Lack of Pharmacokinetic Interaction between Antipsychotics (Quetiapine, Clozapine and Olanzapine) and the Beta-Blockers Metoprolol, Propranolol, Bisoprolol and Nebivolol Except between Carvedilol and the Antipsychotics Quetiapine and Clozapine
Received: 24-Oct-2017 / Accepted Date: 03-Nov-2017 / Published Date: 06-Nov-2017 DOI: 10.4172/2155-9872.1000387
Abstract
The purpose of this study was to evaluate a potential pharmacokinetic interaction between antipsychotics (quetiapine QUE, clozapine CLO and olanzapine OLA) and beta-blockers (metoprolol MET, propranolol PRO, bisoprolol BIS, nebivolol NEB and carvedilol CAR). Antipsychotics and beta-blockers are metabolized by the same cytochrome-P450 (CYP)-isoenzymes, which are inhibited by representatives of both substance classes. Pooled human liver microsomes (HLM) and recombinant CYP isoenzymes were used to determine whether the investigated antipsychotics and beta-blockers inhibit the metabolism of each other. Drug concentrations have been measured by high performance liquid chromatography (HPLC) with ultraviolet (UV) detection. Experiments with HLM showed that the metabolism of QUE as well as CLO slowed down in presence of CAR. There was no significant difference between the metabolism of the antipsychotics alone and the metabolism in combination with BIS, NEB and the two known CYP2D6-inhibitors MET and PRO. Experiments with recombinant CYP2D6 demonstrated an inhibitory effect on the metabolism of QUE and CLO by MET, PRO, NEB and CAR. The results suggest that CAR is also an inhibitor of other CYP enzymes, which are involved in the metabolism of CLO and QUE. It is assumed that CYP2D6 is a minor pathway of the antipsychotics and that the CYP2D6-inhibitory-effect of MET, PRO and NEB is compensated through a higher metabolism rate via the metabolic pathways of the other CYP-isoforms.
Keywords: High performance liquid chromatography; Pharmacokinetic; Antipsychotics; Metabolism
Abbreviations
BIS: Bisoprolol; CAR: Carvedilol; CLO: Clozapine; CYP: Cytochrome-P450; HLM: Human Liver Microsomes; HPLC: High Performance Liquid Chromatography; MET: Metoprolol; NEB: Nebivolol; OLA: Olanzapine; PRO: Propranolol; QUE: Quetiapine; UV: Ultraviolet.
Introduction
Approximately 10% of the schizophrenic patients, whose serum concentrations for CLO, OLA or QUE are tested in our therapeutic drug monitoring laboratory, are co-medicated with one of these betablockers: MET, PRO, BIS, NEB or CAR.
One possible complication of using this combination may be pharmacokinetic interactions. Both substance classes are metabolized by the same CYP-isoenzymes, especially CYP2D6, which is inhibited by representatives of both substance classes. CLO and PRO are both described as CYP2D6 inhibitors [1-5]. Data concerning a CYP2D6 inhibitory effect of MET are inconsistent. In some studies MET is characterized as a weak inhibitor [3,6], in other references no inhibitory effect could be observed [5,7,8]. All considered drugs are substrates of CYP2D6 [9-17] and therefore could be affected by the inhibitory effect of CLO, PRO and MET. OLA is an inhibitor of CYP1A2 and can cause interactions with PRO and CAR, which are both metabolized via CYP1A2 [18]. Inhibition of these enzymes could slow down the metabolism of these drugs, eventually resulting in increased serum concentration and increased risk of side effects. Altered serum concentrations and increased adverse side effects caused by the CYP2D6-inhibitors cimetidine and quinidine are described for MET [19-23], PRO [20,21,24,25] NEB [26] and CLO [27].
In addition to pharmacokinetic interaction between the described antipsychotics and beta-blocking agents, there could be pharmacodynamic interactions, too. Both substance classes can cause similar adverse events, which may lead to additive effects. Antipsychotics as well as beta-blockers are associated with weight gain [28-31]. and increase of serum triglycerides [32-39]. Several studies investigated the onset of Type II diabetes mellitus in patients treated with CLO and OLA [39-43]. Antipsychotics and beta-blockers can alter serum glucose levels [29,44-46]. Further adverse effects include impaired glucose tolerance caused by CLO and PRO [28,40] and decreased insulin sensitivity induced by MET and PRO [38,47]. Concomitant treatment with antipsychotics and beta-blockers increases the adverse effects of both, which may be a potential risk factor for metabolic syndrome, may increase the risk of new onset of Type II diabetes mellitus and impair management of existing diabetes mellitus.
The purpose of the present study was to investigate the pharmacokinetic interactions between different combinations of antipsychotics and beta-blockers to prevent unnecessary adverse effects.
Materials and Methods
Chemicals and reagents
Metoprolol tartrate, propranolol hydrochloride, carvedilol, nebivolol hydrochloride, olanzapine and clozapine were purchased from Sigma-Aldrich (Seelze, Germany). Quetiapine hemifumarate was kindly supplied by Astra Zeneca GmbH (Wedel, Germany). Bisoprolol hemifumarate, di-potassium hydrogen phosphate trihydrate, dimethyl sulfoxide, HPLC-grade acetonitrile, HPLC-grade methanol and orthophosphoric acid were acquired from Merck (Darmstadt, Germany). Potassium dihydrogen phosphate was purchased from Carl Roth (Karlsruhe, Germany). Pooled human liver microsomes, recombinant CYP Supersomes and NADPH regenerating system were purchased from Corning (Woburn, MA). Water used for preparation of stock solutions, buffer and mobile phases, was deionized and filtered by the water purification system Arium® basic from Sartorius (Goettingen, Germany).
Enzyme assay
The experiment was carried out according to the CYP Corning- SupersomesTM and Mammalian Liver Microsomes Guidelines for Use. Incubation mixtures contained 40 μl NADPH Regenerating System Solutions A (1.3 mM NADP+, 3.3 mM glucose 6-phosphate and 3.3 mM MgCl2, final concentrations), 8 μl NADPH Regenerating System Solutions B (0.4 U/ml glucose-6-phosphate dehydrogenase, final concentration) and 3.2 μl substrate (10 μM, final concentration) in 0.5 M potassium phosphate buffer (pH 7.4) to a final volume of 800 μl. After pre-incubation (5 min at 37°C), the reaction was initiated by addition of 20 μl HLM (0.5 mg of protein/ml, final concentration) or recombinant CYP Supersome (0.025 pmol of P450/ml, final concentration). After mixing, a reference sample was withdrawn immediately, and the reaction was stopped by adding the sample to the same volume of iced acetonitrile. The remaining mixture was incubated at 37°C and 300 rpm in a Thermomixer comfort (Eppendorf, Hamburg, Germany). After 15, 30, 45, 60, 75 and 90 minutes, 90 μl from the incubated mixture was withdrawn and added to 90 μl iced acetonitrile. The mixture was centrifuged at 13.000 × g, at 5°C for 3 minutes. The supernatant was withdrawn from the protein pellet and analyzed by HPLC-UV.
HPLC assay
Analyses of CLO, QUE, OLA, MET, PRO, BIS, NEB and CAR were performed with a validated HPLC-UV method. The HPLC system consisted of an Ultimate 3000 autosampler, a binary low-pressure gradient pump P680, a programmable column heater TCC100 and a variable wavelength photodiode array detector UVD 170U, all from ThermoFisher (Idstein, Germany). All analyses were performed with a Security Guard Cartridge Gemini Phenyl-Hexyl 4 × 3.0 mm precolumn coupled to the analytical column Gemini Phenyl Hexyl 110A 250 × 4.6 mm (5 μm), both obtained from Phenomenex (Aschaffenburg. Germany). Pretreatment was carried out on a LiChrospher RP-4 ADS extraction column (25 μm) from Merck (Darmstadt, Germany). The process of switching from extraction column to the analytical column was executed by an electric 6-port valve. The volume of injection was 125 μl. Autosampler temperature was set to 20°C and column heater temperature to 30°C. Three different UV wavelengths were used for detection of the analytes: PRO, NEB, CLO and QUE at 215 nm, MET and BIS at 226 nm and OLA and CAR at 242 nm. The flow rate was set to 1.0 mL/min. The extraction column eluent (8% acetonitrile in water) was mixed by the measurement system. Until minute 2, samples were delivered to the pre-cleaning column. At minute 2, the switching valve was set to the analytical position and the analytical eluent delivered the sample to the analytical column in backflush mode. At minute 4, the valve was set back to the starting position. Chromatographic separation was performed applying a gradient consisting of 10% methanol in buffer pH 3.1 and acetonitrile. The acetonitrile content was set to 8% until 8 minutes. Then the content of acetonitrile was increased by 1% acetonitrile/min, to 40% at the end of the total run time of 40 minutes. Retention times of OLA, MET, BIS, PRO, CLO, QUE, CAR and NEB were 10.89 min, 20.30 min, 25.97 min, 29.72 min, 30.39 min, 32.19 min, 36.97 min and 39.21 min, respectively. Calibration curves were constructed by spiking a potassium phosphate buffer (pH 7.4)/ acetonitrile mixture (1:1) with OLA, MET, BIS, PRO, CLO, QUE, CAR and NEB in 3 concentrations: 62.5 ng/ml, 250 ng/ml and 1000 ng/ ml, respectively. A quality control sample was measured in each run at a concentration of 500 ng/ml. The chromatograms were monitored and integrated by the Chromeleon software version 6.80 SR13 from ThermoFisher (Idstein, Germany).
Statistical analysis
All results are expressed as mean (± S. D.). Statistically significant differences in concentrations were tested with a t test using SigmaPlot 10.0 (Systat Software Inc., San Jose, CA, USA).
Results
Influence of beta-blockers on the metabolism of antipsychotics via HLM
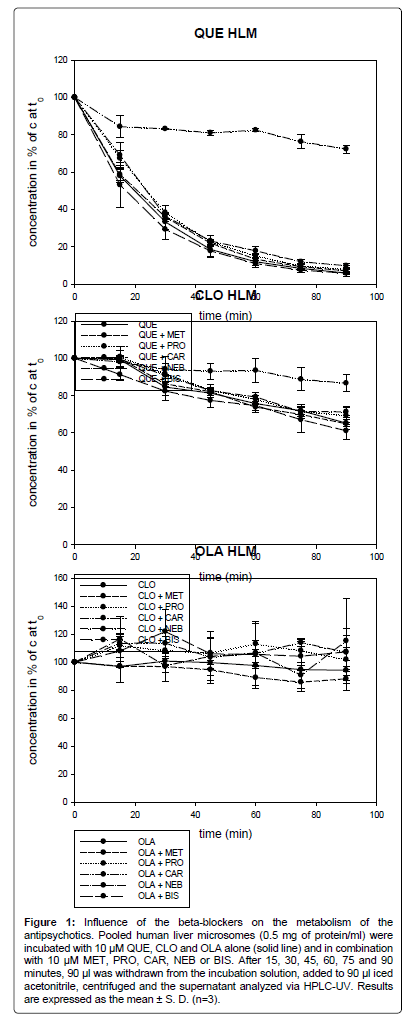
As shown in Figure 1, in experiments using HLM, only CAR inhibited the metabolism of QUE and CLO. The metabolism of OLA was not affected. Even the two known CYP2D6 inhibitors MET and PRO did not slow down the metabolism of the antipsychotics.
Figure 1: Influence of the beta-blockers on the metabolism of the antipsychotics. Pooled human liver microsomes (0.5 mg of protein/ml) were incubated with 10 μM QUE, CLO and OLA alone (solid line) and in combination with 10 μM MET, PRO, CAR, NEB or BIS. After 15, 30, 45, 60, 75 and 90 minutes, 90 μl was withdrawn from the incubation solution, added to 90 µl iced acetonitrile, centrifuged and the supernatant analyzed via HPLC-UV. Results are expressed as the mean ± S. D. (n=3).
OLA was slowly metabolized, after 90 min only 6% (7.2) of OLA was degraded. No significant difference could be observed between the metabolism of OLA alone and in combination with the beta-blockers.
After 20 min, 50% of QUE was metabolized. After 90 min, only 6% (1.8) of the parent drug remained. In combination with CAR, 72% (2.1) of QUE was left after 90 min, which represents a statistically significant difference (p<0.001). In combination with BIS, MET, PRO and NEB the QUE concentration after 90 min was at 6% (0.9), 7% (1.3), 8% (1.8) and 10% (1.1). The four beta-blockers had no significant effect on the metabolism of QUE.
After 90 min, 65% (1.7) of CLO remained. In combination with CAR, 87% (4.4) of CLO was left. CAR decreased the metabolism of CLO by 34%. The difference in CLO metabolism between CLO alone and in combination with CAR was not significant at 15 min. However, after 30 min the inhibition of the metabolism of CLO was significant with p<0.05 and at 60 min and 90 min with p<0.01. In combination with BIS, MET, PRO and NEB, the concentration of CLO after 90 min was at 61% (4.4), 65% (3.2), 69% (5.0) and 71% (2.2) of the concentration at time zero, respectively. The four beta-blockers had no significant influence on the metabolism of CLO.
The metabolism rate of QUE was higher than for CLO. Six percent (1.8) of QUE was left after 90 min. In comparison, 65% (1.7) of CLO remained after 90 min. OLA was metabolized to the lowest extent. 94% (7.2) of OLA was left after 90 min.
Influence of beta-blockers on the metabolism of the QUE and CLO via recombinant CYP2D6
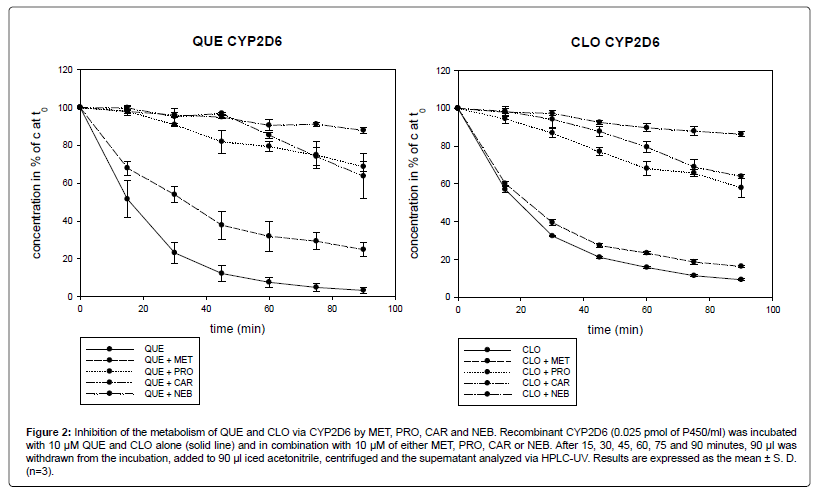
In the experiments with recombinant CYP2D6, the four betablockers MET, PRO, CAR and NEB slowed down the metabolism of QUE and CLO (Figure 2).
Figure 2: Inhibition of the metabolism of QUE and CLO via CYP2D6 by MET, PRO, CAR and NEB. Recombinant CYP2D6 (0.025 pmol of P450/ml) was incubated with 10 μM QUE and CLO alone (solid line) and in combination with 10 μM of either MET, PRO, CAR or NEB. After 15, 30, 45, 60, 75 and 90 minutes, 90 μl was withdrawn from the incubation, added to 90 μl iced acetonitrile, centrifuged and the supernatant analyzed via HPLC-UV. Results are expressed as the mean ± S. D.(n=3).
After 90 min, 3% (1.6) of QUE remained. In combination with MET, PRO, CAR and NEB, the remaining concentration of QUE after 90 min was 23% (6.7), 69% (2.6), 64% (12.0) and 88% (1.7) of the concentration at time zero, respectively. Concomitant with MET, PRO, CAR and NEB, there was a 7.7-fold, 23.0-fold, 21.3-fold and 29.3-fold reduced metabolism rate of QUE, respectively. The difference between QUE alone and in combination with MET was not significant at 15 min. A significant difference was documented after 30 min (p<0.01) and 90 min (p<0.001). In combination with PRO, CAR and NEB, the inhibition of the QUE metabolism was statistically significant at 15 min (p<0.01) and after 30 min (p<0.001).
After 90 min, 9% (0.5) of CLO remained. In combination with MET, PRO, CAR and NEB, the concentration of CLO after 90 min stayed at 16% (0.5), 58% (5.2), 64% (1.1) and 86% (1.3) of the concentration at time zero, respectively. Incubated with MET, PRO, CAR and NEB, there was a 1.8-fold, 6.4-fold, 7.1-fold and 9.5-fold increase of the concentration of CLO, respectively. The difference between CLO alone and in combination with MET was not significant at 15 min. After 30 min the difference was significant with p<0.01 and after 60 min with p<0.001. In combination with PRO, CAR and NEB, the inhibition of the metabolism of CLO was at any time statistically significant (p<0.001).
QUE was metabolized to a slightly higher extent than CLO via CYP2D6. After 90 min 3% (1.6) of QUE and 9% (0.5) of CLO was left. MET proved to be a weak CYP2D6-inhibitor, whereas NEB showed the strongest inhibitory properties.
Influence of antipsychotics on the metabolism of betablockers via HLM
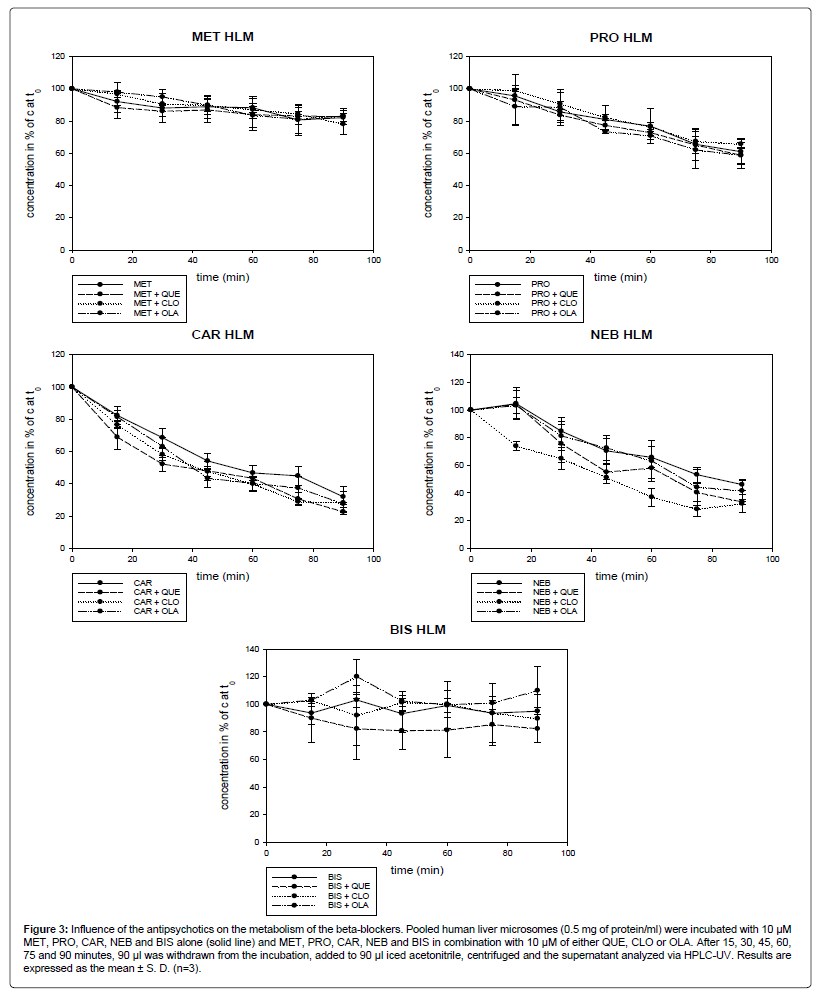
As shown in Figure 3, the antipsychotics QUE, CLO and OLA did not inhibit the metabolism of MET, PRO, CAR, NEB and BIS. In combination with CLO, the concentration of NEB (32%, 6.5) after 90 min was significantly lower than the concentration of NEB when incubated alone (46%; 4.0). The difference was statistically significant (p<0.05) at any time, at 60 min and 75 min with p<0.01.
Figure 3: Influence of the antipsychotics on the metabolism of the beta-blockers. Pooled human liver microsomes (0.5 mg of protein/ml) were incubated with 10 μM MET, PRO, CAR, NEB and BIS alone (solid line) and MET, PRO, CAR, NEB and BIS in combination with 10 μM of either QUE, CLO or OLA. After 15, 30, 45, 60, 75 and 90 minutes, 90 μl was withdrawn from the incubation, added to 90 μl iced acetonitrile, centrifuged and the supernatant analyzed via HPLC-UV. Results are expressed as the mean ± S. D. (n=3).
After 90 min, 82% (4.6), 61% (8.1), 32% (6.5), 46% (4.0) and 95% (2.6) of MET, PRO, CAR, NEB and BIS remained, respectively. CAR and NEB were metabolized to the highest extent, whereas the metabolism of BIS was low.
Influence of OLA on the metabolism of PRO and CAR via recombinant CYP1A2
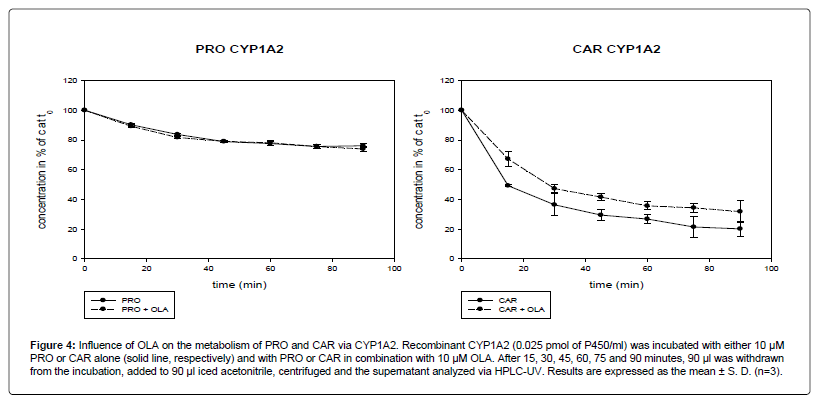
In the experiment with recombinant CYP1A2 it could be documented that OLA slowed down the metabolism of CAR. The metabolism of PRO was not affected (Figure 4).
Figure 4: Influence of OLA on the metabolism of PRO and CAR via CYP1A2. Recombinant CYP1A2 (0.025 pmol of P450/ml) was incubated with either 10 μM PRO or CAR alone (solid line, respectively) and with PRO or CAR in combination with 10 μM OLA. After 15, 30, 45, 60, 75 and 90 minutes, 90 μl was withdrawn from the incubation, added to 90 μl iced acetonitrile, centrifuged and the supernatant analyzed via HPLC-UV. Results are expressed as the mean ± S. D. (n=3).
After 90 min, 20% (5.2) of CAR remained. In combination with OLA, the concentration of CAR after 90 min decreased to 32% (7.6) of the concentration at time zero. The difference between CAR alone and in combination with OLA was not statistically significant at 30 min (p=0.08) and 90 min (p=0.09). A significant difference was found at 15 min, 60 min and 75 min (p<0.05) and at 45 min (p<0.01).
The metabolism rate via CYP1A2 for PRO seemed to be lower than for CAR. 76% (1.3) of PRO and 20% (5.2) of CAR was left after 90 min.
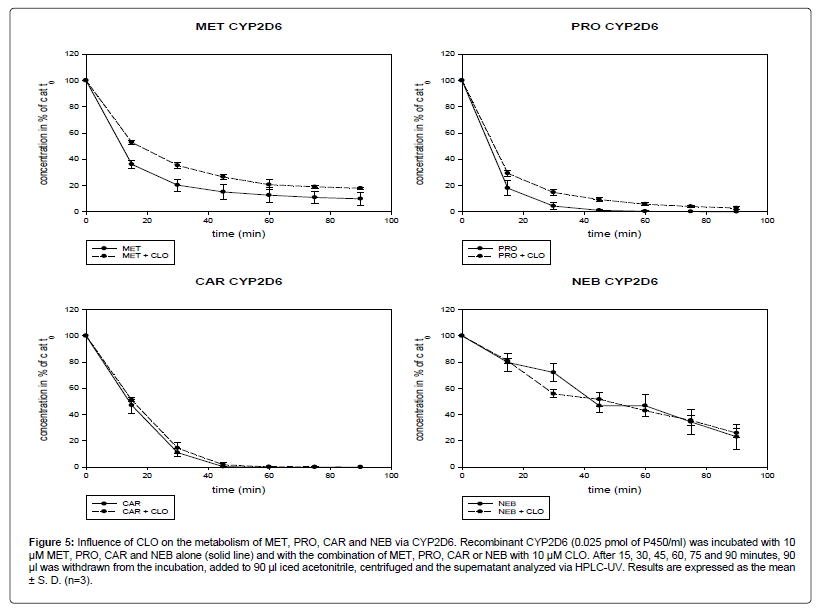
Influence of CLO on the metabolism of the beta-blockers via recombinant CYP2D6
In the experiment with recombinant CYP2D6, CLO inhibited the metabolism of MET and PRO but not of CAR and NEB (Figure 5).
Figure 5: Influence of CLO on the metabolism of MET, PRO, CAR and NEB via CYP2D6. Recombinant CYP2D6 (0.025 pmol of P450/ml) was incubated with 10 μM MET, PRO, CAR and NEB alone (solid line) and with the combination of MET, PRO, CAR or NEB with 10 μM CLO. After 15, 30, 45, 60, 75 and 90 minutes, 90 μl was withdrawn from the incubation, added to 90 μl iced acetonitrile, centrifuged and the supernatant analyzed via HPLC-UV. Results are expressed as the mean ± S. D. (n=3).
After 90 min, 10% (4.8) of MET was left. Combined with CLO, the concentration of MET after 90 min decreased to 18% (0.9). The difference between MET alone and in combination with CLO was statistically significant at all time points (After 15 min and 30 min: p<0.001; after 45 min, 75 min and 90 min: p<0.01; after 60 min: p<0.05).
After 90 min, 0.03% (0.051) of PRO remained when used alone. In combination with CLO, 3% (1.1) of the initial PRO was left. The inhibition of the metabolism of PRO by CLO was significant (at 15 min: p<0.05; at 30 min: p<0.01; at 45 min: p<0.001).
CAR was completely metabolized after 75 min and therefore showed the highest metabolism rate. Of the four beta-blockers investigated, NEB was metabolized as the lowest rate. After 90 min, 23% (9.6) of NEB was left. The metabolism of CAR and NEB was not affected by CLO.
Discussion
The present study using HLM showed that only CAR altered the metabolism of QUE and CLO. The two CYP2D6-inhibitors MET and PRO did not show the expected alteration in metabolism rate of the antipsychotics. The experiments with recombinant CYP2D6 confirmed an inhibitory effect of MET and PRO on CYP2D6. The non recognizable CYP2D6-inhibition by MET and PRO in this study with HLM suggests that CYP2D6 plays only a minor role in the metabolism of the antipsychotics and the CYP2D6-inhibition is compensated through a higher metabolism rate via the metabolic pathways of other CYP-isoforms. Also other studies showed that CYP2D6 inhibitors like cimetidine, haloperidol, risperidone and imipramine did not increase the serum concentration of QUE (DeVane and Nemeroff), while the CYP3A4-inhibitor ketoconazole and the CYP3A4-inductors phenytoin and thioridazine increased or decreased the concentration of QUE [48] The main metabolic pathway of QUE is via CYP3A4 with a contribution of 89% [49]. Also for CLO, CYP2D6 presents only a minor pathway with 6% contribution. CYP2D6 polymorphism did not influence CLO pharmacokinetics [50,51]. However, in another report the CYP2D6-inhibitor cimetidine increased serum clozapine levels and the risk of adverse effects [27]. CLO seems to be mainly metabolized via CYP1A2 with a contribution of 30-50% [18,50]. Further degradation pathways are CYP2C19, CYP3A4 and CYP2C9 [50]. OLA, similar to CLO, is mainly metabolized via CYP1A2 (contribution of 30-40%). CYP2D6 has only a minor role [9,18] CYP2D6 genotypes do not seem to have any influence on the clearance of OLA [52]. In summary, the studies mentioned above suggest that the three antipsychotics OLA, CLO and QUE are metabolized to a lesser extent via CYP2D6 and polymorphisms of CYP2D6 normally do not have any influence on the clearance of these antipsychotics. These observations are consistent with the results of the present study that the CYP2D6-inhibitors MET and PRO did not have any effect on the metabolism of the antipsychotics. To the best of the authors’ knowledge, no literature on inhibitory effects of CAR is available to explain the slower metabolism of CLO and QUE in combination with CAR. One potential explanation is that CAR inhibits CYP3A4 (the main pathway of QUE) and CYP1A2 (the main pathway of CLO) in addition to CYP2D6. Potential inhibition of CYP2C19 should also be taken into consideration, as CYP2C19 accounts for 24% of the CLO metabolism [50]. Further investigations are required to determine which CYP-isoforms are inhibited by CAR.
No inhibitory effect of the antipsychotics on the concentrations of the beta-blockers could be documented in the present study. An altered metabolism rate would be expected in combination with CLO, which was previously shown to have inhibitory activity on CYP2D6 [1,2]. Because CYP2D6 is the main pathway of beta-blocker metabolism, in contrast to the antipsychotics, increased concentrations of the beta-blockers are more likely. In addition, CYP2D6-inhibitors like cimetidine and quinidine increased the serum concentration of MET, PRO, CAR and NEB [19,20,23,25,26,53]. However, since no decreased metabolism could be observed, it is assumed that the CYP2D6- inhibitory effect by CLO is relatively low. In the present study, CLO did not show a strong inhibitory effect on the CYP2D6-activity when using recombinant CYP2D6. CLO slightly increased the concentration of MET and PRO, but not of CAR and NEB.
OLA, which is assumed to be a CYP1A2-inhibitor, mildly decreased the CAR metabolism in our tests with recombinant CYP1A2. The concentration of PRO was not affected, although CYP1A2 accounts with 30-50% for a higher percentage of the PRO metabolism than for the CAR metabolism with 5-10% [18]. In addition, CAR was metabolized to a greater extent as PRO by CYP1A2. In contrast to [18]. our results suggest that CYP1A2 plays a greater role in the metabolism of CAR than in the metabolism of PRO.
OLA and BIS were metabolized at a lower rate under our test conditions. With a bioavailability of 90%, hepatic metabolism was responsible for only 10% of the BIS metabolism [54]. The first-passeffect of OLA is with approximately 20-40% (bioavailability 60-80%) greater than that of BIS, but due to its long half-life of 30 hours (20- 70 hours) a very slow degradation of OLA was observed in our study [55]. In microsomal experiments, CYP-enzymes showed only a limited long-term stability, which is why studies were not performed over a longer period than 90 min. The other substances have higher first-passeffects and shorter half-life compared to OLA and BIS. Therefore, they are more suitable for microsomal experiments.
One limitation of the present study is that our experiments were in-vitro experiments with the use of high drug concentrations (10 μM). Further in-vivo investigations are required using therapeutic drug concentrations, conducted on healthy volunteers, for evaluating the clinical relevance. In addition, tests should be performed to identify the other CYP-isoforms inhibited by CAR. To allow a better comparison with other substrates and inhibitors, the determination of enzyme kinetic parameters, like Michaelis constant Km, maximal rate Vmax and inhibition constant Ki would be necessary.
In summary, the present study using recombinant CYP2D6 demonstrated an inhibited metabolism of QUE and CLO caused by the beta-blockers MET, PRO, CAR and NEB. While MET and PRO were already known CYP2D6-inhibitors, to the author’s knowledge, no literature exists on CYP2D6 inhibitory properties of CAR and NEB. Experiments using HLM did not show any pharmacokinetic interaction between the antipsychotics QUE, CLO and OLA and the beta-blockers MET, PRO, BIS and NEB, except for CAR. It is assumed that CYP2D6 represents only a minor pathway of the antipsychotics degradation and that the CYP2D6-inhibitory-effect of MET, PRO and NEB is compensated through a higher metabolism rate via the metabolic pathways of the other CYP-isoforms. In microsomal tests, only CAR inhibited the metabolism of the antipsychotics QUE and CLO and increased their concentration 12-fold and 1.3-fold, respectively. The results suggest that CAR is also an inhibitor of other CYP-isoenzymes, which are involved in the metabolism of CLO and QUE.
Acknowledgements
We like to thank Alexandra Köppl, Sandra Unholzer, Sebastian Böhr and Carina Rothammer (Clinical Pharmacology, University of Regensburg) for their support.
Authorship Contributions
Participated in research design: Margarete Silva Gracia, Regina Brandl. Conducted experiments: Margarete Silva Gracia. Performed data analysis: Margarete Silva Gracia. Wrote or contributed to the writing of the manuscript: Margarete Silva Gracia, Ekkehard Haen.
References
- Fischer V, Vogels B, Maurer G, Tynes RE (1992) The antipsychotic clozapine is metabolized by the polymorphic human microsomal and recombinant cytochrome P450 2D6. J Pharmacol Exp Ther 260: 1355-1360.
- Shin JG, Soukhova N, Flockhart DA (1999) Effect of antipsychotic drugs on human liver cytochrome P-450 (CYP) isoforms in vitro: preferential inhibition of CYP2D6. Drug Metab Dispos 27: 1078-1084.
- Otton SV, Inaba T, Kalow W (1984) Competitive inhibition of sparteine oxidation in human liver by beta-adrenoceptor antagonists and other cardiovascular drugs. Life Sci 34: 73-80.
- Bax ND, Lennard MS, Tucker GT (1981) Inhibition of antipyrine metabolism by beta-adrenoceptor antagonists. Br J Clin Pharmacol 12: 779-784.
- Perrild H, Kayser L, Poulsen HE, Skovsted L, Jorgensen B, et al. (1989) Differential effect of continuous administration of beta-adrenoceptor antagonists on antipyrine and phenytoin clearance. Br J Clin Pharmacol 28: 551-554.
- Polasek TM, Lin FPY, Miners JO, Doogue MP (2011) Perpetrators of pharmacokinetic drug-drug interactions arising from altered cytochrome P450 activity: a criteria-based assessment. Br J Clin Pharmacol 71: 727-736.
- Kallio J, Huupponen R, Seppala M, Sako E, Iisalo E (1990) The effects of beta-adrenoceptor antagonists and levomepromazine on the metabolic ratio of debrisoquine. Br J Clin Pharmacol 30: 638-643.
- Hefner G, Unterecker S, Shams MEE, Wolf M, Falter T, et al. (2015) Melperone but not bisoprolol or metoprolol is a clinically relevant inhibitor of CYP2D6. J Neural Transm 122: 1609-1617.
- Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM (1999) Olanzapine. Pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet 37: 177-193.
- Grimm SW, Richtand NM, Winter HR, Stams KR, Reele SB (2006) Effects of cytochrome P450 3A modulators ketoconazole and carbamazepine on quetiapine pharmacokinetics. Br J Clin Pharmacol 61: 58-69.
- Horikiri Y, Suzuki T, Mizobe M (1998) Pharmacokinetics and Metabolism of Bisoprolol Enantiomers in Humans. Journal of Pharmaceutical Sciences 87: 289-294.
- Lefebvre J, Poirier L, Poirier P, Turgeon J, Lacourciere Y (2007) The influence of CYP2D6 phenotype on the clinical response of nebivolol in patients with essential hypertension. Br J Clin Pharmacol 63: 575-582.
- Peer A, Snoeck E, Woestenborghs R, Velde V, Mannens G, et al. (1991) Clinical Pharmacokinetics of Nebivolol. Drug Invest 3: 25-30.
- Lennard MS, Silas JH, Freestone S, Ramsay LE, Tucker GT, et al. (1982) Oxidation Phenotype - A Major Determinant of Metoprolol Metabolism and Response. N Engl J Med 307: 1558-1560.
- Rowland K, WYNNE ELLIS S, Lennard MS, Tucker GT (1996) Variable contribution of CYP2D6 to the N-Dealkylation of S-(-)-propranolol by human liver microsomes. Br J Clin Pharmacol 42: 390-393.
- Volotinen M, Turpeinen M, Tolonen A, Uusitalo J, Maenpaa J, et al. (2007) Timolol Metabolism in Human Liver Microsomes Is Mediated Principally by CYP2D6. Drug Metabolism and Disposition 35: 1135-1141.
- Oldham HG, Clarke SE (1997) In vitro identification of the human cytochrome P450 enzymes involved in the metabolism of R(+)- and S(-)-carvedilol. Drug Metab Dispos 25: 970-977.
- Zhou S-F, Yang L-P, Zhou Z-W, Liu Y-H, Chan E (2009) Insights into the Substrate Specificity, Inhibitors, Regulation, and Polymorphisms and the Clinical Impact of Human Cytochrome P450 1A2. AAPS J 11: 481-494.
- Chellingsworth MC, Laugher S, Akhlaghi S, Jack DB, Kendall MJ (1988) The effects of ranitidine and cimetidine on the pharmacokinetics and pharmacodynamics of metoprolol. Aliment Pharmacol Ther 2: 521-527.
- Kirch W, Spahn H, Kohler H, Ohnhaus EE, Mutschler E (1982) Interaction of metoprolol, propranolol and atenolol with concurrent administration of cimetidine. Klin Wochenschr 60: 1401-1407.
- Mutschler E, Spahn H, Kirch W (1984) The interaction between H2-receptor antagonists and beta-adrenoceptor blockers. Br J Clin Pharmacol 17 Suppl 1: 51-57.
- Toon S, Davidson EM, Garstang FM, Batra H, Bowes RJ, et al. (1988) The racemic metoprolol H2-antagonist interaction. Clin Pharmacol Ther 43: 283-289.
- Leemann TD, Devi KP, Dayer P (1993) Similar effect of oxidation deficiency (debrisoquine polymorphism) and quinidine on the apparent volume of distribution of (+/-)-metoprolol. Eur J Clin Pharmacol 45: 65-71.
- Yasuhara M, Yatsuzuka A, Yamada K, Okumura K, Hori R, et al. (1990) Alteration of propranolol pharmacokinetics and pharmacodynamics by quinidine in man. J Pharmacobiodyn 13: 681-687.
- Zhou HH, Anthony LB, Roden DM, Wood AJ (1990) Quinidine reduces clearance of (+)-propranolol more than (-)-propranolol through marked reduction in 4-hydroxylation. Clin Pharmacol Ther 47: 686-693.
- Kamali F, Howes A, Thomas SH, Ford GA, Snoeck E (1997) A pharmacokinetic and pharmacodynamic interaction study between nebivolol and the H2-receptor antagonists cimetidine and ranitidine. Br J Clin Pharmacol 43: 201-204.
- Szymanski S, Lieberman JA, Picou D, Masiar S, Cooper T (1991) A case report of cimetidine-induced clozapine toxicity. J Clin Psychiatry 52: 21-22.
- Micossi P, Pollavini G, Raggi U, Librenti MC, Garimberti B, et al. (1984) Effects of metoprolol and propranolol on glucose tolerance and insulin secretion in diabetes mellitus. Horm Metab Res 16: 59-63.
- Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder, SR, et al. (2002) The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry 63: 856-865.
- Lainscak M, Keber I, Anker SD (2006) Body composition changes in patients with systolic heart failure treated with beta blockers: a pilot study. Int J Cardiol 106: 319-322.
- Boxall BW, Clark AL (2012) Beta-blockers and weight change in patients with chronic heart failure. J Card Fail 18: 233-237.
- Byington RP, Worthy J, Craven T, Furberg CD (1990) Propranolol-induced lipid changes and their prognostic significance after a myocardial infarction: the Beta-Blocker Heart Attack Trial experience. Am J Cardiol 65: 1287-1291.
- Dursun SM, Szemis A, Andrews H, Reveley MA (1999) The effects of clozapine on levels of total cholesterol and related lipids in serum of patients with schizophrenia: a prospective study. J Psychiatry Neurosci 24: 453-455.
- Fogari R, Zoppi A, Pasotti C, Poletti L, Tettamanti F, et al. (1989) Plasma lipids during chronic antihypertensive therapy with different beta-blockers. J Cardiovasc Pharmacol 7: 32.
- Gaulin BD, Markowitz JS, Caley CF, La Nesbitt, Dufresne RL (1999) Clozapine-associated elevation in serum triglycerides. Am J Psychiatry 156: 1270-1272.
- Leon J de, Susce MT, Johnson M, Hardin M, Pointer L, et al. (2007) A clinical study of the association of antipsychotics with hyperlipidemia. Schizophr Res 92: 95-102.
- Osser DN, Najarian DM, Dufresne RL (1999) Olanzapine increases weight and serum triglyceride levels. J Clin Psychiatry 60: 767-770.
- Pollare T, Lithell H, Selinus I, Berne C (1989) Sensitivity to insulin during treatment with atenolol and metoprolol: a randomised, double blind study of effects on carbohydrate and lipoprotein metabolism in hypertensive patients. BMJ 298: 1152-1157.
- Wirshing DA, Spellberg BJ, Erhart SM, Marder, SR, Wirshing WC (1998) Novel antipsychotics and new onset diabetes. Biol Psychiatry 44: 778-783.
- Hagg S, Joelsson L, Mjorndal T, Spigset O, Oja G, et al. (1998) Prevalence of diabetes and impaired glucose tolerance in patients treated with clozapine compared with patients treated with conventional depot neuroleptic medications. J Clin Psychiatry 59: 294-299.
- Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, et al. (2000) Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am J Psychiatry 157: 975-981.
- Lund BC, Perry PJ, Brooks JM, Arndt S (2001) Clozapine use in patients with schizophrenia and the risk of diabetes, hyperlipidemia, and hypertension: a claims-based approach. Arch Gen Psychiatry 58: 1172-1176.
- Popli AP, Konicki PE, Jurjus GJ, Fuller MA, Jaskiw GE (1997) Clozapine and associated diabetes mellitus. J Clin Psychiatry 58: 108-111.
- Baymiller SP, Ball P, McMahon RP, Buchanan RW (2003) Serum glucose and lipid changes during the course of clozapine treatment: the effect of concurrent beta-adrenergic antagonist treatment. Schizophr Res 59: 49-57.
- Grajower MM, Walter L, Albin J (1980) Hypoglycemia in chronic hemodialysis patients: association with propranolol use.Nephron 26: 126-129.
- Kotler MN, Berman L, Rubenstein AH (1966) Hypoglycaemia precipitated by propranolol. Lancet 2: 1389-1390.
- Lithell H, Pollare T, Vessby B (1992) Metabolic effects of pindolol and propranolol in a double-blind cross-over study in hypertensive patients. Blood Press 1: 92-101.
- DeVane CL, Nemeroff CB (2001) Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet 40: 509-522.
- Hasselstrom J, Linnet K (2006) In vitro studies on quetiapine metabolism using the substrate depletion approach with focus on drug-drug interactions. Drug Metabol Drug Interact 21: 187-211.
- Olesen OV, Linnet K (2001) Contributions of five human cytochrome P450 isoforms to the N-demethylation of clozapine in vitro at low and high concentrations. J Clin Pharmacol 41: 823-832.
- Jaquenoud Sirot E, Knezevic B, Morena GP, Harenberg S, Oneda B, et al. (2009) ABCB1 and cytochrome P450 polymorphisms: clinical pharmacogenetics of clozapine. J Clin Psychopharmacol 29: 319-326.
- Okubo M, Narita M, Murayama N, Akimoto Y, Goto A, et al. (2016) Individual differences in in vitro and in vivo metabolic clearances of the antipsychotic drug olanzapine from non-smoking and smoking Japanese subjects genotyped for cytochrome P4502D6 and flavincontaining monooxygenase 3. Hum Psychopharmacol.
- Ishida K, Taira S, Morishita H, Kayano Y, Taguchi M, et al. (2008) Stereoselective oxidation and glucuronidation of carvedilol in human liver and intestinal microsomes. Biol Pharm Bull 31: 1297-1300.
- Leopold G (1986) Balanced pharmacokinetics and metabolism of bisoprolol. J Cardiovasc Pharmacol 8 Suppl 11: S16-20.
- Markowitz JS, Brown CS, Moore TR (1999) Atypical antipsychotics. Part I: Pharmacology, pharmacokinetics, and efficacy. Ann Pharmacother 33: 73-85.
Citation: Gracia MS, Brandl R, Haen E (2017) Lack of Pharmacokinetic Interaction between Antipsychotics (Quetiapine, Clozapine and Olanzapine) and the Beta-Blockers Metoprolol, Propranolol, Bisoprolol and Nebivolol Except between Carvedilol and the Antipsychotics Quetiapine and Clozapine. J Anal Bioanal Tech 8: 387. DOI: 10.4172/2155-9872.1000387
Copyright: © 2017 Gracia MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 8451
- [From(publication date): 0-2017 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 7424
- PDF downloads: 1027