Research Article Open Access
Incidental Cholangiocarcinoma is Associated with Poor Outcome in Patients Transplanted for Hepatocellular Carcinoma
Rustam Khan1,2, Safia Awan2, Peng Chung Cheow1, Chee Kiat Tan1 and Pik Eu Chang1*
1Department of Gastroenterology and Hepatology, Singapore General Hospital, Outram Road, 169608, Singapore
2Aga Khan University Hospital, Stadium Road, Karachi 74800, Pakistan
- *Corresponding Author:
- Dr. Jason Chang Pik Eu
Department of Gastroenterology and Hepatology
Singapore General Hospital
Outram Road, 169608, Singapore
Tel: (+65) 6321 4684
Fax: (+65) 6227 3623
E-mail: jason.chang@sgh.com.sg
Received date: July 15, 2015 Accepted date: August 12, 2015 Published date: August 18, 2015
Citation:Khan R, Awan S, Cheow PC, Tan CK, Chang PE (2015) Incidental Cholangiocarcinoma is Associated with Poor Outcome in Patients Transplanted for Hepatocellular Carcinoma. J Gastrointest Dig Sys 5:322. doi:10.4172/2161-069X.1000322
Copyright: © 2015 Khan R et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution; and reproduction in any medium; provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Hepatocellular carcinoma (HCC) is a common malignancy in Asia for which orthotopic liver transplantation (OLT) offers curative treatment in selected patients. Incidental HCC-cholangiocarcinoma (HCC-CLC) has been associated with poor OLT outcomes. Aims: To examine the prevalence of incidental HCC-CLC in liver explants from patients undergoing liver transplantation for HCC in our center and to examine its association with post-transplant disease recurrence and mortality. Methods: Medical records of all liver transplant patients in our center were reviewed. The presence of HCC-CLC in liver explants was reviewed by experienced pathologists. Factors associated with recurrence of HCC post OLT and post-transplant mortality were analyzed. Survival in patients with incidental HCC-CLC was compared against those without.
Abstract
Background: Hepatocellular carcinoma (HCC) is a common malignancy in Asia for which orthotopic liver transplantation (OLT) offers curative treatment in selected patients. Incidental HCC-cholangiocarcinoma (HCC-CLC) has been associated with poor OLT outcomes.
Aims: To examine the prevalence of incidental HCC-CLC in liver explants from patients undergoing liver transplantation for HCC in our center and to examine its association with post-transplant disease recurrence and mortality.
Methods: Medical records of all liver transplant patients in our center were reviewed. The presence of HCC-CLC in liver explants was reviewed by experienced pathologists. Factors associated with recurrence of HCC post OLT and post-transplant mortality were analyzed. Survival in patients with incidental HCC-CLC was compared against those without.
Results: A total of 54 transplants were performed during the study period, of which 24 were transplanted for HCC. Mean age was 57 ± 9 years and 87.5% were male. Incidental HCC-CLC was documented in the explants of 4 patients (16.7%). Of these, 2 developed HCC recurrence and 3 died within 24 months. Over a median follow-up of 36 months, 3(12.5%) developed HCC recurrence and 8(33%) died. Factors associated with HCC recurrence and death were number and size of HCC lesions, microvascular invasion and incidental HCC-CLC. Age and AFP level were associated with recurrence but not with mortality. Mean survival of patients with HCC-CLC was poorer compared to those without.
Conclusions: Incidental HCC-CLC is not uncommon in OLT for HCC and is associated with tumor recurrence and poor survival.
Keywords:
Predictors; Mortality; Recurrence; Intrahepatic; Incidental; Cholangiocarcinoma
Introduction
Hepatocellular carcinoma (HCC) is the third commonest cause of cancer-related death worldwide [1]. Orthotopic liver transplantation (OLT) can be regarded as the best treatment modality where both tumor as well as diseased liver are removed and replaced with a new, disease-free liver. In OLT for HCC, United Network for Organ Sharing (UNOS) system for allocating organs in the United States and many other liver transplant centers in the world select patients based on Milan criteria i.e. single HCC lesion ≤5 cm or 2-3 lesions ≤3 cm each with no vascular invasion or distant metastasis [2]. Another commonly adopted selection criteria is that proposed by the University of California San Francisco (UCSF) that is a slight expansion of Milan criteria to benefit more patients with HCC for OLT (single HCC lesion ≤6.5 cm and 2-3 lesions ≤4.5 cm each and cumulative diameter not more than 8 cm with no local or systemic spread) [3-5]. Proper selection of HCC patients with the Milan and UCSF criteria has improved the recurrence-free survival and survival rates at 5-years post liver transplant to approximately 83% and 75% respectively.
Outcome of OLT for HCC depends on recurrence of HCC and site of recurrence [4,5]. The reported rate of recurrence of HCC following OLT varies between 10-20% despite strict adherence to the proposed criteria [6,7]. Recognized factors associated with recurrence of HCC in transplanted livers include tumor size beyond Milan and UCSF criteria, vascular invasion on explanted liver, poor differentiation of HCC on histopathology, previous hepatectomy and pretransplant alpha-fetoprotein (AFP) level ≥1000 ng/ml [8-15]. Post-transplant recurrence of HCC is associated with reduced survival [8,9].
Several recent studies have observed that unrecognized mixed HCC-cholangiocarcinoma are associated with poor post-transplant outcomes with increased recurrence rates and reduced survival [16-22]. The aim of our study was to examine the prevalence of incidental HCC-cholangiocarcinoma in liver explants from patients undergoing liver transplantation for HCC in our center and to examine its association with post-transplant disease recurrence and mortality.
Methods
A retrospective review was performed from the medical records of all patients who underwent liver transplantation at the Singapore General Hospital (SGH) since the inception of the liver transplant program in February 2006 to June 2012. SGH is the largest tertiary hospital and one of two government-funded liver transplant centers in Singapore. Majority of OLT in SGH are deceased donor liver transplantations (DDLT). Our program adopts the UCSF selection criteria to enroll HCC patients for OLT (single tumor <6.5 cm, maximum of 3 total tumors with none >4.5 cm, and a cumulative tumor size <8 cm). Patients who did not fulfill criteria for liver transplant were excluded from the study.
The socio-demographic data of patients transplanted for HCC were evaluated (age, race, gender, etiology of liver disease and AFP level). Pre-transplant imaging that included ultrasound, quadriphasic CT scan and contrast enhanced dynamic MRI scan were reviewed for number of lesions, size of individual and largest lesion, cumulative size of the lesions, vascular invasion, portal vein thrombosis, tumor thrombosis and evidence of distant metastasis. UCSF criteria was used to enroll the patients for transplant. These features were compared with operative findings, gross and histo-pathological evaluation of the explanted liver. Presence of mixed HCC-cholangiocarcinoma in the explanted livers was evaluated by experienced pathologists. Treatment of HCC prior to transplant with liver resection and loco-regional therapeutic procedures such as percutaneous ethanol injection (PEI), trans-arterial chemoembolization (TACE), trans-arterial embolization (TAE), radiofrequency ablation (RFA) were documented.
After OLT, patients were prospectively monitored for recurrence of disease. Post-transplant HCC surveillance was performed via 3-monthly AFP and CT scan or ultrasound abdomen. In cases of HCC recurrence, the site of recurrence and time interval of recurrence after OLT were recorded. Type of immunosuppressant medications used and in cases with underlying viral etiology, hepatitis B DNA and hepatitis C RNA quantitative levels were documented before and after transplantation. In patients who died, the cause of death and time interval from OLT was recorded.
IBM SPSS Statistics (version 19) was used for data analyses. Descriptive analyses of demographic variables were performed. Continuous variables were expressed as mean and standard deviations (SD) and categorical variables as proportions. Continuous variables were compared using Student t test and categorical variables were compared by chi-square or Fisher exact test and 95% confidence intervals (CI) was calculated for each association. All p-values were two sided and considered as statistically significant if p <0.05. Survival comparisons were performed using Kaplan Meier analysis and compared using the log-rank association.
Results
A total of 54 patients underwent liver transplantation at our center during the period of study. Of these, 24 (44.4%) were performed for HCC as the primary indication. In patients who underwent OLT for HCC, 19 (79.2%) had DDLT and 5 (20.8%) had living donor liver transplantation (LDLT). Mean age of patients was 57 ± 9 years and 87.5% were male. Underlying liver diseases leading to HCC were hepatitis B cirrhosis in 16 (66.7%), hepatitis C cirrhosis in 4 (16.6%) and cryptogenic cirrhosis in 4 (16.7%). None of the patients had underlying primary sclerosing cholangitis. Mean MELD score was a HCC-assigned score of 15 as the indication of transplantation was HCC rather than severity of disease in majority of these cases (Table 1).
| Demographics | N=24 |
| Age in years (mean ± SD) | 57 ± 9 |
| Male gender | 21 (87.5%) |
| Etiology of liver disease | |
| Hepatitis B | 16 (66.7%) |
| Hepatitis C | 4 (16.7%) |
| Cryptogenic Cirrhosis | 4 (16.7%) |
| Pre-transplant imaging features | |
| Number of lesions | 2.8 ± 2.8 |
| Size of largest lesion (cm) | 3.4 ± 3.0 |
| Cumulative size of lesions (cm) | 3.6 ± 3.1 |
| Portal vein thrombosis | 2 (8.3%) |
| Pre-transplant loco-regional therapies | |
| TACE | 20 (83%) |
| RFA | 13 (54%) |
| Resection | 6 (25%) |
| Type of liver transplant | |
| Deceased donor | 18 (75%) |
| Living donor | 6 (25%) |
| Features on explanted liver | |
| Microvascular invasion | 3 (12.5%) |
| Associated cholangiocarcinoma | 4 (16.7%) |
| Tumor thrombosis | 1 (4.2%) |
| Outcome | |
| Recurrence of HCC | 3 (12.5%) |
| Recurrence within 6 months | 1 (4.2%) |
| Recurrence within 18 to 24 months | 2 (8.4%) |
| Number of deaths | 8 (33%) |
| Number of deaths with HCC recurrence | 2 (8.3%) |
Table 1: Characteristics of patients transplanted for hepatocellular carcinoma. Data presented as mean ± SD or n(%). HCC: Hepatocellular Carcinoma, UCSF: University of California San Francisco, TACE: Trans Arterial Chemoembolization, RFA: Radiofrequency Ablation.
Incidental HCC-cholangiocarcinoma (HCC-CLC) was detected in 4 (16.7%) of the explanted livers on histological examination. All were males, with a mean age of 55 ± 7 years. There were no significant differences in the age, gender, etiology of liver disease, AFP level, number of lesions, size of HCC, presence of portal vein thrombosis, microvascular invasion or pre-transplant HCC treatment between patients with and without incidental HCC-CLC. There were no distinctive features on the pre-transplant imaging features that could have distinguished HCC-CLC from routine diagnosis of HCC. Two patients with HCC-CLC had recurrence of HCC and three died at 1, 12 and 24 months after OLT (Table 2).
| Sex | Age | Etiology | Type of OLT | No. of lesions | Size of biggest lesion (cm) | Micro-vascular invasion | AFP (IU/ml) | Recurrence | Death | Cause of death |
| Male | 47 | HCV+HCC | DDLT | 5 | 5.5 | Yes | 1838 | Yes | Yes | HCC recurrence |
| Male | 64 | HBV+HCC | LDLT | 7 | 5.5 | No | 44.7 | Yes | Yes | HCC recurrence |
| Male | 52 | HCV+HCC | DDLT | 2 | 1.1 | No | 2.6 | No | Yes | Hepatic artery thrombosis |
| Male | 57 | HBV+HCC | DDLT | 2 | 1.5 | No | 9.3 | No | No | - |
Table 2: Clinical characteristics of patients with incidental HCC-CLC. HCV: Chronic Hepatitis C Virus infection,HBV: Chronic Hepatitis B Virus Infection.
Of the 24 patients who underwent OLT for HCC, 3 (12.5%) patients had recurrence of HCC over a median follow-up period of 36 months. The first had early recurrence at 6 months post-OLT. This patient had multiple lesions (beyond UCSF criteria) and microvascular invasion of tumor into portal vein in the explanted liver which were not apparent on pre-transplant imaging. The patient was treated with loco-regional ablative therapy but developed multiple recurrences and eventually died 4.4 years after OLT. In the second case, recurrence occurred at 18 months after OLT and the patient died within 2 years with lung metastasis. Histological review of the explanted liver revealed multiple liver nodules, evidence of micro-vascular invasion of HCC and an associated 3.3 cm HCC-CLC nodule. The third patient had recurrence of HCC at 2 years. This patient had several liver nodules that were interpreted as indeterminate at pre-transplant imaging. However, on histological examination of the explanted liver, these nodules were confirmed to be HCC. There were a total of five HCC lesions in the explanted liver, which was beyond Milan and UCSF criteria. In this case there was no evidence of microvascular invasion of the HCC but a 2 cm nodule of HCC-CLC was detected. This patient had recurrence of HCC in regional lymph nodes at the porta hepatis and peritoneal cavity and died due to complications of HCC. On univariate analysis, factors associated with HCC recurrence included presence of microvascular invasion on liver explant (67% vs. 4.8%, p=0.03), incidental HCC-CLC (67% vs. 9.5%, p=0.06), age (45 ± 19 vs. 58 ± 6 years, p=0.02), number of HCC lesions on liver explant (5.7 ± 1.1 vs. 2.4 ± 2.7, p=0.06) and AFP level (846 ± 911 vs. 164 ± 376 IU/ml, p=0.02). Disease-free survival at one, three and five years was 96%, 87% and 87% respectively.
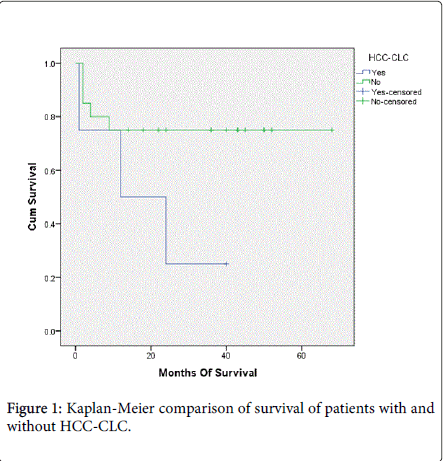
A total of 8 patients died over a median follow-up period of 36 months (range 1 to 68 months). Two (8.3%) patients died from HCC recurrence. The other six demised from sepsis with multi-organ dysfunction syndrome (MODS), primary non-function of liver graft, portal vein thrombosis and hepatic artery thrombosis (Table 3). On univariate analysis, factors associated with mortality in patients transplanted for HCC include >3 HCC lesions, largest HCC lesion >6.5 cm, LDLT, presence of microvascular invasion and incidental HCC-CLC in the liver explant (Tables 3 and 4). Pre-transplant AFP>1000 ug/L was not associated with mortality in our study. Multivariable analysis could not be meaningfully calculated due to small sample size. Overall median survival at one, three and five years was 79%, 71% and 71% respectively. None of the patients was subjected to re-transplantation and there was no living donor mortality. Patients with HCC-CLC demonstrated a trend for reduced survival compared to those without HCC-CLC (mean survival 19.2 Vs. 52.0 months, p=0.06 by log-rank comparison) (Figure 1).
| Patient number | Cause of death | Type of Transplantation | Interval between OLT and death |
| 1 | Primary non-function of graft | DDLT | Day 2 |
| 2 | Sepsis | LDLT | 38 months |
| 3 | Sepsis, portal vein thrombosis and MODS | LDLT | 21 months |
| 4 | HCC recurrence and MODS | DDLT | 36 months |
| 5 | Liver failure due to hepatic artery thrombosis | DDLT | Day 5 |
| 6 | Sepsis and MODS | LDLT | 9 months |
| 7 | HCC recurrence and MODS | LDLT | 32 months |
| 8 | Sepsis and MODS | DDLT | 38 months |
Table 3: Causes of death after liver transplantation for hepatocellular carcinoma. HCC: Hepatocellular Carcinoma, LDLT: Living Donor Liver Transplantation, DDLT: Deceased Donor Liver Transplantation, MODS: Multi-Organ Dysfunction Syndrome.
| Alive (n=16) | Died (n=8) | 95% CI | p | |
| >3 HCC lesions | 2 (12.5) | 3 (37.5%) | 1.29-174 | <0.001 |
| Largest tumor >6.5 cm | 3 (18.8%) | 5 (62.5%) | 0.92-53.23 | <0.001 |
| Fulfilled UCSF criteria | 15 (93.7%) | 5 (62.5%) | 0.04-3.79 | <0.001 |
| DDLT | 14 (87.5%) | 4 (50%) | 1.29-174 | <0.02 |
| AFP ≥1000 IU/ml | 1 (6.25%) | 2 (25%) | 0.38-17.45 | 0.72 |
| HCC-CLC | 2 (12.5%) | 2 (25%) | 0.009-1.32 | <0.001 |
| Microvascular invasion | 1 (6.25%) | 2 (25%) | 0.01-2.64 | <0.01 |
Table 4: Factors associated with mortality in patients transplanted for HCC.
Discussion
Orthotopic liver transplantation is a standard of care in properly selected patients with hepatocellular carcinoma. Milan and UCSF criteria are currently used to enlist the patients with HCC for OLT in majority of transplant centers [23]. Nevertheless, despite strict adherence to these criteria, recurrence of HCC is still reported in 10 to 20% of the patients after OLT [6,7]. These figures highlight that apart from size, number and metastasis of the HCC, other factors may also play role in the recurrence of HCC post-OLT.
In this review of liver transplantation for HCC from a single center, we observed a prevalence of incidental HCC-CLC of 16.7% in the liver explants. Despite being a low volume transplant center, we encountered a relatively high prevalence of HCC-CLC. Sapisochin et al. reported incidental cholangiocarcinoma in the explants of 10 of 302 patients (3.3%) who underwent liver transplant for HCC in a large North American center [18]. A small study from Saudi Arabia reported incidental cholangiocarcinoma in 2 out of 23 patients (8.7%) who had liver transplant for HCC [19]. In comparison, Vallin et al. [20] and Patkowski et al. [21] reported lower HCC-CLC prevalence rates of 1% and 0.8% respectively. However in these latter studies, the low prevalence rates were observed from a denominator of all liver transplants performed for both HCC and non-HCC indications.
Incidental HCC-CLC is associated with recurrence of HCC post OLT [16]. In our series, two out of three cases of HCC recurrence had incidental HCC-CLC in the explanted liver. In both cases, the recurrences were proven on biopsy to be HCC without histological evidence of associated cholangiocarcinoma. In addition, AFP was elevated in both cases of HCC recurrence. This interesting association has been reported in other studies demonstrating increased risk of HCC recurrence and more aggressive disease in patients with coexisting cholangiocarcinoma and HCC [17]. Our results are consistent with existing literature on the association of incidental HCC-CLC and increased risk of HCC recurrence. Sapisochin and colleagues have described a tumor recurrence rate of 57% in liver transplant patients with incidental HCC-CLC [18]. A French multicenter study [20] similarly reported a post-transplant recurrence rate of 50% amongst 10 cases of incidental HCC-CLC whereas the Polish study [21] reported recurrence rates of 73% in liver transplant patients with incidental cholangiocarcinoma. The reason for this observation remains elusive; further studies are required to determine if there is a direct effect of the presence of concurrent cholangiocarcinoma on the recurrence of HCC.
The results from our study also demonstrate a trend towards reduced survival after OLT in HCC patients with a concurrent focus of cholangiocarcinoma. Average survival of patients with HCC-CLC was only 19 months compared to 52 months in OLT patients without HCC-CLC. Although the log-rank comparison did not reach statistical significance due to the small number of subjects, the poorer prognosis observed in HCC-CLC patients is consistent with reports from other centers [18-21]. In fact, post-transplantation cholangiocarcinoma recurrence has been reported to have a dismal recurrence-free survival of 0% at 3 years [21].
Other factors associated with recurrence of HCC in this study on univariate analysis were size of largest lesion more than 6.5 cm, number of lesions more than three and micro vascular invasion on explanted liver. These findings were consistent with previously reported risk factors for recurrence of HCC after OLT [10-15]. In this series, our numbers were too low to calculate meaningful multivariate analysis 95% confidence interval. Many studies have also suggested that aggressiveness of HCC (as evident by poor differentiation of tumor on histopathology) are associated with increased recurrence. [10-15]. However, all 3 cases of recurrence in our study had well-differentiated HCC and this difference may be due to small number of HCC recurrence cases in this study. Contrary to reports that a pre-transplant alpha-fetoprotein (AFP) level of 1000 ng/ml or more is a predictor of recurrence, all of our cases of HCC recurrence had AFP level ≤1000 ng/ml [24,25]. In this series, all the recurrences of HCC occurred within two years of transplantation and none of the patients transplanted for HCC had recurrence beyond two years and similar observation was reported in other studies [10-15]. Therefore close surveillance for recurrence is recommended in the initial post transplantation period in order to initiate effective therapy earlier.
In our study, the median and disease-free survival at one and five years were 79%, 71%, and 96%, 88% respectively, which are similar to other reviews [10-15]. Pre-transplant evaluation of the HCC was by ultrasound, quadriphasic CT scan and thorax and dynamic contrast enhanced MRI scan. However despite advances in imaging techniques, there remains a discrepancy in pre-operative staging of tumor and actual number of tumor lesions in the explanted liver. In all the three patients with recurrence of HCC in this study, pre-operative imaging had underestimated the number, size and vascular invasion of the HCC. Disparity between pre-operative imaging and disease staging in the explanted liver has been reported in up to 20% of cases by Decaens et al. [5]; the authors suggest caution in expanding selection criteria based upon tumor size and number on pre-transplant imaging.
The immunosuppressant regime used in majority of our patients was a combination of prednisolone, tacrolimus and mycophenolate mofetyl (MMF). Sirolimus was used in only one patient because of significant renal insufficiency and low cell counts. Several studies in the literature has suggested that a mTOR inhibitor like sirolimus may have anti-neoplastic activity against HCC recurrence [26,27].
We acknowledge that the interpretation of the findings described in this retrospective single-center study is limited due to the small sample size and require further validation in a larger multi-center prospective study.
In conclusion, we have demonstrated that incidental HCC-CLC is not uncommon in OLT for HCC and is associated with tumor recurrence and poor survival. The role of pre-emptive treatment with combination of chemotherapy and radiotherapy in an attempt to reduce rate of HCC recurrence remains to be explored.
Compliance with Ethical Requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [5]. In this study, data was obtained from medical chart review and computer based recorded Information so patients’ consent was not required. No identifying information about patients is included in the article.
References
- Botha JF, Langnas AN (2006) Liver transplantation for hepatocellular carcinoma: an update. J Natl Compr Canc Netw 4: 762-767.
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, et al. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334: 693-699.
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, et al. (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33: 1394-1403.
- Leung JY, Zhu AX, Gordon FD, Pratt DS, Mithoefer A, et al. (2004) Liver transplantation outcomes for early-stage hepatocellular carcinoma: results of a multicenter study. Liver Transpl 11: 1343–1354.
- Decaens T, Roudot-Thoraval F, Hadni-Bresson S, Meyer C, Gugenheim J, et al. (2006) Impact of UCSF criteria according to pre- and post-OLT tumor features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl 12: 1761–1769.
- Zou WL, Zang YJ, Chen XG, Shen ZY (2008) Risk factors for fatal recurrence of hepatocellular carcinoma and their role in selecting candidates for liver transplantation. Hepatobiliary Pancreat Dis Int 7: 145-151.
- Merli M, Nicolini G, Gentili F, Novelli G, Iappelli M, et al. (2005) Predictive factors of outcome after liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Transplant Proc 37: 2535-2540.
- Escartin A, Sapisochin G, Bilbao I, Vilallonga R, Bueno J, et al. (2007) Recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc 39: 2308-2310.
- Lai Q1, Merli M, Ginanni Corradini S, Mennini G, Gentili F, et al. (2009) Predictive factors of recurrence of hepatocellular carcinoma after liver transplantation: a multivariate analysis. Transplant Proc 41: 1306-1309.
- Teixeira AC, Mente ED, Castro-e-Silva O, Sankarankutty AK, Cantão CA, et al. (2010) Treatment of hepatocellular carcinoma with liver transplantation: a single-center experience from Brazil. Transplant Proc 42: 502-504.
- Nakamura Y, Hama K, Iwamoto H, Yokoyama T, Kihara Y, et al. (2012) Long-term recurrence-free survival after liver transplantation from an ABO-incompatible living donor for treatment of hepatocellular carcinoma exceeding milano criteria in a patient with hepatitis B virus cirrhosis: a case report. Transplant Proc 44: 565-569.
- Choi HJ, Kim DG, Na GH, Hong TH, You YK (2012) Extended criteria for living donor liver transplantation in patients with advanced hepatocellular carcinoma. Transplant Proc 44: 399-402.
- Yu CY1, Ou HY, Huang TL, Chen TY, Tsang LL, et al. (2012) Hepatocellular carcinoma downstaging in liver transplantation. Transplant Proc 44: 412-414.
- Kim JM, Kwon CH, Joh JW, Choi MS, Lee JH, e al. (2012) Effectiveness of locoregional therapy before living donor liver transplantation in patients with hepatocellular carcinoma who meet the Milan criteria. Transplant Proc 44: 403-408.
- Lee S, Ahn C, Ha T, Moon D, Choi K, et al. (2010) Liver transplantation for hepatocellular carcinoma: Korean experience. J Hepatobiliary Pancreat Sci 17: 539-547.
- Zuo HQ, Yan LN, Zeng Y, Yang JY, Luo HZ, et al. (2007) Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary & pancreatic diseases international : HBPD INT 6: 161-165.
- Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, et al. (2006) Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today 36: 892-897.
- Sapisochin G, Fidelman N, Roberts JP, Yao FY (2011) Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl 17: 934-942.
- Allam N, Khalaf H, Fagih M, Al-Sebayel M (2008) Liver transplant for hepatocellular carcinoma: experience in a Saudi population. Exp Clin Transplant 6: 14-24.
- Vallin M, Sturm N, Lamblin G, Guillaud O, Hilleret MN, et al. (2013) Unrecognized intrahepatic cholangiocarcinoma: an analysis of 993 adult cirrhotic liver explants. Clin Transplant 27: 403-409.
- Patkowski W, Stankiewicz R, Grt M, Krasnodbski M, Kornasiewicz O, et al. (2014) Poor outcomes after liver transplantation in patients with incidental cholangiocarcinoma irrespective of tumor localization. Transplant Proc 46: 2774-2776.
- Ali JM, Bonomo L, Brais R, Griffiths WJ, Lomas DJ, et al. (2011) Outcomes and diagnostic challenges posed by incidental cholangiocarcinoma after liver transplantation. Transplantation 91: 1392-1397.
- Abdelfattah MR, Abaalkhail F, Al-Manea H2 (2015) Misdiagnosed or Incidentally Detected Hepatocellular Carcinoma in Explanted Livers: Lessons Learned. Ann Transplant 20: 366-372.
- Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR (2012) Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 35: 987-999.
- Maggs JR, Suddle AR, Aluvihare V, Heneghan MA (2012) Systematic review: the role of liver transplantation in the management of hepatocellular carcinoma. Aliment Pharmacol Ther 35: 1113-1134.
- Soll C, Clavien PA (2011) Inhibition of mammalian target of rapamycin: two goals with one shot? J Hepatol 54: 182-183.
- Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, et al. (2009) Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl 15: 1834-1842.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15004
- [From(publication date):
August-2015 - Aug 02, 2025] - Breakdown by view type
- HTML page views : 10420
- PDF downloads : 4584