonderup MW, Cook NA, et al. (2017) Hepatitis C treatment: where are we now? Int J Gen Med 10: 39-52.
[Crossref] [Google scholar] [PubMed]
- Seifert LL, Perumpail RB, Ahmed A (2015) Update on hepatitis C: Direct-acting antivirals. World J Hepatol 7: 2829-2833. [Crossref] [Google scholar] [PubMed]
- Tefferi A, Hanson CA, Inwards DJ (2005) How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc 80: 923-936.
[Crossref] [Google scholar] [PubMed]
- Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, et al. (2010) The LiMAx test: A new liver function test for predicting postoperative outcome in liver surgery. HPB 8: 450.
[Crossref] [Google scholar] [PubMed]
- Pockros PJ (2018) Management of patients who have achieved sustained virologic response for hepatitis C virus infection. Gastroenterol Hepatol (N Y) 14: 305-307.
- Jacobson IM, Lim JK, Fried MW (2017) American gastroenterological association institute clinical practice update—Expert review: Care of patients who have achieved a sustained virologic response after antiviral therapy for chronic hepatitis C infection. Gastroenterology 152: 1578-1587.
[Crossref] [Google scholar] [PubMed]
- Hézode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez-Torres M, et al. (2015) Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: A randomised study. Gut 64: 948-956.
[Crossref] [Google scholar] [PubMed]
- Link JO, Taylor JG, Xu L, Mitchell M, Guo H, et al. (2014) Discovery of ledipasvir (GS-5885): A potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J Med Chem 57: 2033-2046.
[Crossref] [Google scholar] [PubMed]
- de Lédinghen V, Vergniol J (2008) Transient elastography (FibroScan). Gastroenterol Clin Biol 32: 58-67.
[Crossref] [Google scholar] [PubMed]
- Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, et al. (2021) Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 65: 741-747.
[Crossref] [Google scholar] [PubMed]
- Foster GR, Irving WL, Cheung MCM, Walker AJ, Hudson BE, et al. (2021) Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 64: 1224-1231. [Crossref]
[Google scholar] [PubMed]
- Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, et al. (2016) Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat 23: 994-1002.
[Crossref] [Google scholar] [PubMed]
Citation: Premkumar K, Panneerselvam S, Pamarthi J (2022) Impact of DAA Therapy in HCV on Thrombocytopenia and Fibrosis-A prospective Study in Tertiary Care Centre. J Infect Dis Ther 10: 485. DOI: 10.4172/2332-0877.1000485
Copyright: © 2022 Premkumar K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4342
- [From(publication date): 0-2022 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 3630
- PDF downloads: 712