Influence of Human, Wildlife and Livestock Husbandry on Epidemiology of Human African Trypanosomiasis at the Transboundary of Western Kenya and Southeast Uganda
Received: 03-May-2018 / Accepted Date: 26-May-2018 / Published Date: 31-May-2018 DOI: 10.4172/2332-0877.1000364
Abstract
Western Kenya and Southeast Uganda have reported different Human African Trypanosomiasis (HAT) incidences in the past more than 3 decades with the latter recording more cases. Here, we describe interactions between socio-economics, tsetse dynamics, livestock husbandry and environmental issues at the transboundary of Kenya and Uganda and how they determine Human African Trypanosomiasis (HAT). Comparative studies were carried out in two districts of each country namely Teso and Busia Districts, of Western Kenya and Tororo and Busia Districts, of Southeast Uganda. In addition, primary data was collected in the selected villages for the livestock numbers and human population. Also structured questionnaire was administered systematically to 384 randomly selected household heads or their representatives in each country. Human population density and types of crops grown, livestock numbers and livestock husbandry in the study villages influenced the occurrence of HAT. Prophylactic administration of tyrpanocides and topical application of insecticides on livestock reduced incidence of HAT. Wildlife abundance was high in villages reporting a history of HAT (69%) than HAT free villages (35.5%). The Glossina pallidipes trapped in the study areas sourced their blood meal exclusively from cattle. Therefore livestock keeping practices, wildlife and environmental factors should be incorporated into tsetse and trypanosomiasis control.
Keywords: HAT; Livestock; Socio-economic; Transboundary; Tsetse; Practices; Wildlife
Abbreviations
HAT: Human African Trypanosomiasis; SS: Sleeping Sickness; T.b.r: Trypanosoma Brucei Rhodesiense; Diseased Villages: Villages which have reported a case of HAT; Disease free village: No reported case of HAT; KARI-TRC: Kenya Agricultural Research Institute, Trypanosomiasis Research Centre; KETRI: Kenya Trypanosomiasis Research Institute; NaLIRI: National Livestock Resource Research Institute; WHO: World Health Organization; χ2: Chi-square; GPS: Global Positioning System; NGOs: Non-Governmental Organization; SPSS: Statistical Package for Social Science; ANOVA: analysis of variance
Introduction
Human African Trypanosomiasis (HAT) or sleeping sickness is an intractable Neglected Tropical Disease (NTD) transmitted by tsetse fly insect vectors in Sub-Saharan Africa. The disease occurs in acute and chronic forms caused by Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense extracellular protozoan hemoparasites, respectively, with the former being found in eastern and southern Africa while the latter is in western Africa and Uganda [1]. The geographic distribution of the tsetse fly is closely linked to land cover [2-4] and its optimal habitat is woodlands containing vegetation greater than 3 cm in diameter and between 1 to 4 meters in height [3]. Anthropogenic activities results in modified environmental conditions which may or may not alter habitats for the vectors at landscape levels and consequently govern the emergence and re-emergence of HAT [5].
Human African Trypanosomiasis (HAT) continue to differentially afflict a community in Uganda and not a comparably poor community, whose socio-cultural background and climatic conditions are similar but are separated by a national border in Kenya [4,6]. Historically, Trypanosoma brucei rhodesiense has been responsible for HAT at the transboundary of Kenya and Uganda and it is solely transmitted by the strictly hematophagus Glossina pallidipes or Glossina fuscipes fuscipes [4,7,8]. The two tsetse species densities at any particular period depend solely on the most recent control methods used and existing land cover systems. Without or limited tsetse control in place the tsetse population surges back to high numbers within a very short period. The vectors find their hosts through olfactory and visual cue and have been shown to have changed preferences of their sources of blood meal over time. Only a proportion of the flies actually carry the infectious T. brucei rhodeseinse and have in fact been established to possess a much higher (>20 times) propensity to host disease (nagana) causing parasites for livestock Trypanosoma vivax, Trypansoma congelenses in pasts studies [9-11]. Disparities in the political and socio-economic factors in the neighboring landscape in the last three decades may have influenced anthropogenic land use practices and modified the transmission of endemic infections [5]. Albeit, livestock husbandry at the transboundary is still facing similar threat of nagana and act as reservoirs of HAT diseasse. The drivers of trypanosomosis include agricultural encroachment, deforestation, drainage system management, human movement, domestic animals (livestock keeping, restocking and trading of livestock), application of insecticides topically on livestock and the environments, chemotherapeutic and chemoprophylactic interventions and wildlife presence. Thus the overall objectives of the study were to determine human, livestock, wildlife, socio-economic activities, and tsetse population interactions in the selected study villages and attempt to explain the disparities in Trypanosoma brucei rhodesiense prevalence at the trans-boundary of western Kenya and south east Uganda.
Materials and Methods
Study area
This study was carried out at the transboundary of Kenya and Uganda delineated by latitude 0° and 0°45’ North and longitude 33°54’ east and 34°25’ east in Kenya and latitude 0° and 0°45’ North and longitude 34° and 34°15' East in Uganda. The Kenyan side covered 1819 km2 with permanent water surface of 7.5% of the total landmass while the Ugandan side covered 3500 km2 with water surface of 13.3% of the total land mass. The study area comprises an equatorial rainforest with thick vegetation and a humid climate, providing a good habitat for breeding of tsetse.
The vegetation in Southeast Uganda districts consists of mixed indigenous trees, savanna grassland interspersed with trees, swamps and wetland vegetation. The vegetation cover was mainly savannah grassland. Glossina fuscipes fuscipes was the commonest tsetse species infesting the area along the vegetation fringing rivers and streams [8,12,13] in Uganda while in Western Kenya Glossina pallidipes predominated and the study area has a moist Combretum-savanna and grassland with large areas under traditionally managed pastures and bush [6,14].
The main occupation in both Kenya and Uganda was small-scale mixed farming with subsistence growing of food crops and livestock rearing including cattle, goats, pigs, sheep and poultry. The types of crops grown are maize, sorghum, sweet potatoes, finger millet, bananas, cassava, groundnuts, irrigated and rain fed rice.
Study design
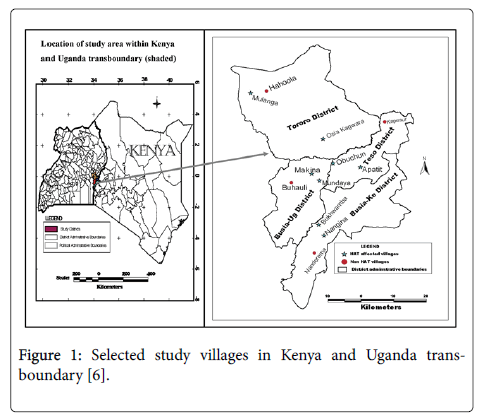
The study was a cross-sectional household survey conducted in subcounties of Busia and Tororo districts in Uganda and sublocations of Busia County (formerly Busia and Teso districts) in Kenya. Also secondary data on human population and HAT trends in the study selected districts in both countries were undertaken. The four districts were purposively selected on the basis of them being the foci for Trypanosoma brucei rhodesiense. Type of HAT in both countries and that they lie adjacent to each other at the international boundary of Kenya and Uganda. Six villages comprising of 2 villages in each of the four districts that had experienced HAT (affected villages) and the other one village in each district that had no reported HAT case (HAT free villages) from 1977 to 2008 (Figure 1) were purposively selected [6] in both countries. The study stations comprised centers of the villages which were a school, church or trading center representing the dispersal point from where systematic sampling was used to identify the households. The radius of the village was approximately 3 kilometres. In order to conduct a detailed analysis of risk factors at the household level, 8 villages that had reported high number of HAT cases ranging from 3 to above 100 HAT cases from 1977 to 2008 were selected and geo-referenced using Garmin 12 XL Global Positioning System (GPS) in the two countries. A similar procedure was undertaken for each of the four HAT free villages in each district in both countries.
Figure 1: Selected study villages in Kenya and Uganda transboundary [6].
Human African trypanosomiasis incidence from 1977 to 2008
Secondary data on HAT incidence in each district were obtained from the sleeping sickness referral hospitals in both Western Kenya and Southeast Uganda from 1978 to 2008 as described previously [6]. The hospital records of HAT affected villages in both Kenya and Uganda from 1977 to 2008 kept and stored by the Trypanosomiasis Research Centre (TRC) in Kikuyu, Kenya, and the National Livestock Health Research Institute (NALIRI) in Tororo, Uganda were used. All the HAT affected and HAT free villages (Disease free villages) were mapped and the points marked in locations within the study area. Details of the procedures followed are given in our earlier paper [6].
Population of tsetse hosts (Human, Livestock and wildlife), land cover patterns and HAT prevalence
Interviews were conducted randomly to selected household heads or their representatives. The questionnaire was first designed in English and then translated and pre-tested in local languages that was well-understood by the participants [6]. Thereafter, every third household until a total of 384 was sampled. The total number of cattle, goats, sheep, pigs, poultry (ducks, chicken and turkey), cats and dogs in all the households within the circumference of the selected villages were counted and recorded. The households also provided estimates on the numbers and types of wildlife presence. The wildlife of interest in the study were monkeys, baboons, foxes, mongoose, squirrels, rats hare, warthogs, antelopes, snakes, lizards, hippopotamus, and crocodiles. Other wildlife not included in the worksheet but was present in the village was also recorded. The enumerators translated animals sighted from the respondents’ local name into English. If the enumerator did not know the animal name and species in English they documented using the local name for translation later from knowledgeable members of the village. Secondary data for human population trends of 1979 to 2008 in each district in Western Kenya and Southeast Uganda were collected from statutory records [15-24].
Population and types of tsetse flies
To give an indication on the relative population and type of tsetse in the selected villages, 10 biconical [15] and Ngu traps (5 traps of each type) baited with acetone and phenol [25] were laid after every 200 meters in each of the 12 study villages for 24 hours. Each trap was geo-referenced using a Global Positioning System (GPS) for later incorporation in Geographical Information System (GIS). Tsetse flies from each trap were counted, species, apparent density (AD) of the flies calculated as the number flies trapped per day (ftd) and determination of trypanosomes infections through dissection of the salivary glands. Only fresh non-teneral tsetse flies were dissected to determine their trypanosome infection status. For blood meal assessment, the trapped flies were recorded hourly indicating the following characteristics: engorgement, species, and locality. Guts of non-teneral fed tsetse flies were pulled out of the midgut using clean forceps and the contents expressed on sodium azide treated Whatman No.1® filter paper, air-dried, temporarily placed in desiccators containing silica gel and later stored at 4°C within 24 h of collection until processed. The contents of the engorged gut were collected to establish the possible hosts and reported elsewhere [26].
Management and analysis of data
Both qualitative and quantitative data collection methods were used in this study. The data were entered into Excel 2003 and transferred to Statistical Package for Social Science (SPSS) version 14.0 for Windows for further statistical analyses. The characteristics of the households in the two countries were described using tables of sum, frequencies and means. Livestock numbers, human population and treatment practices, crops grown and tsetse dynamics were compared statistically. Comparisons of the two countries and correlation among the variables were analyzed using Pearson’s Chi-squared tests. The relationship between land use patterns in HAT affected and unaffected villages on the occurrence of diseases was investigated through Analysis of variance (ANOVA) tests. Estimation of relative HAT risk was made at 95% confidence level (CL) and set at a significance level of 5% to correlate the strength of HAT and the variable of interest in the two countries, HAT affected and unaffected villages [6]. The mean number of wildlife daily seen was calculated.
Results
Demographic characteristics
The respondents comprised 50.7% males as household heads and 49.3% females either as household heads or wives (25.4%; 23.9%). The other demographic factors of the study sample included household heads’ age, education, ethnicity, gender and occupation as indicated in our earlier paper [6].
Temporal and geographical distribution of HAT
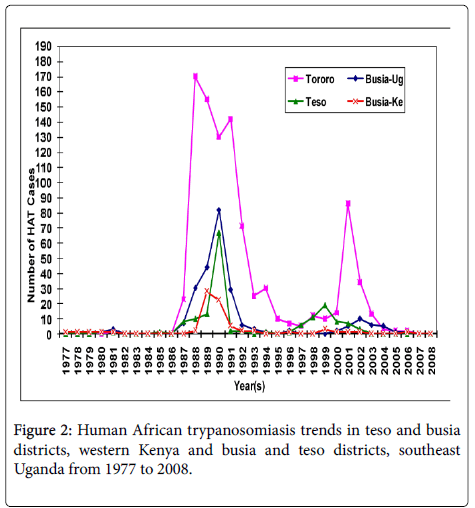
The villages that have experienced HAT in the two countries’ trans-boundary in the past over three decades differ greatly (Figure 2). In Southeast Uganda, the villages that have reported HAT were distributed evenly in Busia and Tororo Districts while in Kenya they were unevenly distributed mainly to the western parts of the districts bordering Uganda. The affected villages in Busia and Tororo Districts in Uganda have historically reported higher past HAT incidences compared to Teso and Busia Districts located in Western Kenya. In Teso and Busia Districts, Western Kenya there were 74 villages recorded in the HAT database, 70 were geo-referenced from Busia and Teso Districts. Four villages could not be located for geo-referencing either due to wrong entry of the village name or the patient gave a non-existent village name. In Busia and Tororo Districts, Southeast Uganda the villages that were affected in the study districts were 260 as recorded in the HAT database. Only 250 villages were geo-referenced. However, nine villages could not be traced in Busia District and one village in Butaleja District forming part of the greater Tororo District.
Teso and Busia Districts in Western Kenya and Busia and Tororo Districts in Southeast Uganda trans-boundary have experienced different annual HAT incidences over the past four decades (Figure 2). The occurrence of HAT over the past 30 years differed for each year with the highest recorded HAT cases from 1987 to 1992 for both countries.
From 1977 to 2008 confirmed HAT cases that were admitted to the referral hospitals were 74 and 161 cases for Busia and Teso Districts in Western Kenya, respectively. The Human African Trypanosomiasis cases in the four study districts significantly differed (χ2=7.518; df 3, P<0.01). From 1977 to 2008 trypanosomiasis in Western Kenya persisted at low levels recording a mean of 2.3 (Busia District) and 5.3 (Teso District) cases annually compared to Southeast Uganda with a mean of 8.2 and 32.6 cases from Busia and Tororo Districts respectively.
From 1977 to 1983 and 1987 to 2004, Tororo and Busia Districts in Southeast Uganda have admitted 1,184 cases for treatment. However from 1983 to 1986 the HAT incidences in Uganda were low due to missing data for the period, therefore it is an under-estimation of the true incidence. The greater Tororo District experienced higher prevalence’s of HAT of 944 cases (Tororo n=825 and Butaleja n=119) throughout the study period, followed by Busia District (239), Uganda. The epidemic waves or annual peaks occurred simultaneously in all the study districts in both countries although the peaks in Uganda were higher. Villages such as Apatit and Poyameri/Poyawo in Kenya and Uganda respectively recorded the highest HAT cases over the study period. In all the study districts in both Western Kenya and Southeast Uganda in the year 2008 to October 2009 only one HAT case was reported from Obekai village in Teso District, Kenya which was less than ten kilometers from the transboundary.
Demographic influence on HAT occurrence
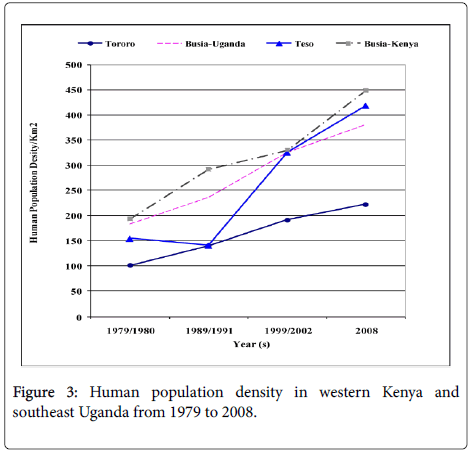
Human population census in both countries is carried at an interval of ten years and there was a registered increase from 1970s to 2008. In 1979 the Kenyan human population in Busia and Teso districts were 216,759 and 81,082 within an area of 1,124 and 559 kilometers squares, respectively. In Uganda in 1980 the human population were 126,184 in Busia and 281,043 in Tororo districts within an area of 692 kilomters square and 2808 kilometers square respectively. The human population of Busia and Teso district, Kenya in 1999 was 503,274 and 233,505 while the human population in Busia and Torroro districs, Uganda were 264,062 and 623,283 in the year 2002. When we compared the human population density, Busia district, currently in Busia County in Kenya had the highest population density with 447 people per kilometers square. The Ugandan side of the border experienced less population growth with Tororo District recording less than 200 people per kilometers square in most of the years since the late 1970s (Figure 3) reaching 222 in the year 2002. HAT only persisted in the areas where the population density did not surpass 300 persons/km2. Nevertherless, the human population numbers in the selected study villages were more in Southeast Uganda than Western Kenya with a total of 5,905 and 3144 persons respectively (Table 1). The HAT affected villages in Southeast Uganda had more human population compared to HAT unaffected villages while in Western Kenya the scenario was slightly different with one HAT unaffected village in Teso District (Kapesur/Kolait) reporting more human population than HAT affected villages.
| Study Area | Livestock Numbers | Human Numbers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | District | Village | Cattle | Goats | Sheep | Pigs | Total | Number of Households | Adults | (<18 yrs) | Total Population |
| Southeast Uganda | Tororo | Osia-Kagwara | 234 | 421 | 65 | 165 | 885 | 267 | 574 | 893 | 1467 |
| Mulanga | 200 | 259 | 28 | 3 | 490 | 177 | 424 | 745 | 1169 | ||
| Hahoola | 172 | 159 | 8 | 8 | 347 | 145 | 289 | 467 | 756 | ||
| Busia-Uganda | Makina | 27 | 250 | 2 | 69 | 348 | 155 | 324 | 475 | 799 | |
| Mundaya | 47 | 164 | 13 | 31 | 255 | 186 | 406 | 573 | 979 | ||
| Buahuli | 98 | 304 | 7 | 47 | 456 | 146 | 285 | 450 | 735 | ||
| Total | 778 | 1557 | 123 | 323 | 2781 | 1076 | 2302 | 3603 | 5905 | ||
| Western Kenya | District | Village | Cattle | Goats | Sheep | Pigs | Total | Number of Households | Adults | (<18 yrs) | Total Population |
| Teso | Apatit | 173 | 155 | 30 | 49 | 407 | 98 | 237 | 258 | 495 | |
| Obuchun | 164 | 178 | 18 | 47 | 407 | 88 | 217 | 268 | 485 | ||
| Kapesur/Kolait | 60 | 93 | 6 | 13 | 172 | 92 | 259 | 379 | 638 | ||
| Busia-Kenya | Bukhuwamba | 165 | 94 | 12 | 24 | 295 | 95 | 212 | 278 | 490 | |
| Nangina | 114 | 184 | 32 | 48 | 378 | 152 | 110 | 138 | 788 | ||
| Nanderema | 49 | 66 | 13 | 36 | 164 | 47 | 349 | 439 | 248 | ||
| Total Livestock Numbers | 552 | 615 | 81 | 168 | 1416 | 572 | 1384 | 1760 | 3144 | ||
Table 1: Total livestock and human population in the study villages, western Kenya and southeast Uganda (2008).
Livestock numbers and household interactions with livestock
The study villages in Southeast Uganda had more livestock than Western Kenya with respondent farmers reporting twice the number of livestock. The total livestock reared in the sampled study villages were 1,416 and 2,781 in Western Kenya and Southeast Uganda, respectively (Table 1). The diseased villages that reported HAT had more cattle, sheep and pigs than non-diseased villages in both countries. Cattle numbers within households were low with farmers having an average of one to two animals per household. Farmers with more than ten cattle were very few. Eighty percent (n=608) of the households in the selected villages in both countries owned livestock. The communities in the study area live in close proximity to their animals with 97% (n=737) having the kraals within the homestead compound and 2.6% (n=20) outside the homestead. Livestock sheltered in sheds which were attached to the family house especially shoats sheltered near the kitchen. Most households (97.4%, n=740) had their livestock sheds or kraals or bomas less than 15 meters away from the homestead. Cattle were tethered or enclosed with barbed wire within the household compound or less than 15 meters from the household. The farmers with more than ten cattle kept their livestock in bomas or sheds enclosed with barbed wire or made of twigs.
Chemotherapeutic, chemoprophylactic and topical insecticides used in livestock
Sixty five percent of the respondents in Western Kenya and 35% in Southeast Uganda treated their livestock with trypanocides. The most commonly used trypanocides was Veriben (33.0%), and the rest were Noviduim, Berenil and Samorin at low percentage of (0.9%) each. The respondents in Western Kenya undertook more tsetse control activities such as bush clearing, live bait technology using insecticides applied on livestock host with the exception of trapping compared to their counterparts in Southeast Uganda, reported also in [6]. More respondents from Kenya (72.7%) sprayed their livestock with topical insecticide than their counterparts in Southeast Uganda (27.3%).
Wildlife presence in the study villages
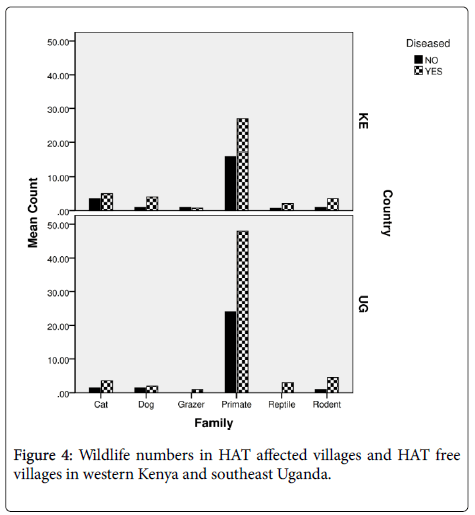
In both countries primates were more in villages that had reported HAT than those villages that were disease free (Figure 4). There was also a strong significant correlation of wildlife with HAT affected villages (F[8]=15.056; P<0.05). Wildlife abundance was high in villages reporting a history of HAT (69%) than HAT free villages (35.5%). Responses on wildlife presence differed between the various respondents depending on the location of the household since the respondents living in more woody or shrubland which was condusive wildlife and tsetse habitats sighted much wildlife than those open areas. The respondents reported that sighting of larger wildlife in their villages were more rampant in the 1970s to 1980s. The respondents reported that wildlife had decreased due to anthropogenic activities as the human population increased. The changes were also significant among the four districts especially from 1980 (F[3]=24.691; P<0.001) to 1990s (F[3]=39.453; P<0.001) where it had the most significant differences between districts. In Busia county of Western Kenya relative frequency of reported wildlife over time were 60.3% (monkeys), 7.3%, (mongoose), 5.0% (squirrels), 4.3% (snakes), 4.3% (foxes), 1.4% (rats), 1.7% (lizards) and 0.7% (warthogs). The other wildlife that had reduced or were extinct contributed to 15% such as the hare, antelopes, leopards and buffaloes. High abundance of wildlife was reported in the villages that had a history of HAT (64.5%) than HAT free villages at (34.8%) (F[15] =22.438; P<0.05) according to the survey. While at Busia and Tororo Districts, Southeast Uganda the relative frequency of reported wildlife over time were 69.2% (monkeys), 8.7% (mongoose), 7.0% (rats), 5.8% (foxes), 2.9% (squirrels), 2.9% (snakes), 2.0% (lizards) and 1.0% (warthogs).
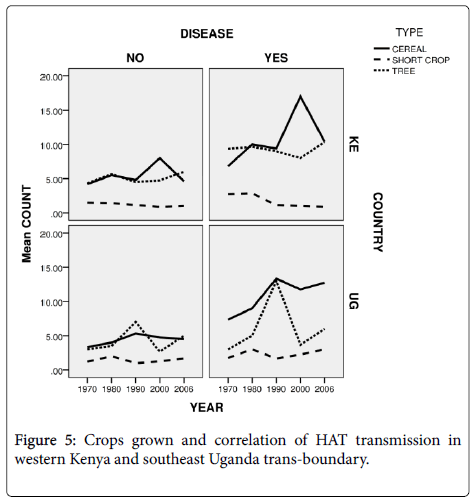
Crops grown and HAT transmission in western Kenya and southeast Uganda trans-boundary
Perennial crops which included bananas and cassava (under category of tree) that can act as peri-domestic tsetse habitats were also grown by 33.8% of the respondents. The tree crops (Figure 5) were more in Southeast Uganda than in Western Kenya. In both countries the HAT affected villages had more tree crops than HAT free villages. Also in both countries cereal crops such as maize were more grown than in HAT free villages.
Trypanosome infection rates
There was no risk of transmission of human infective trypanosomes as indicated in the results of caught tsetse flies infection rates from study villages (Table 2). No Trypanosoma brucei species were found circulating in the tsetse flies. The trypanosome parasites circulating within the study area in Western Kenya was T. vivax contributing 12% of the infection recorded in Kapesur while in Southeast Uganda T. congolense was reported in Hahoola. Kapesur and Hahoola were our control study villages in Western Kenya and Southeast Uganda respectively.
| Tsetse species numbers | Tsetse Species | Trypanosome Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | District | HAT Status | Village | No traps | G. pd | Ftd | G. ff | Ftd | No dissected | G. pd | G. ff | T. vivax* | T. c** |
| Kenya | Teso-Kenya | Diseased villages | Obuchun | 10 | 3 | 0.3 | - | - | 3 | 3 | -ve | -ve | |
| Apatit | 10 | - | 0 | - | - | 0 | 0 | 0 | 0 | 0 | |||
| Non-diseased villages | Kapesur/Kolait | 10 | 2380 | 238 | - | - | 50 | 6 | 6 | - | |||
| Busia-Kenya | Diseased villages | Nangina | 10 | 6 | 0.6 | - | - | 2 | 0 | - | - | ||
| Bukhwuamba | 10 | - | 0 | - | 0 | 0 | 0 | 0 | 0 | ||||
| Non-diseased villages | Nanderema | 10 | 1 | 0.1 | 1 | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Uganda | Busia-Uganda | Diseased villages | Makina | 10 | 1 | 0.1 | - | - | 0 | 0 | 0 | 0 | 0 |
| Mundaya | 10 | 14 | 1.4 | - | - | 7 | 0 | -ve | -ve | -ve | |||
| Non-diseased villages | Buhauli | 10 | 2 | 0.2 | - | - | 0 | 0 | 0 | 0 | 0 | ||
| Tororo-Uganda | Diseased villages | Osia Kagwara | 10 | 13 | 1.3 | 1 | 0.1 | 7 | 6 | 1 | -ve | 2 | |
| Mulanga | 10 | 2 | 0.2 | 34 | 3.4 | 30 | 2 | 28 | - | -ve | |||
| Non-diseased villages | Hahoola | 10 | 3 | 0.3 | 1 | 0.1 | 4 | 3 | 1 | -ve | 1 | ||
Table 2: Tsetse catches in 24 hour trapping and infection rates in tsetse flies at study villages in Kenya and Uganda trans-boundary in 2008.
Tsetse fly blood meals
Our results showed that tsetse preference was on domestic livestock recording 100% cattle blood from PCR blood meal analysis (Table 3) of engorged flies. We could not obtain the last blood meals from the 47% of the field caught flies as their midgut contents were inadequate. The blood meals were solely from Glossina pallidipes as the Glossina fuscipes fuscipes caught were few and had negligible blood meal contents in their midguts.
| Country | Sampling Sites | Sample Name | Genus and Species | Common Name | % Similarity |
|---|---|---|---|---|---|
| Kenya | Kapesur | A | Bos taurus | Cattle | 96.12 |
| Kenya | Kapesur | B | Bos taurus | Cattle | 97.16 |
| Kenya | Kapesur | C | Bos taurus | Cattle | 100 |
| Kenya | Kapesur | E | Bos taurus | Cattle | 97.74 |
| Kenya | Kapesur | F | Bos taurus | Cattle | 99.44 |
| Kenya | Kapesur | G | Bos taurus | Cattle | 99.21 |
| Kenya | Kapesur | H | Bos taurus | Cattle | 100 |
| Kenya | Nangina | I | Bos taurus | Cattle | 100 |
| Uganda | Mundaya | K | Bos taurus | Cattle | 100 |
Table 3: Blood meal analysis of trapped tsetse flies.
Discussion
We postulated a conceptual framework that explains interactions of various socio-economic, cultural, biological and physical factors that predispose individuals and populations at the transboundary of Kenya and Uganda to HAT [6] and herein explore factors that are related to the hosts of trypanosomes.
Demographic influence on HAT occurrence
The population density in the study distrcts has increased from 100-200 persons per kilometers square in 1980 to a range of 220 to 450 persons per kilometers square with a higher increase in the density in Western Kenya than Southeast Uganda and specifically much lower increase in Tororo district (Figure 3). The latter location registered the largest number of HAT cases during the period 1980-2008 and cumulative number of reported cases of HAT in the study districts (Figure 2) negatively correlates to increase of population density a fact that was also corroborated by outcome of our interviews in this study. However, even if the human population numbers increased in HAT affected villages, G. fucscipes fuscipes can persist due environmental legislation and policies of riverine habitats protection. Rhodesian HAT is typically acute and febrile with pathognomonic trypanosomal chancre at the bite site, progressing to second stage with neuropsychiatric signs within a few weeks and to death within 6 months [27]. The existence of comparable medical facilities and referral hospitals at the locations of study in both countries, peculiar clinical signs of the HAT, and community’s level of knowledge of the clinical signs of sleeping sickness because of past experiences debunks any claims of biased underreporting of cases in Kenya and therefore we seek to elaborate on the key host factors that may have caused the differences in the prevalence in studied districts at the trans-boundary. In this study, as human population increased wooded shrubland, shrubland, natural forest, wetland and other woody vegetation reduced because the communities cleared them to create room for settlement, built up infrastructure or agriculture. The density of tsetse fly population and proportion of the infected vectors with human infective trypanosomes determines the vector load and therefore the potential risk of disease transmission to the host [28,29]. Increasing human population has been observed as one of the key factors in land use and vegetation change which alters tsetse and its vertebrate hosts’ habitats in addition to hunting pressure on key hosts of tsetse and reservoirs of trypanosome [3,30-32]. It is predicted to eventually cause the extinction of tsetse flies. However, in the selected study villages there were no major differences in total human population in HAT affected villages and non-HAT villages, a fact that could be explained by tsetse’s flying range which could exceed one kilometer per day [33]. Albeit, the savana type tsetse, Glossina pallidipes has three times longer daily flight range than the riverine G fuscipes fuscipes [33,34].
Certain crops cultures such as cotton that rely on heavy use of chemicals and probably influence tsetse flies abundance were cultivated in 1970s and 1980s but the coverage decreased in 1990s to late 2000s due to lack of markets and change of agricultural support policies [6]. In this study cotton growing correlated negatively with HAT occurrence and in 1970s crop’s farming significantly differed (F[22]=17.490, p<0.05) in HAT affected villages (Diseased) and HAT free villages (Not diseased).
Livestock and wildlife host related factors
The Ugandan communities kept more livestock than the Kenyans (Table 1) even though the Kenyans residents observed more wildlife (except monitor lizards) than their Ugandan neighbors. Obviously the Ugandan HAT, therefore is associated with the key reservoirs, the cattle, than the Kenyans and more so because the Ugandan cattle were less treated with trypanocides or protected with insecticide than those in Kenya. The communities in both countries related closely with cattle, 97% sheltering their animals within 15 meters from their homesteads. Cattle could either pose risk or averts risks of HAT dependent on the intricate balance of roles it has been made to serve by the prevailing husbandry system. If the animal is sprayed with an effective insecticide as is the case in Kenya, where 73% of the farmers as opposed to less 30% by their Ugandan counterparts, then the animals will act as a cost effective live trap for the tsetse fly furnished with appropriate sight and olfactory cue, and lethal insecticide [35]. Circulating trypanocides in the blood of the reservoirs will decrease the load of trypanosomes in the population of reservoirs and insect hosts and ensure less infectivity of the Glossina in a locality. It has been suggested that topical application of insecticide would be more effective than admnistratation of prophylactic trypanoside and that in absence of wild host control of sleeping sickness could be achieved by treating 65% of cattle with trypanocides or applying topical insecticide on 20% of cattle [36]. In Kenya (6.7%) applied chemoprophylaxis and chemotherapeutic controls against trypanosomiasis to a larger extent than Uganda (2.1%) [6]. This also agrees with an outcome of a single intervention of application of insecticides in Soroti and Kamuli districts of Uganda, where a piloted mass chemotherapeutic intervention reduced HAT by more than 8 fold in 12 months post intervention [37].
Tsetse fly related factors
The key vectors for the Rhodesian sleeping sickness at the transboundary are the savannah Glossina pallidipes and the riverine Glossina fuscipes fuscipes. The riverine tsetse (G. fuscipes fuscipes) plays a bigger role in Ugandan side than in Kenya side of transboundary because Uganda has more water mass while Glossina pallidipesare more in Kenya, hence a important vector playing a bigger role in HAT. The savannah fly are strongly repelled by human and unlike the riverine flies respond a lot more strongly to olfactory cue [34,38], consequently more G. pallidipes were trapped in this study and much less G. fuscipes fuscipes (Table 2). Our traps smelt more than looked like the potential reservoirs and hence were more effective at trapping the savannah flies, in addition we were impeded by the fact most flies caught had little gut content consistent with previous observations that tsetse sampling tools are biased towards hungry flies [39]. Only G. pallidipes trapped were amenable to tests on the sources of their blood-meal because they had adequate gut contents and the fact that 100% of the contents were cattle blood with no infective Trypanosoma bruceispecies (Table 2) confirms the lower likelihood of human infection in western Kenya and southeast Uganda during the period. Only G. pallidipes flies were caught during the survey in the study villages. The tsetse fly density in the selected districts and villages were low with the exception of Kolait in Teso District, Kenya. Historical tsetse numbers of the two species (G. fuscipes fucipes and G. pallidipes were limited and could not be used to correlate with HAT trends. For one to pick HAT disease there are very many dynamics at play which includes, trypanosomes in circulation in both domestic and wildlife, tsetse competence and exposure of hosts to infected tsetse. Historically tsetse blood meals in the study area from 1950 to 2008 indicated changes of feeding habits of tsetse over time for Glossina fuscipes fuscipes and Glossina pallidipes from monitor lizards which has been reducing over the years ranging from 59% in 1990 to 4% in 2004 and other animals such domestic cattle, bush buck and suidae to mainly domestic livestock as a source of feed [26,40-42].
There are probably key differences in the stability of Trypanosoma brucei rhodesiense in the different vectors and reservoirs [43]. Experimental studies have documented that G. pallidipes has less vector competence than G. fuscipes fucipes (0 to 2.7% of G. pallidipes) to became infected after feeding on hosts infected with T. brucei strains [44]. The monitor lizards and G. pallidipes are refractory to the infection of T.b.rhodesiense due to low body temperatures of the reptiles [1,45] and innate resistance of the fly. Teneral or starved flies are more susceptible to infections by the parasite possibly due to compromised immunity [46] and it is possible that the potential reservoirs with sterile blood could ironically serve as good barriers against the transmission of HAT. When infection of the fly occurs, then the infected fly exponentially increases its ability to infect susceptible human and animal hosts by increased frequencies of bites due to an impaired feeding ability caused by the ensuing reduction of hemostatic factors [47,48] and the relatively longer lifespan of upto 35 days [1]. This may explain the large differences that are observed in areas with and without effective interventions for example in this study and elsewhere in Uganda [37].
Conclusion
Human African Trypanosomiasis persists in Uganda and not in the neighboring locations in Kenya due to factors that include vector, and hosts/reservoir factors. The undulating occurrences in past with simultaneous occurrence of peaks suggest a factor that is common in all the four studied locations and could be climatic. The fact that nagana persists in all the study locations indicates that conducive conditions for survival of the vector of HAT persists with an impending flare up of HAT should the control measures in place relent. Optimistically if benchmark are applied a control of HAT in South East Uganda and other HAT endemic areas is foreseeable.
Acknowledgements
I would like to thank Drs. Grace Murilla (former Centre Director KARLRO-BRI), Joseph Ndungu (former Director KETRI), Reto Brun, Swiss Tropical Institute (STI) and Edward Ssewannyana (former Director NaLIRRI, Uganda) for their support and facilitating field activities of the study.
Funding Statement
The study received funding from WHO/TDR (Grant ID: A60159), Swiss Tropical Institute (STI), and Eastern Africa Network for Tsetse and Trypanosomiasis (EANETT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conceived and designed the experiments: Jane Jemeli Rutto, Odipo Osano, Victor Odenyo; Performed the experiments: Jane Jemeli Rutto, Odipo Osano; Analyzed the data: Jane Jemeli Rutto, Odipo Osano; Wrote the paper: Jane Jemeli Rutto, Odipo Osano.
Competing Interests
The authors have declared that no competing interests exist.
References
- Franco JR, Simarro PP, Diarra A, Jannin JG (2014) Epidemiology of human African trypanosomiasis. Clin Epidemiol 6: 257-275.
- Cecchi G (2011) Biogeographical patterns of African trypanosomoses for improved planning and implementation of fieldinterventions. Université libre de Bruxelles.
- Jordan AM (1986) Trypanosomiasis control and African rural development. Trends Parasitol 2: 362.
- Mumford JD (2005) Application of benefit/cost analysis to insect pest control using the sterile insect technique. Sterile Insect Technique. Springer pp 481-498.
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, et al. (2004) Unhealthy Landscapes: Policy Recommendations on Land Use Change and Infectious Disease Emergence. Environ Health Perspect 112: 1092-1098.
- Rutto JJ, Osano O, Thuranira EG, Kurgat RK, Odenyo VAO (2013) Socio-Economic and Cultural Determinants of Human African Trypanosomiasis at the Kenya-Uganda Transboundary. PLoS Negl Trop Dis 7: e2186.
- Cecchi G, Paone M, Argilés Herrero R, Vreysen MJB, Mattioli RC (2015) Developing a continental atlas of the distribution and trypanosomal infection of tsetse flies (Glossina species). Parasit Vectors 8: 284.
- Magona J, Okuna NM, Katabazi B, Omollo P, Okoth J, et al. (1998) Control of tsetse and animal trypanosomiasis using a combination of tsetse trapping, pour-on and chemotherapy along the Uganda-Kenya border. Revue Élev Méd vét Pays trop 51: 311-315.
- Klassen-Fischer MK, Meyers WM, Neafie RC (2011) African Trypanosomiasis. INOVA CENTRAL LAB FAIRFAX VA.
- Berrang-Ford L, Waltner-Toews D, Charron D, Odiit M, McDermott J, et al. (2005) Sleeping Sickness in Southeastern Uganda: A Systems Approach. Eco Health 2: 183-194.
- Mbahin N, Affognon H, Andoke J, Tiberius M, Mbuvi D, et al. (2013) Parasitological prevalence of bovine trypanosomosis in Kubo division of Kwale county of coastal: baseline survey. Am J Anim Vet Sci 8: 28-36.
- Waiswa C, Picozzi K, Katunguka-Rwakishaya E, Olaho-Mukani W, Musoke RA, et al. (2006) Glossina fuscipes fuscipes in the trypanosomiasis endemic areas of south eastern Uganda: Apparent density, trypanosome infection rates and host feeding preferences. Acta Trop 99: 23-29.
- Gloria-Soria A, Dunn WA, Telleria EL, Evans BR, Okedi L, et al. (2016) Patterns of genome-wide variation in Glossina fuscipes fuscipes Tsetse Flies from Uganda. G3 (Bethesda) 6: 1573-1584.
- Weiss BL, Mouchotte R, Rio RV, Wu Y-n, Wu Z, et al. (2006) Interspecific transfer of bacterial endosymbionts between tsetse fly species: infection establishment and effect on host fitness. Appl Environ Microbiol 72: 7013-7021.
- Challier A (1982) The ecology of tsetse (Glossina spp.) (Diptera, Glossinidae): A review (1970-1981). Int J Trop Insect Sci 3: 97-145.
- MacLean L, Reiber H, Kennedy PG, Sternberg JM (2012) Stage progression and neurological symptoms in Trypanosoma brucei rhodesiense sleeping sickness: role of the CNS inflammatory response. PLoS Negl Trop Dis 6: e1857.
- Vale GA, Torr SJ (2005) User-friendly models of the costs and efficacy of tsetse control: application to sterilizing and insecticidal techniques. Med Vet Entomol 19: 293-305.
- Feldmann U, Dyck VA, Mattioli RC, Jannin J (2005) Potential impact of tsetse fly control involving the sterile insect technique. Sterile Insect Technique pp. 701-723.
- GoK (2002) Teso District: Effective management for sustainable economic growth and poverty reduction. The Republic of Kenya Nairobi Kenya.
- Rogers DJ, Hendrickx G, Slingenbergh JH (1994) Tsetse flies and their control. Rev Sci Tech 13: 1075-1124.
- GoU (2006) Tororo District Development Plan 2006-2009. Minstry of Planning and Development. The Republic of Uganda, Kampala, Uganda.
- GoU (2006) Busia District Development Plan 2006-2009. Minstry of Planning and Development, Kampala, Uganda.
- GoU (2002) Busia district 2002. Population and housing, Kampala, Uganda.
- GoU (2002) Tororo Population and Housing Census. Minstry of Planning and Development, Tororo District, Kampala, Uganda.
- Baylis M, Nambiro CO (2009) The responses of Glossina pallidipes and G. longipennis (Diptera: Glossinidae) to odour-baited traps and targets at Galana Ranch, south-eastern Kenya. Bull Entomol Res 83: 145-151.
- Muturi CN, Ouma JO, Malele II, Ngure RM, Rutto JJ, et al. (2011) Tracking the feeding patterns of tsetse flies (Glossina genus) by analysis of bloodmeals using mitochondrial cytochromes genes. PLoS One 6: e17284.
- Odiit M, Kansiime F, Enyaru CK (1997) Duration of Symptoms and Case Fatality of Sleeping Sickness Caused by Trypanosoma brucei rhodesiense in Tororo Uganda. East Afr Med J 74: 792-795.
- Okoth SO (2006) Transmission dynamics and epidemiology of Rhodesian sleeping sickness in allopatric populations of Glossina pallidipes of Kenya. PhD Kenyatta University.
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, et al. (2004) Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect 112: 1092-1098.
- Reid R, Kruska RL, Deichman U, Thornton PK, Leak SGA (2000) Human Population Growth and the Extinction of the Tsetse Fly. Agric Ecosyst Environ 77: 227-236.
- Rutto JJ, Karuga JW (2009) Temporal and Spatial Epidemiology of Sleeping Sickness and the Use of Geographical Information System (GIS) in Kenya. J Vector Borne Dis 46: 18-25.
- Bourn D, Reid R, Rogers D, Snow B, Wint W (2001) Environmental change and the autonomous control of tsetse and trypanosomiasis in sub-Saharan Africa: case histories from Ethiopia, The Gambia, Kenya, Nigeria and Zimbabwe. ERGO Oxford.
- Vale GA, Hursey BS, Hargrove JW, Torr SJ, Allsopp R (2011) The use of small plots to study populations of tsetse (Diptera: Glossinidae): Difficulties associated with population dispersal. Int J Trop Insect Sci 5: 403-410.
- Vale GA, Hargrove JW, Solano P, Courtin F, Rayaisse JB, et al. (2014) Explaining the Host-Finding Behavior of Blood-Sucking Insects: Computerized Simulation of the Effects of Habitat Geometry on Tsetse Fly Movement. PLoS Negl Trop Dis 8: e2901.
- Torr SJ, Vale GA (2011) Is the Even Distribution of Insecticide-Treated Cattle Essential for Tsetse Control? Modelling the Impact of Baits in Heterogeneous Environments. PLoS Negl Trop Dis 5: e1360.
- Hargrove JW, Ouifki R, Kajunguri D, Vale GA, Torr SJ (2012) Modeling the Control of Trypanosomiasis Using Trypanocides or Insecticide-Treated Livestock. PLoS Negl Trop Dis 6: e1615.
- Fyfe J, Picozzi K, Waiswa C, Bardosh KL, Welburn SC (2017) Impact of mass chemotherapy in domestic livestock for control of zoonotic T. b. rhodesiense human African trypanosomiasis in Eastern Uganda. Acta Trop 165: 216-229.
- Weiss BL, Wang J, Maltz MA, Wu Y, Aksoy S (2013) Trypanosome Infection Establishment in the Tsetse Fly Gut Is Influenced by Microbiome-Regulated Host Immune Barriers. PLoS Pathog 9: e1003318.
- Langley PA, Wall R (1990) The implications of hunger in the tsetse fly, Glossina pallidipes, in relation to its availability to trapping techniques. J Insect Physiol 36: 903-908.
- Okoth S, Kokwaro E, Kiragu J, Murilla A (2007) Glossina pallidipes and host interactions: implications of host preference on transmission risk of Rhodesian sleeping sickness in Kenya. Trends Appl Sci Res 2: 386-394.
- Weitz B (1963) The feeding habits of Glossina. Bull World Health Organ 28: 711-729.
- Karanja S (2005) Epidemiology and Importance of Trypanosomiasis, Helminthosis and Tick-borne Diseases on the Performance of Cattle in Busia District, Kenya. PhD. Frieien University, Berlin, Germany.
- Coleman PG, Welburn SC (2004) Are fitness costs associated with resistance to human serum in Trypanosoma brucei rhodesiense? Trends Parasitol 20: 311-315.
- Auty H, Morrison LJ, Torr SJ, Lord J (2016) Transmission dynamics of Rhodesian sleeping sickness at the interface of wildlife and livestock areas. Trends Parasitol 32: 608-621.
- Njagu Z, Mihok S, Kokwaro E, Verloo D (1999) Isolation of Trypanosoma brucei from the monitor lizard (Varanus niloticus) in an endemic focus of rhodesian sleeping sickness in Kenya. Acta Trop 72: 137-148.
- Kubi C, van den Abbele J, de Deken R, Marcotty T, Dorny P, et al. (2006) The effect of starvation on the susceptibility of teneral and nonâ€teneral tsetse flies to trypanosome infection. Med Vet Entomol 20: 388-392.
- Telleria EL, Benoit JB, Zhao X, Savage AF, Regmi S, et al. (2014) Insights into the trypanosome-host interactions revealed through transcriptomic analysis of parasitized tsetse fly salivary glands. PLoS Negl Trop Dis 8: e2649.
- Van Den Abbeele J, Caljon G, De Ridder K, De Baetselier P, Coosemans M (2010) Trypanosoma brucei Modifies the Tsetse Salivary Composition, Altering the Fly Feeding Behavior That Favors Parasite Transmission. PLoS Pathog 6: e1000926.
Citation: Rutto JJ, Osano O, Odenyo V (2018) Influence of Human, Wildlife and Livestock Husbandry on Epidemiology of Human African Trypanosomiasis at the Transboundary of Western Kenya and Southeast Uganda. J Infect Dis Ther 6: 364. DOI: 10.4172/2332-0877.1000364
Copyright: © 2018 Rutto JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4923
- [From(publication date): 0-2018 - Oct 21, 2025]
- Breakdown by view type
- HTML page views: 4008
- PDF downloads: 915