Invasive Acremonium Infection with Gastrointestinal Dissemination: A Case Report
Received: 25-Jan-2019 / Accepted Date: 21-Feb-2019 / Published Date: 28-Feb-2019 DOI: 10.4172/2332-0877.1000395
Abstract
Acremonium is rare fungal specie that causes mycetoma, mycotic keratitis and onychomycosis, and it may cause disseminated infection in immunocompromised patients. Herein, we report a 17-year-old immuno competent male diagnosed with invasive gastrointestinal acremonium infection. He presented to us with fever and abdominal pain with marked eosinophilia. Radiological scans revealed multiple hepatic lesions and thickening of the gut wall. He previously had a biopsy done which reported necrotizing granulomatous inflammation with septate hyphae and he was put on voriconazole for eight months. The patient underwent exploratory laparotomy for his continuous abdominal and biopsy of the resected mass showed the same necroinflammatory changes with septate hyphae. Subsequent culture of the biopsy specimens yielded Acremonium cephalosporium specie. He was subsequently resumed on voriconazole and the patient showed marked clinical improvement. This is a first case in the literature of acremonium infection presented with gastrointestinal dissemination.
Keywords: Acremonium; Gastrointestinal; Hepatic; Fungal
Introduction
Acremonium species are mostly environmental organisms; however certain species including A.faclifrome , A. kiliense , A. recifei , A. alabamensis , A. roseogriseum and A. strictum cause mycetoma, mycotic keratitis and onychomyocosis, and may cause disseminated infection in immunocompromised patients [1].
Clinical manifestations vary from localized lesions to invasive dissemination. Acremonium species have been reported in immune compromised patients with local and disseminated infections with predisposing conditions such as catheters, immunosuppression, IV drug users and anatomic defects [2].
We report a case of Acremonium (cephalosporium) infection in a 17-year-old male presented with fever and abdominal pain for the last eight months. His previous scans showed multi focal gut circumferential wall thickening with hepatic lesions, needle biopsy at that time reported necrotizing granulomatous inflammation secondary to fungal infection. Repeat CT scan revealed the same findings. Due to lack of response to treatment patient underwent laparotomy and had surgical resection of the mass in transverse colon. Histopathology showed granulomatous inflammation with septate hyphae. Subsequent fungal culture revealed the growth of Acremonium cephalosporium specie and he was restarted on voriconazole.
In our knowledge, this is the first case report of gastrointestinal acremonium infection with hepatic dissemination.
Case Report
A 17-year-old male from lower Dir district was presented to Northwest General Hospital, Peshawar in the month of October 2018, with a five-day history of low grade fever, abdominal pain and twenty day history of melena which had now settled. The patient denied NSAID intake or iron supplementation. The patient was febrile with a temperature of 100°F and pale conjunctiva. Abdominal examination showed mild right upper quadrant tenderness, hepatomegaly with span of 17 cm. There was no evidence of lymphadenopathy, splenomegaly or any mucosal site bleeding and rest of the systemic examination was normal.
His past medical history was significant for long standing fever, weight loss of 14 kg and recurrent abdominal pain. The patient had forty-five pet pigeons at his home. He had extensive workup done around eight months back. His previous radiological scans showed hepatosplenomegaly, lesions in the liver and hepatic flexure with multi focal gut circumferential wall thickening. Colonoscopy had ulcerated stricture growth at hepatic flexure; biopsy taken from it showed colonic mucosa with associated necroinflammatory slough and septate hyphae fungal organisms identified with candida specie. Biopsy from the hepatic lesion revealed necrotizing granulomatous inflammation secondary to fungal infection with septate hyphae. He was subsequently started on Voriconazole 200 mg BD which he had been taking for the last eight months. According to the patient he improved, his abdominal pain settled, he gained weight and his appetite improved. A CT abdomen was repeated after two months of the treatment which showed no regression.
Laboratory investigations on admission were notable for peripheral leukocytosis with total count of 28.60 x 10^12/l and marked eosinophilia (absolute count of 16.02 x 10^12/l). Microcytic hypochromic anaemia with Hb of 8.82 g/dl, MCV of 64.63 fl. CRP 7.51 mg/dL and ESR 40 mm/hr, both were slightly raised. Urinalysis, liver function tests, renal profile, pancultures and calcium level were normal. HIV serology was negative, Hb electrophoresis showed no abnormal bands, CEA level normal as well. His laboratory results are shown in Table 1.
| Laboratory Investigations | Value | Normal Range* |
|---|---|---|
| Hemoglobin | 8.82 | 13-18 g/dL |
| Red blood cell count | 4.43 | 4.5-5.5x10^12/L |
| Hematocrit | 28.6 | 40-54% |
| MCV | 64.63 | 83-101 fl |
| Platelets | 468.6 | 150-450x10^9/L |
| White cell count | 28.6 | 4-11x10^9/L |
| Differential Leukocytes | ||
| Neutrophils | 7.72 | 1.65-8.25 x10^9/L |
| Lymphocytes | 4 | 0.8-4.95 x10^9/L |
| Eosinophils | 16.02 | 0.04-0.66 x10^9/L |
| Monocytes | 0.86 | 0.24-1.1 x10^9/L |
| Other tests | ||
| Creatinine | 0.67 | 0.2-1.2 mg/dl |
| Random glucose | 83 | 110-165 mg/dl |
| PT | 12.4 | 10.0 sec |
| ALT | 18 | 10-40 U/L |
| Malarial Parasite | Not seen | |
| ESR | 40 | 0-15 mm/hr |
| CRP | 7.51 | 7.51 mg/dl |
| Calcium | 9.1 | 8.5-10.5 mg/dl |
| HCV Antibody | Non-Reactive | |
| Hbs Ag | Non-Reactive | |
| HIV Antibody | Non-Reactive | |
| EBV | Negative | |
| CMV | Negative | |
| Blood culture | No growth | |
| Urine culture | No growth | |
| Immunoelectrophoresis | No abdnormal bands | |
| Urine R/E | Normal |
Table 1: Relevant investigations done (*normal values for male, MCV=mean corpuscular volume, PT=Prothrombin time,ALT=alanine aminotransferase, ESR= Erythrocyte Sedimentation rate, CRP=C-reactive protein)
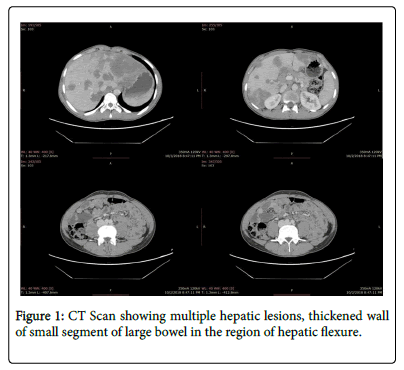
Abdominal CT showed multiple hepatic lesions, thickened wall of small segment of large bowel in the region of hepatic flexure and upper abdominal lymphadenopathy along with few right upper lobe pulmonary nodules suggesting infection and possibility of malignancy (Figure 1).
Ultrasound guided biopsy from the hepatic lesions was done. Initial histopathological assessment ruled out lymphoma and reported the same findings necroinflammatory granulomas with septate hyphae. A bone marrow biopsy was also done to rule out malignancy, the biopsy showed eosinophilic hyperplasia with no blast cells and iron deficiency anemia. Immunohistochemistry was negative for malignancy.
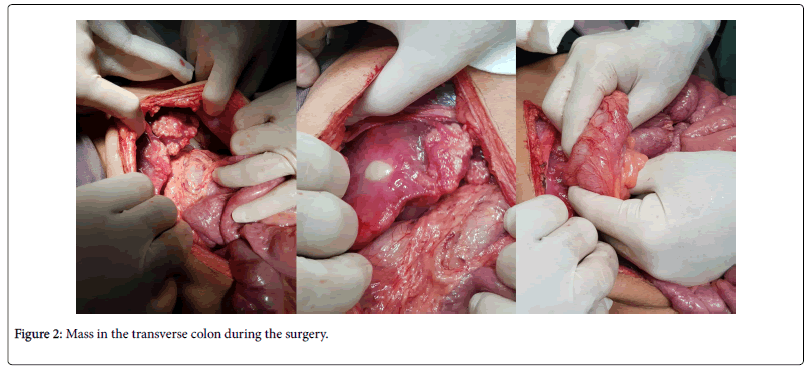
The patient was discharged with advice to follow up in two weeks and his antifungals were put on hold. The patient was readmitted with severe abdominal pain and underwent exploratory laparotomy. A mass in the transverse colon was resected and right sided hemicolectomy was performed (Figure 2). The specimens were sent for histopathology as well as fungal culture.
Histopathology report of the intestinal mass reported chronic granulomatous inflammation with necrosis. Branching septate fungal hyphae and the recovered lymph nodes showed benign reactive changes. Fungal culture after four weeks showed the growth of acremonium cephalosporium species. Macroscopically the colonies had smooth yeast like appearance. The slides were reviewed again with the microbiologist and histopathologists and the culture confirmed the diagnosis of acremonium cephalosporium specie. Based on these specific histopathological features and fungal culture, the diagnosis of invasive infection due to acremonium species was established. Immediately, he was restarted on vorcionazole. This was followed by dramatic improvement of fever and the abdominal manifestations. His leukocyte count and eosinophil count normalized and the patient was discharged on oral voriconazole 200 mg bid. The patient has been on regular follow-up and showed a marked improvement after surgery. His post-op scan after three months showed only two residual lesions in the liver. He is advised to have a CT scan repeated after six months.
Discussion
Acremonium is a saprophytic fungus found worldwide. Several species which have been identified in humans are A. falciforme , A. kiliense , A. alabamensis, A. roseogriseum , A. strictum , A. potronii and A. recifei . It usually manifests as mycetoma, mycotic keratitis, onychomycosis, and disseminated infection in immunocompromised patients [1,3]
The pathogenesis and risk factors of Acremonium is poorly understood. It is an opportunistic fungus found in the soil and dead plants [4]. It is still unclear regarding the mode of transmission but it is believed to occur through penetration of damaged skin or mucous membranes causing mycetoma and keratitis [5]. It generally do not invade blood vessels and rarely disseminate. In immune compromised patients it can occur though catheters and inhalation [2]. Our patient lived in the district of lower Dir, which is a fairly high altitude in the lower hills of Himalaya Mountains and he had close contact with his pet pigeons. The clinical spectrum of acremonium infection depends on the type of the species. Our patient had abdominal mass which may give a false diagnosis of malignancy or inflammatory diseases such as inflammatory bowel disease, appendicitis, peritoneal tuberculosis, amebiasis, and sarcoidosis.
Leukocytosis, marked eosinophilia, and an elevated ESR and CRP were present in the current case while it was not found in other case reports in literature. It follows that peripheral eosinophilia should prompt the clinician to evaluate for an on-going source of infection and alert for a possible fungal infection.
For the diagnosis, culture of Acremonium cephalosporium is considered the gold standard. It is a slow growing fungus and usually takes two weeks to be detected. Macroscopically colonies have a flat smooth pastel color, yeast-like appearance, and evolving to powdery white and cottony. The reverse is colorless, pale yellow, or pink-grey. Microscopically it is characterized by fine narrow septate hyphae which are unbranched, delicate phialides which extends at right angles from hyphae and hyaline, clustered conidia. The grains are oblong and may appear dense hyphal packs [6].
In the medical literature, disseminated infection caused by acremonium in immuno competent patients has rarely been reported worldwide. Disseminated infection has been described in patients with immunodeficiency but our patient was immuno competent and we believe it is the first case to have manifested in the GI tract with hepatic dissemination. In a review by Guarro et al. (1997), there have been 36 cases reported without any GI dissemination. There have been case reports of invasive infections of mycetoma, dialysis fistula infection, hematological, pulmonary infection in children, osteomyelitis, septic arthritis, esophageal, cerebritis and endocarditis in an IV drug abuser. While it was also found in the patients with predisposing conditions of neutropenia, chronic granulomatous disease and Addison’s disease [5,7-16].
As more cases are reported, larger studies may improve our understanding of the risk factors for this disease and methods to prevent it. No optimal treatment has been established yet for this rare infection. There have been some reports of clinical improvement with antifungal therapy alone; most patients with disseminated infection have received a combination of surgical and medical therapies. The best choice of antifungal agent is not clear. ESCMID and ECMM has joint guidelines on diagnosis and management of acremonium based on limited case reports. The guideline recommends treatment with voriconazole, amphotericin B and posaconazole, with voriconazole being superior and recommended for at least one year with disseminated infection [1]. The long-term prognosis in such cases remains unclear.
Conclusion
GI acremonium infection may masquerade as another clinical disease that leads to diagnostic confusion, morbidity and mortality. Diagnosis of this disease requires a high index of suspicion and awareness. Findings of this case report i.e. abdominal mass and peripheral eosinophilia could assist clinicians to diagnose and manage patients with invasive disseminated acremonium infection and with the aim of avoiding any complications associated with this rare disease.
Conflicts of Interest
A written consent was taken and the authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, et al. (2014) ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. ClinMicrobiol Infect 3: 27-46.
- Pastorino AC, Menezes UP, Marques HH, Vallada MG, Cappellozi VL, et al. (2005) Acremoniumkiliense infection in a child with chronic granulomatous disease. Braz J Infect Dis 9: 529-534.
- Perdomo H, Sutton DA, GarcÃa D, Fothergill AW, Cano J, et al. (2011) Spectrum of clinically relevant Acremonium species in the United States. J ClinMicrobiol 49: 243-256.
- Klimko NN, Khostelidi SN, Melekhina YE, Gornostaev DA, Semelev VN (2016) Acremonium Pneumonia Successfully Treated in Patient with Acute Myeloid Leukemia: A Case Report. J BacteriolMycol Open Access 2: 116-119.
- Fincher RM, Fisher JF, Lovell RD, Newman CL, Espinel-Ingroff A, et al. (1991) Infection due to the fungus Acremonium (cephalosporium). Medicine 70: 398-409.
- Collier L (1998) Topley&Wilsons microbiology and microbial infections: 1. Virology; 2. Systematic bacteriology; 3. Bacterial infections; 4. Medical mycology; 5. Parasitology; 6. Cumulative index. Arnold;
- Das S, Saha R, Dar SA, Ramachandran VG (2010) Acremonium species: a review of the etiological agents of emerging hyalohyphomycosis. Mycopathologia 170: 361-375.
- Geyer AS, Fox LP, Husain S, Della-Latta P, Grossman ME (2006) Acremoniummycetoma in a heart transplant recipient. J Am AcaDermatol 55: 1095-1100.
- Khan Z, Al-Obaid K, Ahmad S, Ghani AA, Joseph L, et al. (2011) Acremoniumkiliense: reappraisal of its clinical significance. J clin microbial 49(6):2342-2347.
- Miyakis S, Velegraki A, Delikou S, Parcharidou A, Papadakis V, et al. (2006) Invasive Acremoniumstrictum infection in a bone marrow transplant recipient. Pediatr Infect Dis J 25: 273-275.
- Warris A, Wesenberg F, Gaustad P, Verweij PE, Abrahamsen TG (2000) Acremoniumstrictumfungaemia in a paediatric patient with acute leukaemia. Scand j infect dis 32: 442-444.
- Weissgold DJ, Orlin SE, Sulewski ME, Frayer WC, Eagle Jr RC (105) Delayed-onset fungal keratitis after endophthalmitis. Ophthalmology 105: 258-262.
- Boltansky H, Kwon-Chung KJ, Macher AM, Gallin JI (1984) Acremoniumstrictum-related pulmonary infection in a patient with chronic granulomatous disease. J Infect Dis 149: 653.
- Szombathy SP, Chez MG, Laxer RM (1988) Acute septic arthritis due to Acremonium. J Rheumatol 15: 714-715.
- Simon G, Rakoczy G, Galgoczy J, Verebely T, Bokay J (1991) Acretnoniumkiliense in oesophagus stenosis: Ösophagus-StenosedurchAcremoniumkiliense. Mycoses 34: 257-260.
- Jeffrey WR, Hernandez JE, Zarraga AL, Oley GE, Kitchen LW (1993) Disseminated infection due to Acremonium species in a patient with Addison's disease. Clin infect dis 16: 170.
Citation: Khan K and Qureshi S (2019) Invasive Acremonium Infection with Gastrointestinal Dissemination: A Case Report. J Infect Dis Ther 7: 395. DOI: 10.4172/2332-0877.1000395
Copyright: © 2019 Khan K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4024
- [From(publication date): 0-2019 - Nov 20, 2025]
- Breakdown by view type
- HTML page views: 3135
- PDF downloads: 889