Research Article Open Access
Influence of Nevirapine on Gastrointestinal Function
Umoren EB*, Obembe AO and Osim EE
Department of Physiology, College of Medical Sciences, University of Calabar, Calabar, Nigeria
- *Corresponding Author:
- Umoren EB
Research Student, University of Calabar
Physiology 4 Etta-AGbor Street, Calabar
Cross River State 540001, Nigeria
Tel: +234-806-770-9327
E-mail: lizzyumoren@yahoo.com
Received date: July 30, 2015 Accepted date: August 08, 2015 Published date: August 17, 2015
Citation: Umoren EB, Obembe AO, Osim EE (2015) Influence of Nevirapine on Gastrointestinal Function. J Gastrointest Dig Syst 5:326. doi: 10.4172/2161-069X.1000326
Copyright: © 2015 Umoren EB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution; and reproduction in any medium; provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
In recent times there has been a growing research interest in Nevirapine an antiretroviral medication that prevents human immunodeficiency virus cells from multiplying in the blood. Nevirapine comes as tablet and a suspension (liquid) often taken by mouth, it is taken with or without once a day for 2 weeks and twice a day after the first 2 weeks. It is best to swallow nevirapine with liquids such as water, milk or soda. Feeding experiments in various animal species and humans have highlighted the beneficial role of nevirapine on health. These benefits include significant reduction in acquired immune deficiency syndrome-related morbidity and mortality. However, nevirapine hypersensitivity reaction can lead to morbidity through treatment interruption, inconvenience and loss of productivity. Additionally, nevirapine has an excellent bioavailability and a long half-life. This increases the risk of non-nucleoside reverse transcriptase inhibitor resistance when patients discontinue all the other antiretroviral drugs in the regimen at the same time. Thus, this can lead to decreased therapeutic options if nevirapine resistance develops. The gastrointestinal tract is a major portal of entry of nutrients into the body and nevirapine first make contact with the gastrointestinal tract. The health of an individual is dependent on the nutrient absorbed and hence functional integrity of the gastrointestinal tract. Studies have revealed that the interaction with drug have led to disruption of gastrointestinal function.
Keywords
Nevirapine; Gastrointestinal tract; Function
Introduction
The antiretroviral (ARV) drug nevirapine (NVP) is a nonnucleoside reverse transcriptase of HIV-1. NVP binds directly to reverse transcriptase and blocks the RNA-dependent and DNAdependent polymerase activities by causing a disruption of the enzyme’s catalytic site [1]. Widespread use of highly active antiretroviral therapy has led to dramatic reductions in morbidity and mortality among individuals infected with HIV-1 [2,3]. It is now clear that long-term remission of HIV-1 diseases can be achieved using various combinations of ARV agents, which suppress plasma viral loads to less than the limit of quantification of the most sensitive commercially available assays [4,5]. The clinical and immunological stabilization of HIV disease that is possible thanks to the availability of a broad spectrum of ARV compounds has its caveats in adherence, resistance, and toxicity problems [6]. NVP, like many other ARV agents, have side effects and toxicities which affect the gastrointestinal system [7].
The gastrointestinal tract (GIT) is a major portal of entry of nutrients into the body and NVP first make contact with GIT. The health of an individual is dependent on the nutrient absorbed and hence functional integrity of the GIT. The interaction with the drug may lead to disruption of its function. However, the emergence of new information on NVP provides the need for proper understanding of the effects of this ARV drug on the GIT (health). This paper therefore attempts to review the effects of NVP on GIT considering recently available information.
Materials and Methods
Drug description
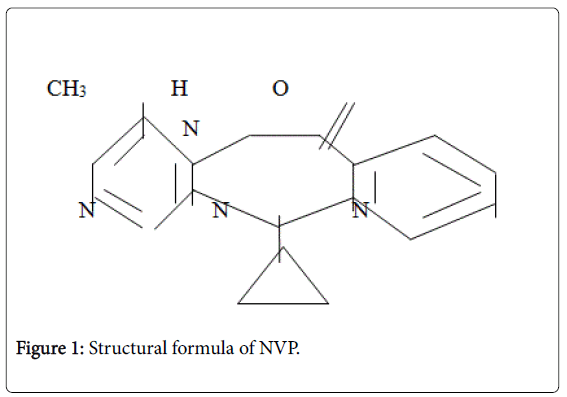
Viramune is the brand name for NVP a non-nucleoside reverse transcriptase inhibitor (NNRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). NVP is structurally a member of the dipyridodiazepinone chemical class of compounds. The chemical name of NVP is 11-cyclopropyl-5,11-dihydro-4-methyl-6Hdipyrido[ 3,2-b: 21,31-e] [1,4]diazepin-6-one. NVP is a white to offwhite crystalline powder with the molecular weight of 266.30 g/mol and the molecular formula C15H14N4O. NVP has a bioavailability of 93 ± 9%. Metabolism is hepatic; it has a half-life of 45 hours. Excretion by renal is <6% (parent drug) while biliary is <5% (parent drug). NVP has the following structural formula (Figure 1).
Viramune tablets are for oral administration. Each tablet contains 200 mg of NVP and the inactive ingredients microcrystalline cellulase, lactose monohydrate, providone, sodium starch glycolate, colloidal silicone dioxide and magnesium stearate. Viramune oral suspension is for oral admimistration. Each 5 ml of viramune suspension contains 50 mg of NVP (as NVP hemihydrates). The suspension also contains Iparaben, sorbitol, sucrose, polysorbate 80, sodium hydroxide and purified water.
Mode of action
NVP is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of HIV-1. NVP is a dipyridodiazepinone that binds directly to the viral reverse transcriptase and blocks the RNA-dependent and DNAdependent DNA polymerase activities by causing a disruption of the enzyme’s catalytic site [8]. NVP falls in the (NNRTI) class of ARV [9]. Both nucleoside and NNRTIs inhibit the same target, the reverse transcriptase enzyme, an essential viral enzyme which transcribes ribonucleic acid (RNA) into deoxoribonucleic acid (DNA). Unlike nucleoside reverse transcriptase inhibitors (NRTIs) which bind at the enzyme’s active site, NNRTIs bind allosterically at a distant site away from the active site termed the NNRTI pocket.
NVP is not effective against HIV-2, as the pocket of the HIV-2 reverse transcriptase has a different structure, which confers intrinsic resistance to the NNRTI class [10]. Resistance to NVP develops rapidly if viral replication is not completely suppressed [11]. The most common mutations observed after NVP treatment are Y 181 C and K 103 N, which are also observed with other NNRTIs [12,13], as all NNRTIs bind within the same pocket, viral strains which are resistant to NVP are usually also resistant to the other NNRTIs, efavirenz (EFV) and delavirdine.
Clinical efficacy
NVP in triple combination therapy has been shown to suppress viral load (VL) effectively when used as initial antiretroviral therapy (ART) (i.e., in ARV-naïve patients) [11]. Some clinical trials have demonstrated comparable HIV suppression with NVP-based regimens to that achieved with protease inhibitors (PIs) [14]. Although concerns have been raised about NVP-based regimens in the starting therapy with high VL or low CD4 count, some analysts suggest that NVP may be effective in these patients [15].
NVP may also form a useful component of salvage regimens after virological failure, usually in combination with one or more PIs as well as nucleoside reverse transcriptase inhibitors (nRTIs), especially in those who have not previously taken an NNRTI.
Adverse effects
While adverse effects of ART have been highlighted in many reports, the pattern and severity of adverse effects are reported to be dependent on the class of ARV agents used in the treatment of HIV/ AIDS patients. Neither the use of monotherapy regimen such as NVP nor the use of cocktail (Triple regimen: NVP, Lamivudine (3TC) and Zidovudine (AZT)) is free from adverse effects. Some of these adverse effects can be life-threatening such as drug eruptions with NVP and Abicar (ABC), pancreatitis with Didanosine (ddi) or lactic acidosis with the nucleoside analogues-3TC and Stavudine (d4T) [15].
Fever, somnolence, redistribution/accumulation of body fat, vomiting, jaundice, fulminant and cholestatic hepatitis, hepatic necrosis, hepatic failure, eosinophilia, neutropenia, arthalgia, paraesthesia, anaphylaxis, angioedema, bullous eruptions, ulcerative stomatitis, urticaria, have all been reported. The most common adverse effect of NVP is the development of mild or moderate rash (13%). Severe or life-threatening skin reactions have been observed in 1.5% of patients, including SJS, toxic epidermal necrolysis and HSR [12]. Concomitant use of st. John’s wort (Hypericum perforatum ) containing products and NVP is not recommended.
NVP may cause severe or life-threatening liver toxicity, usually emerging in the first six weeks of treatment Boehringer Ingelheim Pharmaceuticals, Inc. (2003) and DHHS Panel (2006). In 2000, the U.S. FDA issued a black box label on NVP, warning that it could cause severe liver damage, including liver failure [12]. Unacceptably high risk of serious liver symptoms in certain patient groups (women with CD4 count >250 and men >400) [15,16] has led the U.S. DHSS to recommend the restriction of NVP use to those at lower risk, unless the benefit to the patient clearly outweighs the risk [17] although 2NN study which found those CD4 limits, the effect was seen only in patients recruited from Thailand. More recent studies on the use of NVP in people with higher CD4 cell counts have come to the following conclusion: Treatment-experienced patients who start NVPbased combination therapy with low pre-ART and current CD4 cell counts and n undetectable VL have a similar likelihood for discontinuing NVP therapy because of hypersensitivity reactions (HSRs), compared with treatment-naive patients with low CD4 cell counts. This suggests that NVP-based combination therapy may be safely initiated in such patients. However, in similar patients with a detectable VL, it is prudent to continue to adhere to current CD4 cell count thresholds [18]. The U.S. PHSTF advocates caution in the use of NVP in pregnancy due to toxicity issues, which may be exacerbated during pregnancy [19]. Severe, life-threatening, and fatal cases of hepatotoxicity and skin reactions, as well as allergic reactions have occurred among HIV-infected patients treated with NVP [20,21] and are described in a box warning on the NVP label (ViramuneTM [package insert] [22], Boehringer Ingelheim/Roxane Laboratories, Inc., Ridgefield, Connecticut, 2003). This report suggests that persons taking NVP regimens for post-exposure prophylaxis (PEP) after HIV exposures also are at risk for serious adverse event. In 1996, the U.S. PHS first recommended PEP after certain occupational exposures to HIV [23]. These recommendations, updated in 1998 [24], are being revised to include other ARV agents that have been approved by FDA for use in HIV-infected persons. NVP is not recommended for basic or expanded PEP regimens. However, data on the safe and effective use of single-dose NVP to prevent perinatal HIV transmission [25] and a theoretical advantage of more rapid activity (i.e., NVP does not require phosphorylation for activation) have prompted clinicians to include NVP in PEP regimens following HIV PEP use from October 1995 through March 1999, six cases of serious adverse events related to PEP were reported among 492 registered participants; a severe skin reaction occurred in one of 11 health-care workers taking a regimen that included NVP [26]. In many circumstances, the risks associated with NVP as part of a PEP regimen outweigh the anticipated benefits.
Absorption and bioavailability
NVP is readily absorbed (>90%) after oral administration in healthy volunteers and in adults with HIV-1 infection. Absolute bioavailability in 12 healthy adults following single-dose administration was 93 and pluamn; 9% (mean and pluamn; SD) for a 50 mg tablet and 91 and pluamn; 8% for an oral solution. Peak plasma NVP concentrations of 2 and pluamn; 0.4 μg/mL (7.5 μM) were attained by 4 hours following a single 200 mg dose. Following multiple doses, NVP peak concentrations appear to increase linearly in the dose range of 200 to 400 mg/day. Steady-state trough concentrations of 4.5 and pluamn; 1.9 μg/mL (17 and pluamn; 7 μM), (n=242) were attained at 400 mg/day.
NVP tablets and suspension have been shown to be comparably bio-available and interchangeable at doses up to 200 mg. When viramune (200 mg) was administered to 24 healthy adults (12 female, 12 male) with either a high-fat breakfast (857 Kael, 50 g fat, 53% of calories from fat) or antacid (Maalox R 30 mL), the extent of NVP absorption (AUC) was comparable to that observed under fasting conditions. In a separate study in HIV-1 infected patients (n=6), NVP steady-state systemic exposure (AUCi) was not significantly altered by didanosine, which is formulated with an alkaline buffering agent. Viramune may be administered with or without food, antacid or didanosine.
Distribution
NVP is highly lipophilic and is essentially non-ionized at physiologic pH. Following intravenous administration to healthy adults, the apparent volume of distribution (Vdss) of NVP was 1.21 and pluamn; 0.09 L/kg, suggesting that NVP is widely distributed in humans. NVP readily crosses the placenta and is also found in breast milk [27]. NVP is about 60% bound to plasma proteins in the plasma concentration range of 1-10 μg/mL. NVP concentrations in human cerebrospinal fluid (n=6) were 45% (and pluamn; 5%) of the concentrations in plasma; this ratio is approximately equal to the fraction not bound to plasma protein.
Metabolism and elimination
In vivo studies in humans and in vitro studies with human liver microsomes have shown that NVP is extensively bio-transformed via cytochrome P450 (oxidative) metabolism to several hydroxylated metabolites. In vitro studies with human liver microsomes suggest that oxidative metabolism of NVP is mediated primarily by cytochrome P450 (CYP) isozymes from the CYP3A and CYP2B6 families, although other isozymes may have a secondary role. In a mass balance/excretion study in eight healthy male volunteers dosed to steady state with NVP 200 mg given twice daily followed by a single 50 mg dose of 14CNVP, approximately 91.4 and pluamn; 10.5% of the radiolabel dose was recovered, with urine (81.3 and pluamn; 11.1%) representing the primary route of excretion compared to feces (10.1 and pluamn; 1.5%). Greater than 80% of the radioactivity in urine was made up of glucuronide conjugates of hydroxylated metabolites. Thus cytochrome P450 metabolism, glucuronide conjugation, and urinary excretion of glucuronidated metabolites represent the primary route of NVP biotransformation and elimination in humans. Only a small fraction (<5%) of the radioactivity in urine (representing <3% of the total dose) was made up of parent compound, therefore, renal excretion plays a minor role in elimination of the parent compound.
NVP is an inducer of hepatic cytochrome P450 (CYP) metabolic enzymes 3A and 2B6. NVP induces CYP3A and CYP2B6 by approximately 20-25%, as indicated by erythromycin breath test results and urine metabolites. Auto-induction of CYP3A and CY2B6 mediated metabolism leads to an approximately 1.5 to 2 fold increase in the apparent oral clearance of NVP as treatment continues from a single dose to two- to-four weeks of dosing with 200-400 mg/day. Auto induction also results in a corresponding decrease in the terminal phase half-life of NVP in plasma, from approximately 45 hours (single dose) to approximately 25-30 hours following multiple dosing with 200-400 mg/day.
Source and Formulation of Nevirapine
NVP was obtained from Strides Arcolab Ltd., Bangalore, India.
Effect of nevirapine on body weight, food, and water intake
Animal’s studies have shown that NVP is widely distributed to nearly all tissues and readily crosses the blood-brain barrier [28]. The ARV, NVP at normal concentration causes the release of cytokines and chemokines which permeates the blood-brain barrier to stimulate the vegetative areas of the brain to cause increase thirst, increase hunger, leading to increased food and water intake which means decreased catabolism but increased anabolism resulting in increased pepsinogen production. This means increased production of other tissue proteins, leading to tissue growth [29]. Previous studies have shown that NVP is highly lipophilic and is essentially non-ionized at physiologic pH [27]. Giving rise to increased osmotic pressure of the blood. Increase in osmotic pressure of the blood arouses thirst sensation hence, increased intake of water with NVP consumption [30] Table 1. FRAM (2005) had reported that weight loss or nutritional compromise in HIV-infected patients is often thought to be associated with inadequate food intake, mal-absorption of nutrients, opportunistic infections or malignancies, diarrhea, or hormonal (e.g., testosterone) deficiencies. However, recent studies have shown increased body weight in NVP-treated animal models as compared to control group [30]. This is in line with previous studies [31-37].
| Group | Body weight change (g) | Mean body weight change (g) | Growth rate (g/day) |
|---|---|---|---|
| Control | 200 ± 1.49 | 137.5 ± 3.75 | 1.64 ± 0.04 |
| NVP | 269 ± 8.49 | 164 ± 10.48** | 1.95 ± 0.13 S |
Table 1: Body weight change in control and NVP treated groups. **P<0.01 vs. control S=Significant vs. control; Values are mean ± SEM, n=10.
Effect of NVP on abdominal wall, intestinal motility and transit
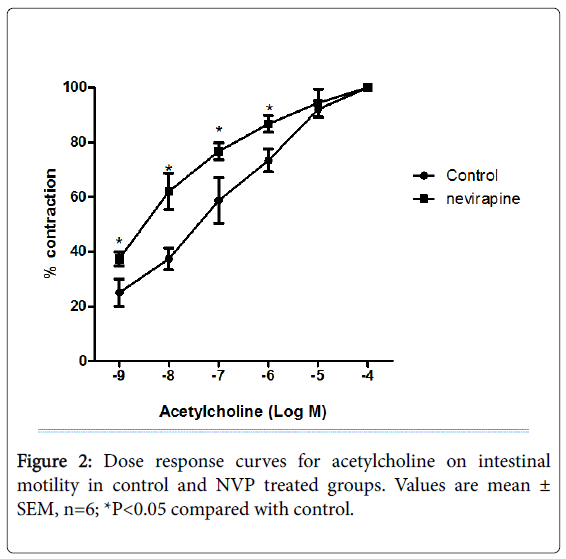
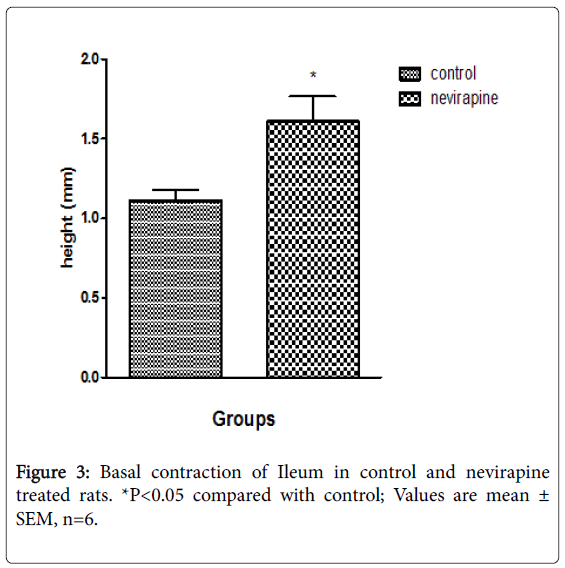
Results obtained from 12 weeks NVP administration showed increased gastric acid secretion, pepsin secretion, adherent mucus secretion, and increased incidence of ulcer spots in animals (Umoren et al.; Table 2). There was also increased basal contraction, intestinal motility and transit as compared to control [38] (Table 3; Figures 2 and 3). The increase in mucus secretion is due to increased gastric acid secretion which apparently stimulates mucus release [39-41]. Earlier [42] had reported that pepsin increases the amount of free (solubilized) mucus in the gastric lumen by eroding the gel adherent to the mucosa, which is in line with our report that increased pepsin secretion might have caused increased solubilization of adherent mucus resulting in increased mucus secretion [38]. This is also in consonance with the report of the Latest Clinical Trials [43] which reported increased mucus secretion but decreased gastric acid secretion. The difference in gastric acid secretion might be due to species specificity. Despite apparent increase in mucus secretion, there was evident ulcer induction in the NVP-treated animals as compared to control [38] (Table 2). This could have been due to autolyzing effect of pepsin on the mucosa. Pepsin potentiates ulcer formation. Increased intestinal motility and transit may be due to a seemingly irritable bowel. NVP may therefore not be beneficial in diarrheal states since it may provoke or aggravate diarrhea [44].
| Group | Gastric mucus (mg/g) | Pepsin (µmol/hr) | Acid secretion (µmol/hr) | Ulceration (µmol/hr) |
|---|---|---|---|---|
| Control | 0.025 ± 0.002 | 37.21 ± 1.13 | 4.2 ± 0.00 | 9.4 ± 0.53 |
| NVP | 0.043 ± 0.002*** | 78.73 ± 0.06*** | 9.8 ± 0.00*** | 13.3 ± 0.56*** |
Table 2: [Gastric mucus and pepsin], acid secretion, and gastric ulceration in control and NVP treated groups. ***p<0.001 vs. control; Values are mean ± SEM, n=10.
| Control | NVP | |
|---|---|---|
| Basal contraction (mm) | 2.0 ± 0.1 | 2.2 ± 0.1* |
| Intestinal transit (%) | 78.15 ± 0.39 | 84.53 ± 1.79** |
Table 3: Intestinal Motility in the control and NVP treated groups. *p<0.05 vs. control; **p<0.01 vs. control; Values are mean ± SEM, n=10.
Effect of NVP on serum liver enzymes profile
Result showed increase levels of alanine phosphatase (ALP), alanine aminotransferase (ALT), aspartate transaminase (AST) and gamma glutamyl transferase (GGT) in NVP-treated animals when compared to controls [45] (Table 4). This is in consonance with the works of [46-53]. Following the introduction of highly active antiretroviral therapy (HAART) as the standard care of HIV disease, multiple pathogenetic mechanisms have been postulated for the emerging liver damage observed during the course of ART. A direct or immunemediated hepatic involvement seems to be caused by NNRTI [12,46,47,53-55] had earlier defined severe liver damage to generally affect liver function tests and to be associated with elevated levels of ALT or AST that are greater than or equal to five times the upper limit of normal (ULN). Alkaline phosphatase levels usually increases remarkably in disease that impair bile formation and to a lesser extent in hepatocellular diseases. Earlier studies [56] (Table 4), have reported increased total bilirubin, conjugate and unconjugated bilirubin as well as elevated levels of cholesterol following NVP administration, pointing towards hepatotoxicity. Result is in line with earlier reports of elevated levels of bilirubin following NVP based treatments [49,52]. Fatal hepatotoxicity including fulminant and cholestatic hepatitis, hepatic necrosis and hepatic failure had also been reported in patients with NVP treatment [7,47,51,53,55,57,58] Increases in liver enzyme (ALT, AST, ALP and GGT) levels imply that NVP administration may impair liver, hamper the integrity and function of the liver in individual users. However, investigation to determine the exact constituent responsible for NVP hepatoxicity is hereby recommended.
| Control | NVP | |
|---|---|---|
| Aspartate aminotransferase (AST) | 67.8 ± 0.99 | 98.4 ± 3.71*** |
| Alanine aminotransferase (ALT) | 56.4 ± 0.81 | 91.6 ± 3.22*** |
| Alkaline phosphatase (ALP) | 98.4 ± 1.15 | 284.4 ± 1.42 |
| Gamma glutamyltransferase (GGT) | 10.76 ± 0.22 | 18.56 ± 0.55*** |
Table 4: Serum liver enzymes in control and NVP treated groups. ***p<0.001 vs. control; Values are mean ± SEM, n=10.
Effect of NVP on Biliary Secretion and its Biochemical Composition
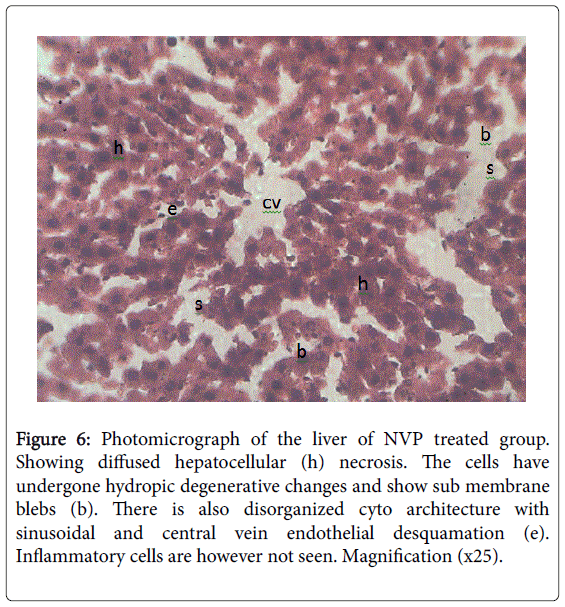
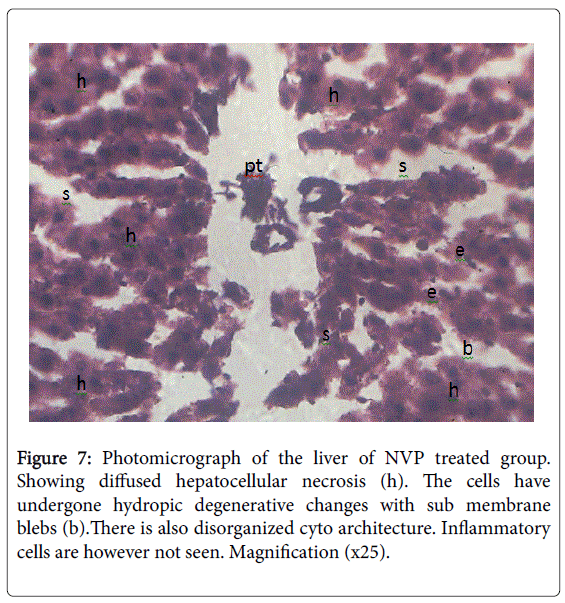
From microscopic examination following 12 weeks of NVP administration in animals, results showed increase in conjugated bilirubin, total cholesterol, total bilirubin, unconjugated bilirubin potassium and bicarbonate ions concentration as compared to controls [58] (Table 5). Decreased biliary secretions and biliary electrolytes (sodium and chloride) concentrations [59] (Table 6). Results showed liver cells had undergone hydropic degenerative changes and sub-membrane blebs; pointing to chemical hepatotoxicity [45] (Figures 4-7). This is in consonance with the work of Degott [60] who reported that liver damage is associated with alteration in bile secretion. Akerlund et al. [61] in support reported that there is always a compensatory increase in cholesterol synthesis when there is a disturbance in bile release and utilization due to liver damage. Result showed elevated levels of cholesterol, conjugated bilirubin and unconjugated bilirubin following NVP administration. Result showed reduction in sodium ion concentration which may be that the site for sodium absorption had been in an unhealthy state to absorb sodium as observed with the duodenal hypertrophy and jejuna hyperplasia [59]. Another reason for the low level in sodium ion concentration might be because increased efflux of potassium ions usually cause increased influx of sodium ions to replace potassium in the blood resulting in hyponatriuemia [62]. The exact mechanism of this action is unclear.Further research on these mechanisms is however recommended. This implies that NVP may provoke liver damage.
| Group | Control | NVP |
|---|---|---|
| Biliary secretion (L/hr) | 0.62 ± 0.04 | 0.47 ± 0.04* |
| Total cholesterol (mmol/L) | 0.78 ± 0.02 | 1.06 ± 0.05*** |
| Total bilirubin (µmol/L) | 9.98 ± 0.75 | 13.96 ± 0.77*** |
| Conjugated bilirubin (µmol/L) | 4.86 ± 0.74 | 6.16 ± 0.61 NS |
| Unconjugated bilirubin (µmol/L) | 5.12 ± 0.38 | 7.8 ± 0.48*** |
Table 5: Biliary secretion in control and NVP treated groups. *P<0.05 vs. control; ***p<0.001 vs. control; NS: Not-Significant vs. control; Values are mean ± SEM, n=10.
| Group | Na+ (mmol/L) | Cl- (mmol/L) | K+ (mmol/L) | HCO3- (mmol/L) |
|---|---|---|---|---|
| Control | 140.4 ± 0.49 | 86.8 ± 0.05 | 4.56 ± 0.05 | 19.6 ± 2.01 |
| NVP | 138.2± 0.33** | 85.2 ± 0.33*** | 5.04 ± 0.05* | 26.6 ± 0.27** |
Table 6: Bile electrolytes in control and NVP treated groups. *p<0.05 vs. control; **p<0.01 vs. control; ***p<0.001 vs. control; Values are mean ± SEM, n=10.
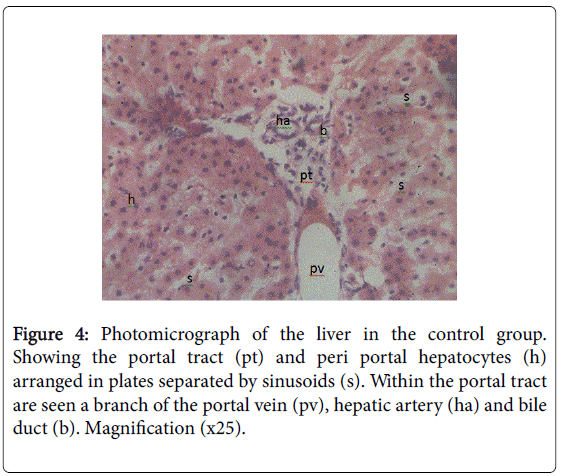
Figure 5: Photomicrograph of the liver in the control group. Showing the hepatocytes arranged in plates radiating outwards from the central vein (cv), separated by sinusoids (s). Hepatocytes (h) are seen as polygonal shaped cells with large one or two centrally placed nucleus. Sinusoids are lined by flattened endothelial cells (e) continuous with that of the central vein. Resident macrophages (kuppfer cells, k) are seen as large irregularly shaped cells within the sinusoids. Magnification (x25).
Figure 6: Photomicrograph of the liver of NVP treated group. Showing diffused hepatocellular (h) necrosis. The cells have undergone hydropic degenerative changes and show sub membrane blebs (b). There is also disorganized cyto architecture with sinusoidal and central vein endothelial desquamation (e). Inflammatory cells are however not seen. Magnification (x25).
Histology of the Liver in the Control and NVP Treated Rats
The histology of the liver showed normal liver in the control and pathological changes in the NVP treated group (Figures 4-7). Taken at different positions with the same magnification (×25).
Effect of NVP on morphology of the small intestine
Results from morphological examination revealed duodenum, jejunum and ileum of NVP-treated animals had hypertrophy of the Brunner’s glands within the sub mucosa; hyperplasia of the mucosal cells and mild desquamation of the epithelia; reductions in density of the Payer’s patches with diffused areas of necrosis of mucosal epithelium as compared to control whose intestinal tissues at different segments appeared normal; Umoren et al. [63] had earlier reported intestinal motility/transit stimulating actions of NVP in albino Wister rats. Disruption and erosion of the intestine of rats fed NVP exposed the muscle coat of intestine to various vaso-active agents causing contraction. Tissue erosion and degeneration can cause leakage of electrolytes such as sodium, potassium and hydrogen ions into the muscle coat layer of the intestine leading to amplification of the electrical activity of the intestine [64]. Deborah et al. [7] had reported gastrointestinal manifestations with protease inhibitor and NVP treatment to include diarrhea. These findings may be due to tissue erosion and degeneration caused by NVP administration, since damage to the ctyo-architecture of the small intestine results in the leakage of various ions, thus, affecting certain ion channels. Opening of calcium-sodium ion channels enhances calcium ion entry which causes contraction of smooth muscle cells [62]. This implies that NVP administration may cause derangement of intestinal tissue leading to increased intestinal motility, contraction and transit [38].
Effect of NVP on intestinal fluid and glucose absorption
Results showed a decrease in fluid and glucose uptake in NVPtreated animals [65] (Table 7). Significant reduction was observed in the Villus height and crypt depth in the small intestine of NVP-treated animals [65] (Table 8). Result is in consonance with the works of [1,6] who attributed toxicity problems to be associated with a broad spectrum of ARV compounds. Umoren et al. [38], Deborah et al. [7] have attributed NVP administration with alteration in gastrointestinal system. Previous studies [66,67] reported early stages of chemotherapy with nucleoside analogs, NNRTIs and protease inhibitors (PIs), symptoms of gastrointestinal disorders, such as abdominal discomfort, loss of appetite, diarrhea, nausea and vomiting, heartburn, abdominal pain, meteorism, dehydration, malnutrition with weight loss, low plasma drug levels and constipation may develop. However, some of their reports are in disparity to some of our results [30]. Damaged morphology of intestinal tissues had been reported [59]. This explains the observed reduction in fluid and glucose absorption in NVP-treated animals. This implies that NVP administration may lead to distortion in villus morphology with a concomitant mal-absorption of fluid and glucose [68].
| Group | Jejunum (GFU) ml/g sac/30mins | Ileum (GFU) ml/g sac/30mins | Jejunum (GGU) mg/g sac/30mis | Ileum (GGU) mg/g sac/30mins |
|---|---|---|---|---|
| Control | 0.269 ± 0.01 | 0.225 ± 0.02 | 8.14 ± 0.17 | 7.82 ± 0.19 |
| NVP | -0.068 ± 0.01*** | -0.064 ± 0.02*** | 2.84 ± 0.24*** | 6.18 ± 0.45* |
Table 7: Intestinal fluid absorption and glucose absorption in control and NVP treated groups. *p<0.05 vs. control; ***p<0.001 vs. control; Values are mean ± SEM, n=10; GFU: Gut Fluid Uptake; GGU: Gut Glucose Uptake.
| Group | Villus height (mm) | Crypt depth (mm) | ||||
|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | |
| Control | 272.01 ± 17 | 359.60 ± 33 | 193.03 ± 57 | 408.17 ± 26 | 255.67 ± 34 | 256.57 ± 81 |
| NVP | 248.83 ± 39** | 259.76 ± 12** | 163.97 ± 18*** | 259.67 ± 49*** | 224.23 ± 89** | 233.20 ± 11** |
Table 8: Villus height and crypt depth in the control and NVP treated groups. **P<0.05 vs. control; ***p<0.001 vs. control; Values are mean ± SEM, n=10.
Effect of NVP on fecal composition and electrolytes
12 weeks of NVP administration resulted in increased fecal composition (Table 9) i.e. fecal weight, fecal moisture loss, fecal glucose, fecal proteins, sodium and potassium ion levels in feces, decreased fecal electrolytes i.e. fecal chloride and fecal bicarbonate ions in animals [68,69].This implies that NVP administration even though may be able to increase body weight gain as a result of improved food and water intake, can also be detrimental to health because of its motility and transit stimulating effect [30,68].
| Control | NVP | |
|---|---|---|
| Fecal glucose (µmol/L) | 0.44 ± 0.009 | 0.314 ± 0.009*** |
| Fecal protein (g/L) | 0.12 ± 0.004 | 0.072 ± 0.002*** |
| Na+ (mmol/L) | 3.98 ± 0.09 | 5.64 ± 0.08*** |
| K+ (mmol/L) | 0.96 ± 0.03 | 1.3 ± 0.03*** |
| Cl- (mmol/L) | 14.8 ± 0.25 | 10.4 ± 0.50*** |
| HCO3-(mmol/L) | 15.6 ± 0.50 | 13.2 ± 0.68* |
Table 9: Fecal glucose, proteins and electrolytes in the control and NVP treated groups. *P<0.05 vs. control; ***p<0.001 vs. control; Values are mean ± SEM, n=10.
Summary and Conclusion
The aim of this paper was to highlight the health effect of nevirapine, an antiretroviral drug that helps to reduce incidences of morbidity and mortality among HIV-1 infected patients with respect to available research and literature. Following 12 weeks of NVP administration in animals, there was observed increase in food intake, water intake and body weight; decreased biliary secretion; reduction in Na+, Cl- levels, but increased levels of K+ and HCO3- in bile, high levels of total bilirubin, conjugated and unconjugated bilirubin as well as elevated cholesterol level as compared to control. Histology of the liver showed damages on liver tissue, with diffused hepatocellular necrosis as well as sinusoidal and central vein endothelial desquamation. Plasma levels of liver enzymes (ALT, AST, ALP and GGT) were also high in NVP-treated animals as compared to control thus, confirming liver impairment. Results also showed increases in adherent mucus, pepsin and gastric acid secretion with attendant incidence of gastric ulcers in NVP-treated animals as compared to control. Increased intestinal motility, basal contractions and transit as well as increased response to cholinergic agonist Acetylcholine (ACh) in NVP-treated animal was observed when compared to control. The administration of Atropine on the ileum of NVP-treated animals showed marked reduction in intestinal motility, but atropine had very little effect on control group. Histological presentations of the duodenum, jejunum and ileum showed hypertrophy of Brunner’s glands within the submucosa; hyperplasia of cells within the core of Villi and mild desquamation of the epithelia as well as reduction in density of the Payer’s patches and diffused areas of necrosis of mucosal epithelium. Reduction in Villus height and crypt depth in NVP-treated animals was observed when compared to control. Decreased gut fluid uptake and glucose absorption in NVP-treated animal was also observed when compared to control. Chronic administration of NVP was found to reduce glucose and protein loss in feces and increased fecal moisture loss. On the other hand, there was an observed increase in glucose and protein loss in feces of control animal with decrease moisture loss.
Experiments using human and animal models have shown that NVP administration may improve food and water intake, thus improve weight gain. Conversely, NVP administration has underlining caveat which ranges from, gastric ulceration, liver toxicity to elevation in liver enzymes. Hence, strict monitoring of HIV patients with hepatic impairment and ulcer incidence should precede NVP administration in order to avoid serious health challenge on individual users.
Acknowledgement
The technical assistance of Mr. Ededet Umoh and financial assistance of Prof E. J. Usua is greatly acknowledged.
Conflict of Interest
The authors stated that there are no conflicts of interest regarding the publication of this article. Research support played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- Ensoli F, Sirianni MC (2002) HIV/HCV co-infection: clinical and therapeutic challenges.AIDS 16: 1419-1420.
- Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, et al. (2000) Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel.JAMA 283: 381-390.
- Hogg RS, Heath KV, Yip B, Craib KJ, O'Shaughnessy MV, et al. (1998) Improved survival among HIV-infected individuals following initiation of antiretroviral therapy.JAMA 279: 450-454.
- Bartlett J, DeMosi R, Quinn J (2000): Meta-analysis of the effectiveness of triple combination therapy in antiretroviral naïve HIV-1 patients. Presented at the 7th Conference on Retroviruses and Opportunistic infections. San Francisco, USA
- Raboud JM, Montaner JS, Conway B, Rae S, Reiss P, et al. (1998) Suppression of plasma viral load below 20 copies/ml is required to achieve a long-term response to therapy.AIDS 12: 1619-1624.
- Bruno R, Sacchi P, Filice G (2002) Mitochondrial toxicity in HIV-HCV coinfection: It depends on the choice of antiretroviral drugs?Hepatology 35: 500-501.
- Deborah JE, Marriott, Jeffrey JP (2005) Gastro-intesinal manifestations: in Immunology/HIV/Infectious Diseases. Clinical Services Unit, St. Vincent’s Hospital, Sydney, NSW.
- Riska P, Lamson M, MacGregor T, Sabo J, Hattox S, et al. (1999) Disposition and biotransformation of the antiretroviral drug nevirapine in humans.Drug MetabDispos 27: 895-901.
- Patel SS, Benfield P (1996) New drug profile: nevirapine. Clinical Immunotherepeutics 6: 307-317.
- Ren J, Bird LE, Chamberlain PP, Stewart-Jones GB, Stuart DI, et al. (2002) Structure of HIV-2 reverse transcriptase at 2.35-A resolution and the mechanism of resistance to non-nucleoside inhibitors.ProcNatlAcadSci U S A 99: 14410-14415.
- Montaner JS, Reiss P, Cooper D, Vella S, Harris M, et al. (1998) A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study.JAMA 279: 930-937.
- (2003) BoehringerIngelheim Pharmaceuticals Inc. Viramune Package Insert. Ridgefield, CT: Boehringer-IngelheimPharmaceuticas Inc.
- Conway B, Wainberg MA, Hall D, Harris M, Reiss P, et al. (2001) Development of drug resistance in patients receiving combinations of zidovudine, didanosine and nevirapine.AIDS 15: 1269-1274.
- van Leeuwen R, Katlama C, Murphy RL, Squires K, Gatell J, et al. (2003) A randomized trial to study first-line combination therapy with or without a protease inhibitor in HIV-1-infected patients.AIDS 17: 987-999.
- van Leth F, Andrews S, Grinsztejn B, Wilkins E, Lazanas MK, et al. (2005) The effect of baseline CD4 cell count and HIV-1 viral load on the efficacy and safety of nevirapine or efavirenz-based first-line HAART.AIDS 19: 463-471.
- Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS (2003) A comprehensive hepatic safety analysis of nevirapine in different populations of HIV-infected patients. Journal of Acquired Immune Deficiency Syndrome 34: S21-S33.
- (2006) DHHS panel. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents.
- Wit FW, Kesselring AM, Gras L (2008) Discontinuation of nevirapine because of hypersensitivity reactions in patients with prior treatment experience, compared with treatment naïve patients: the ATHENA cohort study. Clin Infect Dis 46: 933-940.
- (2015) US Public Health Service. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnantHIV-1 transmission in the United States.
- Cattelan AM, Erne E, Salatino A, Trevenzoli M, Carretta G, et al. (1999) Severe hepatic failure related to nevirapine treatment.Clin Infect Dis 29: 455-456.
- Sidley P (2000) South Africa to tighten control on drug trials after five death.BMJ 320: 1028.
- Johnson S, Baraboutis JG, Sha BE, Proia LA, Kessler HA (2000) Adverse effects associated with use of nevirapine in HIV postexposure for 2 health care workers [letters]. JAMA 284: 2722-2723.
- (1996) CDC Update: provisional Public Health Service recommendations for chemoprophylaxis after occupational exposure to HIV. Mobidity and Mortality Weekly Report45: 468-472.
- (1998) CDC. Public Health Service guidelines for the management of health-care worker exposures to HIV and recommendations for postexposure prophylaxis. Mortality Weekly Report. 47(no. RR-7).
- Guay LA, Musoke P,Fleming T (1999): Intrapartum and neonatal single-dose nevirapine compared with zidovudine for preparation of mother-to- child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomized trial Lancet 354: 795-802.
- Wang SA, Panlilio AL, Doi PA (2000) Experience of healthcare workers taking postexposure prophylaxis after occupational HIV exposures: finding of the HIV Postexposure Prophylaxis Registry. Infect Control HospEpidemiol. 21: 780-785.
- de Maat MM, Ekhart GC, Huitema AD, Koks CH, Mulder JW, et al. (2003) Drug interactions between antiretroviral drugs and comedicated agents.ClinPharmacokinet 42: 223-282.
- Montuale N J (2001) Physician’s desk reference (PDR). Nevirapine (Viramune- Roxane).(55thedn) New York: Medical Economics Company. Inc., 2838-2842.
- Hoffbrand AV, Moss PA, Pettit JE (2006) Essential Haematology. (5thedn) Blackwell Publishing, Inc., 350 Main Street, Malden, Massachusetts 02148-5020, USA 2006: 94-100.
- Umoren EB, Obembe AO, Osim EE (2012) Chronic administration of the antiretroviral nevirapine increases body weight, food, and water intake in albino Wistar rats.J Basic ClinPhysiolPharmacol 23: 89-92.
- Saghayam S, Kumarasamy N, Cecelia AJ, Solomon S, Mayer K, et al. (2007) Weight and body shape changes in a treatment-naive population after 6 months of nevirapine-based generic highly active antiretroviral therapy in South India.Clin Infect Dis 44: 295-300.
- Pujari SN, Dravid A, Naik E (2005) Lipodystrophy and dyslipidemia among patients taking first-line, World Health Organization-recommended highly active antiretroviral therapy regimens in western India. J Acquir Immune DeficSyndr 39: 199-202.
- Carbonnel F, Maslo C, Beaugerie L, Carrat F, Wirbel E, et al. (1998) Effect of indinavir on HIV-related wasting.AIDS 12: 1777-1784.
- Mwamburi DM, Wilson IB, Jacobson DL, Spiegelman D, Gorbach SL, et al. (2005) Understanding the role of HIV load in determining weight change in the era of highly active antiretroviral therapy.Clin Infect Dis 40: 167-173.
- Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, et al. (2005) Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women.Clin Infect Dis 40: 1837-1845.
- Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, et al. (2005) Fat distribution in men with HIV infection.J Acquir Immune DeficSyndr 40: 121-131.
- Lichtenstein KA, Delaney KM, Armon C, Ward DJ, Moorman AC, et al. (2003) Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients.J Acquir Immune DeficSyndr 32: 48-56.
- Umoren EB, Obembe AO, Osim EE (2013) Ulcerogenic and intestinal motility/transit stimulating actions of nevirapine in albino Wistar rats.J PhysiolBiochem 69: 547-557.
- Bickel M, Kauffman GL Jr (1981) Gastric gel mucus thickness: effect of distention, 16,16-dimethyl prostaglandin e2, and carbenoxolone.Gastroenterology 80: 770-775.
- Iijima K, Ara N, Abe Y, Koike T, Iwai W, et al. (2012) Association of gastric acid and mucus secretion level with low-dose aspirin-induced gastropathy.J Gastroenterol 47: 150-158.
- Wilson DE, Quadros E, Rajapaksa T, Adams A, Noar M (1986) Effects of misoprostol on gastric acid and mucus secretion in man.Dig Dis Sci 31: 126S-129S.
- McQueen S, Hutton D, Allen A, Garner A (1983) Gastric and duodenal surface mucus gel thickness in rat: effects of prostaglandins and damaging agents.Am J Physiol 245: G388-393.
- (2012) Latest Clinical Trials. Antiretroviral drug levels during and after pregnancy.
- Ray JE, Marriott D, Bloch MT, McLachlan AJ (2005) Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. British Journal of Clinical Pharmacology 60: 291-299.
- Umoren EB, Obembe AO, Odo MO, Osim, EE (2013) Influence of long term administration of nevirapine on serum liver enzymes profile and morphology of liver tissue in albino Wistar rats. CSJS 1: 50-56.
- den Brinker M, Wit FW, Wertheim-van Dillen PM, Jurriaans S, Weel J, et al. (2000) Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection.AIDS 14: 2895-2902.
- Martínez E, Blanco JL, Arnaiz JA, Pérez-Cuevas JB, Mocroft A, et al. (2001) Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy.AIDS 15: 1261-1268.
- Martín-Carbonero L, Núñez M, González-Lahoz J, Soriano V (2003) Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine.HIV Clin Trials 4: 115-120.
- Mavukani MP (2009) Maternal and fetal outcomes of pregnant women on antiretroviral (ARV) therapy at Dr George Mukhari Hospital: a case-controlled clinical study. PhD Dissertation, University of Limpopo (Medunsa campus) Pg 85.
- Podjanee J, Peninnah O, Viral S (2005) An HIV-infected boy with severe rash after starting highly active antiretroviral therapy. Dept of Pediatrics, Faculty of Medicine, Chiang Mai University Pg 1-2.
- Carocci CA, Martinelli MC, Mastronardi MV, Corsi CP, Leonici LF (2008) Efficacy and tolerability of long-term nevirapine plus nucleoside reverse transcriptase inhibitors for HIV-1 infection. J. Intern. AIDS Soc. (Suppl 1) 11: 69.
- Balasundaram S, Ranganathan K, Umadevi K, Gunaseelan R, Kumaraswamy N, et al. (2011) Oral lesions associated with nevirapine-related Stevens Johnson syndrome: A report of four cases.J Oral MaxillofacPathol 15: 39-45.
- Piliero PJ, Purdy B (2001) Nevirapine-induced hepatitis: a case series and review of the literature.AIDS Read 11: 379-382.
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD (2000) Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection.JAMA 283: 74-80.
- Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD (2002) Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections; Hepato. 35: 182-189.
- Umoren EB, Obembe AO, Odo MO, Osim EE (2014) Effect of nevirapine administration on biliary secretion/its biochemical composition in albino Wistar rats. J. Antivir. Antiretrovir 6: 045-049.
- Clarke S, Harrington P, Condon C, Kelleher D, Smith OP, Mulcahy F (2000) Late onset hepatitis and prolonged deterioration in hepatic function associated with nevirapine therapy. International Journal of STD & AIDS 11: 336-337.
- Soriano V, Martin-Caronero L, Garcia-Samaniego J, Pouti M (2001) Mortality due to chronic viral liver disease among patients infected with human immunodeficiency virus. Clinical Infectious Diseases 33: 1793-1795.
- Umoren, EB and Osim EE (2014): Morphology of the small intestine of albino Wistar rats following long term administration of nevirapine. BiochemPharmacol 3: 2.
- Degott C (1997) Drug-Induced Liver Injury. Cholestatic Injury, Acute and Chronic.PatholOncol Res 3: 260-263.
- Akerlund JE, Björkhem I, Angelin B, Liljeqvist L, Einarsson K (1994) Apparent selective bile acid malabsorption as a consequence of ileal exclusion: effects on bile acid, cholesterol, and lipoprotein metabolism.Gut 35: 1116-1120.
- Guyton AC, Hall JE (2006) Digesstion and absorption in the gastrointestinal tract. In: Schmitt W (ed)textbook of medical physiology.(11thedn) Elsevier Saunders. Philadelphia 812-814.
- Umoren EB, Osim EE (2014): Morphology of the small intestine of albino Wistar rats following long term administration of nevirapine. BiochemPharmacol. 3: 132.
- Ladipo JK, Bradshaw LA, Halter S, Richards WO (2003) Changes in intestinal electrical activity during ischaemia correlate to pathology.West Afr J Med 22: 1-4.
- Umoren EB, Obembe AO (2014) Intestinal fluid and glucose transport in albino Wistar rats following long term administration of nevirapine J. AntivirAntiretrovir 6: 057-063.
- Hoffmann C, Kamps BS (2013) Management of side effects. HIV Medicine. Paris: Flying Publisher.
- Umoren EB (2013) Effect of nevirapine on fecal composition and fecal electrolytes in albino Wistar rats. GARJM 2: 032-038.
- Umoren EB, Obembe AO, Odo AO, Osim EE (2013) Influence of long term administration of nevirapine on serum liver enzymes profile and morphology of liver tissue in albino Wistar rats. Continental Journal of Innovations and sustainable Development 1: 50-56.
- João EC, Calvet GA, Menezes JA, D'Ippolito MM, Cruz ML, et al. (2006) Nevirapine toxicity in a cohort of HIV-1-infected pregnant women.Am J ObstetGynecol 194: 199-202.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15935
- [From(publication date):
October-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 11240
- PDF downloads : 4695