Light/Dark Rhythm of the Pineal Immunomodulating Hormone Melatonin in Autoimmune Diseases and its Relation to Lymphocyte-to-Monocyte Ratio
Received: 17-Oct-2019 / Accepted Date: 02-Nov-2019 / Published Date: 08-Nov-2019
Abstract
It is known that the immune responses are substantially the end-result of interactions between monocytemacrophage and lymphocyte systems, which may be synthetized by the values of lymphocyte-to-monocyte ratio (LMR). The evidence of abnormally low values of LMR values appeared to be the expression of an immunosuppressive status in cancer patients, whereas its significance occurring in the autoimmune diseases is still controversial. Moreover, both lymphocytes and monocytes have appeared to be under a neuroendocrine control. In more detail, lymphocytes are namely stimulated by the pineal hormone melatonin (MLT), and inhibited by cortisol and mu-opioid agonists. On the other hand, the neuroendocrine regulation of macrophage system is more complex, by depending on the subtype of cells macrophage. Cortisol may inhibit M1 and stimulate M2 macrophages, while MLT would inhibit both inflammatory and immunosuppressive actions of macrophages. Moreover, the progressive decline in the pineal function with loss of the physiological light/dark circadian rhythm of MLT represents the main cancer-related endocrine deficiency, while MLT rhythm in autoimmunity has still to be better investigated. This preliminary study was performed to investigate MLT rhythm in autoimmunity. The study included 25 patients with different types of autoimmune pathology, who were investigated during remission phase of their disease. MLT secretion was evaluated by measuring day and night urinary excretion of its main metabolite, the 6-MTS. No patients showed low LMR values. An altered pineal rhythm occurred in 7/25 (28%) patients. LMR mean values were higher in patients with normal MLT rhythm than in those, who showed no MLT rhythm, even though the difference was not statistically significant. These preliminary results study shows the possible occurrence of pineal alterations in autoimmunity, as well as in cancer, whose clinical significance, however, needs to be clarified by monitoring MLT rhythm during the clinical course of the disease. Moreover, this study would seem to exclude the occurrence of low LMR values also in autoimmunity, at least during the remission phase of disease. Therefore, further longitudinal studies, by monitoring patients with autoimmune diseases during both acute and remission phases of their pathology, will be required to establish the clinical and prognostic significance of the alterations of MLT rhythm in relation to the immune status of patients.
Keywords: Autoimmune disease; Chronobiology; Lymphocyte-to- Monocyte ratio; Melatonin; Pineal gland
Introduction
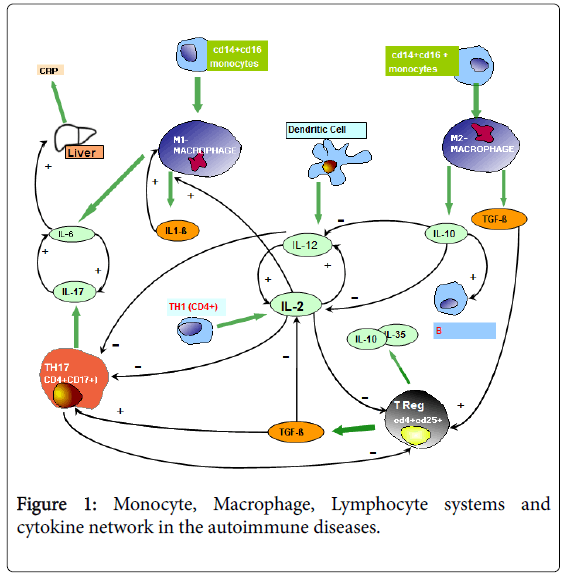
The recent advances in the area of Psycho-neuro-endocrinoimmunology (PNEI) have shown that the immune system is physiologically under a psychoneurondocrine regulatory control, which reflects the psychological and spiritual status of subjects [1]. In more detail, the immune functions are inhibited by corticosteroids and mu-opioid agonists [2], whereas they are stimulated by the pineal hormone melatonin (MLT) [3]. Then, the immune alterations occurring in the systemic diseases, including cancer and autoimmunity, could be due to an altered neuroendocrine control of the immune functions, at least on the onset of disease. MLT may exert several dose-dependent immune effects, namely consisting of stimulation of T helper-1 (TH1) lymphocytes, with a following enhanced IL-2 production, and inhibition of macrophage-mediated inflammatory-immunosuppressive events [3-5], with a following stimulation of the anticancer immunity, which is activated by TH1 lymphocytes, and inhibited by the macrophage system [3-5]. Moreover, the evidence of an abnormally low lymphocyte-to-monocyte ratio (LMR), due to decline in lymphocyte and increase in monocyte counts, has been proven to reflect cancer-related immunosuppression [6], and to predict a worse prognosis in metastatic cancer patients [7]. On the contrary, the prognostic significance of LMR in the autoimmunity is still controversial, since either low or high values have been reported [8-10]. On the same way, the immune-modulatory role of MLT in autoimmunity has still to be better defined. At present, it is known that the functionless of the immune system is based on equilibrium between two major systems, consisting of granulo-monocytemacrophage and lymphocyte systems, which are namely connected by the dendritic cells through the release of IL-12, which stimulates T lymphocyte differentiation into TH1 cells [11]. The main T lymphocyte subsets are represented by TH1 cells (CD4+), cytotoxic T lymphocytes (CD8+), TH17 lymphocytes (CD4+CD17+), which activate the inflammatory response through the release of IL-17 [12], and regulatory T lymphocytes (T reg) ( CD4+CD25+), which in contrast may suppress both TH1 and cytotoxic T lymphocyte functions, while they are inhibited by IL-17 [13]. The classification of monocyte-macrophage subsets is more complex and controversial, even though they are generally subdivided into two main subsets, consisting of M1 and M2 cells [8-10]. M1 and M2 macrophages would originate from the so-called classical monocytes (CD14+CD16-) monocytes, or from the non-classical monocytes (CD4+CD16+), respectively. CD16 antigen corresponds to Fc receptor, while CD14 antigen is only a fragment of Fc receptor [14]. Classical monocytes play a major pro-inflammatory role, whereas non-classical monocytes exerts an anti-inflammatory action. M1 macrophages are activated by gamma-IFN, whereas M2 cells are stimulated by IL-4 and IL-13. Moreover, the differentiation into M1 or M2 macrophages would seem to be induced by GM-CSF and M-CSF, respectively, even though it is more complex [14]. M1 cells play an inflammatory function by secreting several inflammatory cytokines, including IL-1 beta, IL-6, and TNF-alpha. IL-6, whose secretion is stimulated by IL-1 beta itself, promotes CRP production at liver site, as well as the secretion of IL-17 from TH17 lymphocytes. On the contrary, M2 cells play an antiinflammatory action by secreting the two main anti-inflammatory cytokines, IL-10 and TGF-beta [13]. M1 cells may also exert an anticancer cytotoxic activity, but on the other hand the inflammatory cytokines produced by M1 cells may suppress the antitumor immunity. On the same way one, M2 cells, despite their anti-inflammatory activity, may also suppress the anticancer immunity through the secretion of TGF-beta, which constitutes the main endogenous immuno suppressive factor [13]. Then, by considering their overall immune effects, macrophages would exert a major protumoral role. On the contrary, the role of macrophages in the pathogenesis of autoimmune diseases is more complex, because of the opposite effects of the two macrophage subsets on the inflammatory response. Autoimmunity-related alterations of cytokines secretions consist of non-specific increase in the blood levels of both macrophage-related inflammatory cytokines, including IL-1 beta, IL-6, and TNF-alpha [15], with a more evident increase in TNF-alpha levels in the rheumatoid arthritis(RA) [10], and TH17 lymphocyte-derived IL-17 [16], in association with low levels of the anti-inflammatory cytokines IL-10 and TGF-beta [17]. The evidence of low levels of IL-10 and TGFbeta in association with high levels of IL-6 and TNF-alpha would reflect an unbalance within the different macrophage subsets, with M1 hyper-activation in association with a M2 deficiency. The main physiopathological question of autoimmune diseases is that concerning the origin of autoimmunity-related chronic inflammatory status itself, since it could be induced by M1 macrophages, by TH17 lymphocytes [16] or cytokines stimulating TH17 cell functions, such as IL-21 and IL-23 [18], by dendritic cells themselves in the presence of an abnormally high antigen presenting cell activity, with a following potential reaction against auto-antigens, or by TH1 lymphocytes through the release of IL-2, which may activate the whole immune system. On the other side, autoimmunity-related chronic inflammatory status might depend on a diminished T reg cell antiinflammatory immunosuppressive activity. Finally, IL-18, produced by macrophages, endothelial and epithelial cells may also contribute to the pathogenesis of the autoimmune diseases, by enhancing their severity [18]. At present, the main cytokine alteration responsible for the development of an autoimmune reaction is considered to be represented by an increased IL-17 secretion [16]. Then, the pathogenesis of the autoimmunity would by a TH17-dependent phenomenon, because of the inhibitory action of IL-17 on T reg cell generation and activation [13], and its stimulatory effect on the macrophage system [19]. Then, from a clinical point of view, the prognosis of an autoimmune disease is better in the presence of high levels of TGF-beta and IL-10, in association with normal values of IL-6, TNF-alpha and IL-17. The interactions among monocytes, macrophages, lymphocytes and cytokine secretions are illustrated in Figure 1.
As far as the immune effects of MLT in the autoimmunity are concerned, because of its concomitant immune-stimulatory and antiinflammatory effects [3,4], it could either to improve, or to worse the clinical course of the autoimmune diseases, by potentially exerting both positive and negative effects, whereas it has been proven to determine only benefits in cancer cure [20]. MLT may be useful in the treatment of the autoimmunity by inhibiting prostaglandin production and the secretion of macrophage inflammatory cytokines, including IL-6, IL-1 beta and TNF-alpha [21], as well that of IL-17 through its connection with brain endocannabinoid system [22-24], but on the other hand the immune-stimulatory activity of MLT, namely due to a stimulation of IL-2 and IL-12 secretions [3,25], could amplify the severity of autoimmune processes. In any case, it has to be remarked that the role of IL-2 and IL-12 in autoimmunity is still controversial, since either low or high levels have been described [26,27]. In addition, both IL-2 and IL-12 could exert both positive and negative effects on the autoimmune processes, and this evidence is not surprising, since IL-2 may promote the autoimmunity by stimulating TH1 and M1 macrophage functions, but on the same way it may prevents the autoimmunity by stimulating T reg cell generation [26] and inhibiting IL-17 secretion [28], as suggested by preliminary studies. On the same way, IL-12 may exert negative effects on the autoimmune processes due to the stimulation of TH1 differentiation, and to the inhibition of T reg functions, but it could also exert some benefits by inhibiting IL-17 secretion [29,30]. According to preliminary clinical studies, MLT would seem to improve the clinical course of Systemic Lupus Erythematosus (SLE), bowel inflammatory diseases, psoriasis, multiple sclerosis (MS) [31] whereas it could worsen the symptomatology of the RA [32]. Moreover, preliminary clinical studies have also shown an altered light/dark circadian secretion of MLT in the autoimmune diseases, by suggesting the occurrence of an autoimmunity-related altered pineal function [33,34]. Because of the important immunemodulatory properties of MLT, autoimmunity-related pineal alterations could play a role in the pathogenesis of auto-immmunity itself. On these bases, a preliminary study was planned to evaluate the light/dark rhythm of MLT in a group of patients affected by autoimmune diseases in relation to the inflammatory and immune status of patients, as assessed by evaluating LMR values [5-7].
Patients and Methods
The study included 25 consecutive patients affected by autoimmune diseases (M/F: 6/19; median age: 48 years, range 17-68), who were investigated during the remission phase of their disease. According to the common laboratory analyses, the diagnosis of autoimmune disease was, as follows: RA: 4; polymyalgia: 4; SLE: 3; Hashimoto’s thyroiditis: 3; bowel inflammatory diseases: 3; mixed connective tissue disease: 2; multiple sclerosis: 2; psoriasis: 2; Sjogren’s syndrome: 1; scleroderma: 1. No patient was under therapy with corticosteroids or other immunosuppressive agents during the study. The control group consisted of 50 sex- and age-matched healthy subjects. The pineal function was evaluated by determining the light and night urinary excretion of the main MLT metabolite, the 6-sulphatoxy-melatonin (6-MTS), and light and night 6-MTS concentrations were analyzed by an enzyme immunoassay and commercially available kits (Melatonin sulphate Urine – ELISA IBL International GMBH/ Tecan Group Company), and the values were reported as mcg/ml. Urine were collected in two different tubes during the day ( from 8 AM to 8 PM) and during the night (from 8 PM to 8 AM). The circadian rhythm of MLT was considered to be normal when 6-MTS night values were at least two times greater than those found during the light phase of the day. As far as LMR values are concerned, normal values observed in our laboratory (95% confidence limits) were, as follows: lymphocyte count above 1,500/mm3, monocyte count below 500/mm3, LMR greater than 2.1.
Results
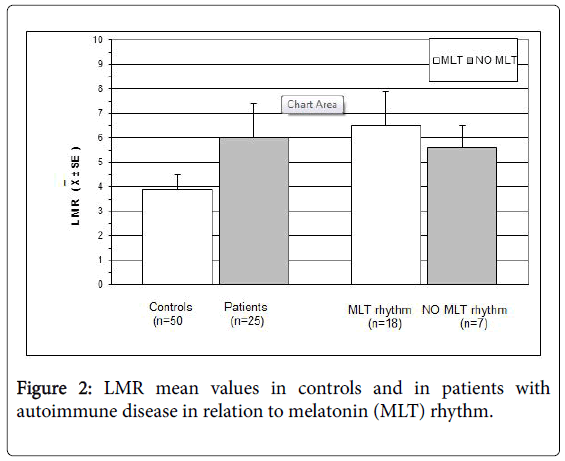
A normal 6-MTS increase in the night was present in 18/25 (72%), whereas the remaining 7 patients (28%) showed an altered pineal circadian rhythm, without any apparent relation to the type of autoimmune pathology( SLE: 1; RA: 1; polymyalgia: 2; multiple sclerosis:1; psoriasis: 1; scleroderma: 1). The alterations of MLT rhythm consisted of lack of the physiological light/dark circadian rhythm in 5 patients, and inverted rhythm, with higher 6-MTS values during the day than during the night, in the remaining 2 cases. Moreover, no patient presented lymphocytopenia. Monocyte count was within the normal range in 22/25 (88%), and abnormally high in the remaining 3 patients (12%). On the same way, no patient showed an abnormally low LMR, whose mean values were higher in patients than in controls, without, however, statistically significant differences. Finally, LMR mean values were higher in patients with a normal circadian rhythm of MLT than in those with an altered MLT rhythm, even though the difference was not significant. LMR mean values observed in patients and in controls are illustrated in Figure 2.
Discussion
According to previous clinical investigations [26,27], this preliminary study, carried out to investigate the pineal function in the autoimmunity and its possible influence on autoimmunity-related immune alterations, seems to confirm the occurrence of an altered pineal activity in autoimmune diseases. Obviously, the too low number of patients does not allow us to establish which relation may occur between the pineal function and specific autoimmune pathology. Moreover, it has to be remarked that all patients with autoimmune diseases considered in the present study were investigated during the only remission phase of their diseases. Therefore, longitudinal studies, by monitoring MLT circadian seretonin relation to the clinical course of patients during both phases of recurrence and remission, will be required to establish the possible clinical and prognostic significance of autoimmunity-related pineal alterations. At present, however, it is not possible to establish whether pineal alterations may precede and predispose to the onset of autoimmune diseases a consequence of an altered neuroendocrine control of cytokine secretions, or whether they simply represent the effect of autoimmunity-related abnormal production of inflammatory cytokines, which has appeared to influence the pineal endocrine activity [34]. Moreover, as far as LMR profile in autoimmunity is concerned, in agreement with previous results of other authors [8], this study would suggest that LMR values may be normal or greater than those found in the healthy controls [8]. In any case, no conclusion may be drawn on the significance of LMR values in patients with autoimmune diseases without a comparison between acute and remission phases of the pathology, as suggested by preliminary clinical studies, which have shown that the evidence of lymphocytopenia and low LMR values are associated with a worse prognosis in autoimmune diseases [10]. Then, an evident decline in LMR values in the autoimmune diseases, as well as in metastatic tumors, would occur during the only exacerbation phases of disease. It would seem to be surprising the fact that the evidence of LMR low values may represent a negative prognostic marker for both autoimmunity and cancer, which are characterized by opposite immune conditions, consisting of immunosuppression in cancer and immune hyper-activation in autoimmunity. Cancer-related decrease in LMR would be mainly due to a decline in lymphocyte count, with a following lymphocytopenia, which constitutes the main biomarker of cancer-related immune-suppression, whereas LMR decline occurring in the autoimmune diseases would be mainly due to a progressive increase in monocyte count [10].
Conclusion
In conclusion, longitudinal studies, by evaluating changes in LMR ratio during the clinical course of the autoimmune diseases in relation to changes in MLT circadian secretion, will be necessary to better define the prognostic significance of LMR as a possible biomarker of the autoimmune pathology and the possible role of the pineal gland in autoimmunity-related immune alterations.
References
- Riley V (1981) Psychoneuroendocrine influences on immunocompetence and neoplasia. Science 212: 1100-1109.
- Manfredi B, Sacerdote P, Bianchi M, Locatelli L, Veljic-Radulovic J, et al. (1993) Evidence for an opioid inhibitory tone on T cell proliferation. J Neuroimmunol 44: 43-46.
- Maestroni GJM (1993) The immunoneuroendocrine role of melatonin. J Pineal Res 14: 1-10.
- Lissoni P, Messina G, Tantarelli R, Lissoni A, Tantarelli O, et al. (2017) The psychoneuroimmunotherapy of human immune-mediated systemic diseases, including cancer and autoimmune diseases. J Mol Oncol Res 1: 7-13.
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454: 436-444.
- Lissoni P, Rovelli F, Vigoré L, Lissoni A, Di Fede G (2018) Low lymphocyte-to-monocyte ratio is associated with an enhanced regulatory T lymphocyte function in metastatic cancer patients. Int J RecAdv Multi Res 5: 3353-3356.
- Gu L, Li H, Chen L, Ma X, Li X, et al. (2016) Prognostic role of lymphocyte-to-monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 7: 31926–31942.
- Burbano C, Vasquez G, Rojas M (2014) Modulatory effects of CD14+CD16++ monocytes on CD14++CD16- monocytes: A possible explanation of monocyte alterations in systemic lupus erythematosus. Arthritis Rheum 66: 3371-3381.
- Cherfane CE, Gessel E, Cirillo D, Zimmerman MB, Polyak S (2015) Monocytosis and a low lymphocyte-to-monocyte ratio are effective biomarkers for ulcerative colitis disease activity. Inflamm Bowel Dis 21: 1769-1775.
- Du J, Chen S, Shi J, Zhu X, Ying H, et al. (2017) The association between the lymphocyte-to-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol 36: 2689-2695.
- Banks RE, Patel PM, Selby PJ (1995)Interleukin-12: A new clinical player in cytokine therapy. Br J Cancer 71: 665-659.
- Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, et al. (2008) Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29: 628-636.
- Zou W (2006) Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol 6: 295-307.
- Mills CD (2012) M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 32: 463-488.
- Arican O, Arat M, Sasmaz S, Ciragil P (2005) Serumlevels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 24: 273-279.
- Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK (2006) Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med 203: 2785-2791.
- Ciric B, El-behi M, Cabreara R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17 producing CD8+ T cells. J Immunol 182: 5296-5305.
- Dinarello A, Kaplanski G (2005) Interleukin-18 treatment options for inflammatory diseases. Expert Rev Clin Immunol 1: 619-632.
- Bettelli E, Carrier y, Gao W, Korn T, Strom TB, et al. (2006) Reciprocal development pathways in the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235-238.
- Bartsch C, Bartsch H (1999) Melatonin in cancer patients and in tumor-bearing animals. Adv Exp Med Biol 467: 274-264.
- Raghavendra V, Singh V, Shajia AV, Vohra H, Kilkarni SK, et al. (2001) Melatonin provides signal 3 to unprimed cells CD4+ T cells, but failed to stimulate LPS primed B cells. Clin Exp Immunol 124: 414-422.
- Lissoni P, Messina G, Cenaj V, Rovelli F, Porro G, et al. (2018) The role of IL-17 secretion in mediating the influence of stress on cancer and other human systemic diseases. MOJ Immunol 2: 31-34.
- Lissoni P, Resentini M, Mauri R, Esposti D, Esposti G, et al. (1986) Effects of tetrahydrocannabinol on melatonin secretion in man. Horm Metab Res 18: 77-78.
- Grothenermen F (2004) Pharmacology of cannabinoids. Neuro Endocrinol Lett 25: 14-23.
- Garcia-Murino S, Pozo D, Carrillo-Vico A, Calvo JR (1999) Melatonin activates Th1 lymphocytes by increasing IL-12 production. Life Sci 65: 2143-2150.
- Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, et al. (2005) Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+ CD25+ regulatory T cells. Nat Med 11: 1238-1240.
- Tokano Y, Morimoto S, Kaneko H, Amano H, Nozawa K, et al. (1999) Levels of IL-12 in the sera of patients with systemic lupus erythematosus (SLE). Relation to Th1-Th2-derived cytokines. Clin Exp Immunol 116: 169-173.
- Lissoni P (2017) Therapy implications of the role of interleukin-2 in cancer. Exp Rev Clin Immunol 13: 491-498.
- Craig A. Murphy, Claire L. Langrish, Yi Chen, Wendy Blumenschein, Terrill McClanahan, Robert A. Kastelein, Jonathon D. Sedgwick, Daniel J. Cua
- Lin GJ, Huang SH, Chen SJ, Wang CH, Chang DM, et al. (2013) Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases. Int J Mol Sci 14: 11742-11766.
- Chen Q, Wei W (2002) Effects and mechanisms of melatonin on inflammatory and immune responses of adjuvant arthritis rat. Int Immunopharmacol 2: 1443-1449.
- West SK, Oosthuizen JM (1992) Melatonin levels are decreased in rheumatoid arthritis. J Basic Clin Physiol Pharmacol 3: 33-40.
- Melamud L, Golan D, Luboshitzky R, LaviI, Miller A (2012) Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci 314: 37-40.
- Lissoni P (1999) Pineal as a central regulator of cytokine network. Neuro Endocrinol Lett 20: 343-349.
Citation: Lissoni P, Porro G, Rovelli F, Messina G, Cenaj V, et al. (2019) Light/Dark Rhythm of the Pineal Immunomodulating Hormone Melatonin in Autoimmune Diseases and its Relation to Lymphocyte-to-Monocyte Ratio . J Mucosal Immunol Res 3: 113.
Copyright: © 2019 Lissoni P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3267
- [From(publication date): 0-2019 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 2365
- PDF downloads: 902