Limbic Encephalitis Caused By Neurospyhilis in a HIV-Positive Male: Case Report and Review
Received: 11-Apr-2014 / Accepted Date: 04-Jun-2014 / Published Date: 12-Jun-2014
Abstract
Neurosyphilis can present with a wide range of different presentations. For this reason, it has been called the “the great imitator”. Limbic encephalitis mimicking HSV encephalitis is an under recognized manifestation of neurosyphilis. Co-infection with HIV may alter the course of neurosyphilis and cause more atypical and aggressive presentations to occur. The author presents a case of mesial temporal encephalitis caused by neurosyphilis in a man with concomitant HIV infection. A review of the literature shows that this is not a sparse presentation of neurosyphilis. The pathophysiology and clinical implications are reviewed.
Keywords: Syphilis; Neurosyphilis; Encephalitis; HIV; PLEDs; Mesial temporal lobe; Limbic; Amnesia
411787Introduction
Case
A 51-year-old man presented with a 3-week history of personality changes, progressive amnesia, and bitemporal headaches. On admission, his vital signs and general physical examination were normal. However, he appeared to be experiencing delusions and visual hallucinations. His short term memory was severely impaired and he required frequent reorientation. He confabulated and claimed to have known each of the medical staff at one point in his life. His speech was otherwise fluent and he was able to follow 3-step complex commands. Neurological exam showed impairments in recall, visuospatial, and executive functions. The rest of his examination was normal.
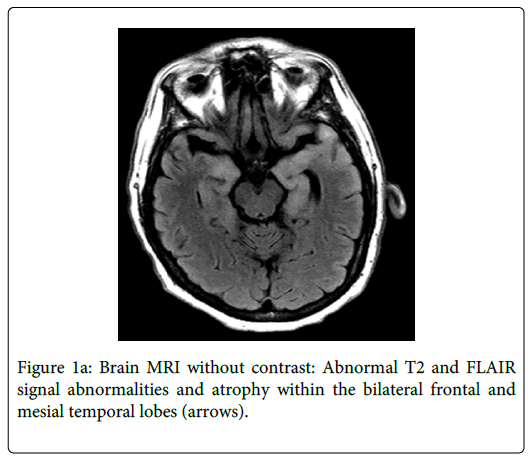
Brain MRI showed increased T2 signal within the bilateral frontal and mesial temporal lobes suspicious for encephalitis (Figure 1a). A lumbar puncture (LP) revealed a cerebrospinal fluid (CSF) glucose of 48 mg/dL, erythrocytes 3/mL, leukocytes 220/mL (lymphocytes 69%, neutrophils 11%, monocytes 20%), and CSF protein of 205 mg/dL. He was started empirically on intravenous acyclovir. CSF analysis for herpes simplex virus (HSV), West Nile virus, and other infectious testing were negative. Malignancy workup including CSF cytology and paraneoplastic antibodies was also negative.
The patient’s wife noted that he was recently engaged in promiscuous sexual behavior. Further testing for syphilis was positive as evidenced by reactive serum rapid plasma reagent (RPR) titer of 1:64), serum and CSF treponema pallidum haemagglutination assay (TPHA) titers were 1:5120 and 1:640, respectively. CSF Venereal Disease Research Laboratory (VDRL) titer was 1:16. The fluorescent treponemal antibody-absorption (FTA-ABS) test also came back positive. Acyclovir was discontinued and he was started on a 21 day course of penicillin G. Further serologic workup revealed that he was HIV-positive with a viral load of 5140 copies and a CD4-lymphocyte count of 396. The CD4 percent was 24% and the CD4/CD8 ratio was 0.6.
His hospital stay was complicated by recurrent generalized tonic-clonic seizures requiring intubation and treatment with Fosphenytoin. Electroencephalogram (EEG) showed independent bitemporal periodic lateralized epileptiform discharges (PLEDs). He was successfully extubated and a repeat LP performed ten days into the course of antibiotic therapy showed marked decrease in the amount of CSF protein (80 mg/dL) and leukocytosis (34/mL).
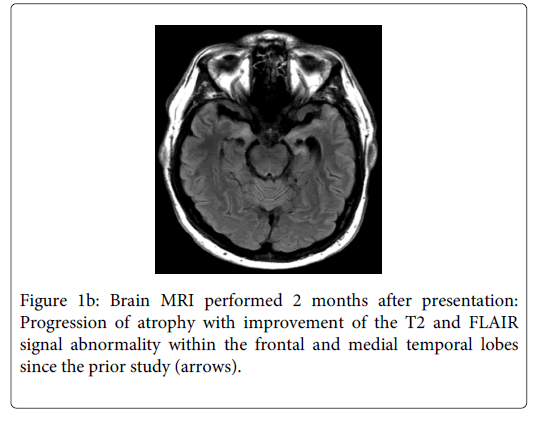
Prior to his hospital discharge, he was started on highly active antiretroviral therapy with tenofovir, efavirenz, and emtricitabine. Six weeks later during outpatient follow-up, he was able to hold a complex conversation. However, he continued to demonstrate severe short-term memory and confabulated about activities he had done earlier in the day and what he had for breakfast. Repeat MRI of the brain showed improvement of the T2 and FLAIR signal abnormality within the frontal and mesial temporal lobes (Figure 1b). However, there was progression of atrophy of the mesial temporal lobes suggesting irreversible damage. At 6 months follow-up, the patient had a repeat LP that showed no pleocytosis, CSF-protein of 42 mg/dL, and CSF-VDRL was nonreactive. The CD4 count had increased from 396 to 625 and CD4 percent increased from 24% to 42%. The HIV viral load was undetectable.
Discussion
Neurosyphilis refers to infection of the central nervous system (CNS) by the spirochete Treponema pallidum. This may occur anytime within weeks of the initial inoculation to years during the late stage of tertiary syphilis. If it occurs acutely, it may be latent and asymptomatic or may present as meningitis, strokes, vertigo, optic neuritis, uveitis, or more rarely as basilar meningitis with cranial neuropathies. The pathophysiology involves an acute meningovascular and ocular inflammation caused by small vessel arteritis [1-3].
More commonly, neurosyphilis occurs many years later in the tertiary stage of syphilis manifested by general paresis and tabes dorsalis. In general paresis, chronic infection of the brain parenchyma causes widespread parenchymal damage leading to forgetfulness and personality changes [1]. Tabes dorsalis is caused by demyelination of the posterior columns, dorsal roots, and dorsal root ganglia leading to impaired proprioception and gait imbalance.
Due to its frequent atypical presentations, syphilis has been commonly called "the great imitator”. The case presented describes neurosyphilis mimicking herpes simplex virus (HSV) encephalitis. The subacute presentation of altered mental status, bilateral mesiotemporal T2 hyperintensity on MR images, and temporal PLEDs on EEG have always been thought of as pathognomonic signs for herpes encephalitis. Other uncommon and rare entities have been reported to have similar imaging findings including paraneoplastic limbic encephalopathy, lupus erythematosus, Hurst hemorrhagic leukoencephalitis, and gliomatosis cerebri [4]. An extensive review of the literature demonstrates 29 cases worldwide with mesial temporal lobe encephalitis as a rare manifestation of neurosyphilis [4–26] (Table 1). All the patients presented similarly with confusion, memory disturbance, or change in personality. Scheid et al. and other authors listed in Table 1 recommend including neurosyphilis in the differential diagnosis of limbic encephalitis [25].
| Case | Year | Patient Age/sex | Symptoms before diagnosis | Labs and HIV status | MRI Findings | Clinician and or MRI Follow up |
|---|---|---|---|---|---|---|
| Bash et al. [4] | 2001 | 50 M | 3 months | CSF cells : 19 WBC/uL, (93% lymphocytes) CSF Glucose 45 mg/dL CSF protein 87 mg/dL Serum RPR titer 1:64 MHA-TP: Positive CSF -VDRL titer 1:16 FTA-ABS: Positive HIV: UK |
Cortical and subcortical increased T2-FLAIR signal in the bilateral mesial temporal region. There was also mild bilateral temporal lobe atrophy. | Four months later, the patient had significant cognitive and radiographic improvement. MRI showed improvement in the previously noted mesial temporal FLAIR and T2 lesions. |
| Elisa et al. [5] | 2011 | 43 M | 1 week | CSF cells: 26.4 WBC/uL, (mostly lymphocytes) CSF protein 92.9?mg/dL Serum RPR titer 1:256 Serum TPHA titer 1:20480 CSF-TPHA titer 1:10240 CSF-VDRL titer 1:32 HIV ½ Ab: Negative |
Asymmetrical bilateral cortical and subcortical increased T2- FLAIR signal in the mesiotemporal region and insula. Atrophy involving the bilateral mesiotemporal region. | 2 weeks after treatment, repeat brain MRI showed extension the T2- FLAIR hyperintensities in the left mesiotemporal region and insula. |
| Denays et al. [6] | 1999 | 51 F | Acute | CSF cells: 23 WBC/uL (80% lymphocytes). CSF protein 46 mg/dL CSF glucose: normal |
Bilateral mesiotemporal lesions, predominantly on the left side | Prior to discharge, there was a marked improvement of memory and regression of MRI lesions. She was able to return to work. |
| PHA titer 1:40960 FTA-ABS titer 1:12800 HIV: UK |
||||||
| Angus et al. [7] | 1998 | 34 M | UK | UK | UK | UK |
| Szilak [8] | 2001 | 55 M | Acute | CSF cells: 79 WBC/uL (93% lymphocytes) CSF protein 71 mg/dL CSF glucose 65 mg/dL. Serum treponemal IgG titer 1 : 64 (Confirmed by microhemagglutination assay). HIV ½ Ab: Negative |
High signal in the left temporal lobe on T2- FLAIR sequences and white matter abnormalities. | Within 1 week of therapy, the patient was oriented and could hold a complex conversation, although short-term memory deficits remained. |
| Bousende et al. [9] | 2012 | 43 M 45 M |
UK | Lab results were noted to be compatible with neurosyphilis (TPHA and VDRL) HIV: UK |
Increased signal in the temporal lobes, amygdala, hippocampus, and insula. | UK |
| Omer et al. [10] | 2012 | 55 M | 6 months | CSF cells: 16 WBC/µL (100 percent mononuclear cells) CSF protein 77mg/d CSF glucose was normal Gram stain was negative. Serum and CSF were noted to have positive TPHA. HIV serology was negative. |
High T2 signal intensity and atrophy of the right frontal and bilateral mesial temporal areas. | Three months later, the patient had improved cognitive function. A repeat MRI showed marked improvement in the previously identified bilateral hyperintensities, with residual atrophy. |
| Fadil et al. [11] | 2006 | UK | UK | UK, noted to have active syphilitic infection | Compatible with mesial temporal sclerosis. | UK |
| Agayeva et al. [12] | 2013 | 51 M | 4 months | Treponemal antibody tests were noted to be positive in serum and CSF. HIV: UK |
Hyperintensity of bilateral mesial temporal structures, insula, and thalami with restricted diffusion. | Six months later, there was improvement in behave iour and memory. |
| Gaud et al. [13] | 2011 | UK | UK | UK, diagnosed with neurosyphilis. | Left mesiotemporal lesions. | UK |
| Jeong et al. [14] | 2008 | 35 M | 1 month | CSF cells: 48 WBCs/uL (92% lymphocytes) CSF protein 88 mg/dL Gram stain negative. CSF-VDRL titer 1:8 FTA-ABS: positive HIV: UK |
High signal changes in the bilateral mesial temporal lobes including both hippocampi and amygdalae on T2- FLAIR sequences. | One month later, noted to have improved cognitive function. |
| Hama et al. [15] | 2008 | 51 M | 12 months | CSF cells: 21 WBCs/µL CSF protein 55 mg/dL CSF glucose 67 mg/dL Serum TPHA : 80,220 U/mL FTA-ABS: positive CSF-TPHA: 4,332 U/mL HIV ½ Ab: Negative |
Cortical and subcortical high T2-FLAIR signal intensity areas in both mesial temporal regions and the right frontal lobe. | 2 months later, there was improved but residual cognitive dysfunction and MRI showed regression of the abnormal signal intensity in the temporal lobes. |
| Chen et al. [16] | 2005 | 52 M | 9 months | CSF cells: 8 WBCs/uL CSF protein 60 mg/dl CSFGlucose 67 mg/dl | High intensity lesions in bilateral mesial | 2 weeks after treatment, the patient became orientated, but still had short-term memory impairment. |
| Serum TPHA titer 1:20480 RPR titer 1: 64 CSF-VDRL: positive HIV: UK |
temporal regions and cortical atrophy without post-contrast enhancement | Two months later, a follow-up MRI was greatly improved. | ||||
| Santos et al. [17] | 2005 | 73 M | 10 days | CSF: 14 WBCs/uL, (94% monocytes) CSF protein 89 mg/dL CSF glucose 64 mg/dL Serum TPHA: positive CSF-VDRL titer 1:160 HIV: UK |
Increased T2-FLAIR cortical and subcortical hyperintensities in the right mesial temporal region, cingulated gyrus, septum, and insula. | 17 days after the patient had been treated with penicillin, MRI showed more circumscribed lesions and focal cerebral atrophy. |
| Marano et al. [18] | 2004 | 48 M | 9 hours | CSF cells: 12 WBCs/ uL CSF protein 63 mg/dL CSF glucose was normal CSF and Serum VDRL and TPHA were all noted to be positive. HIV ½ Ab: Negative |
Hyperintense cortical lesion in the right temporal and basal frontal lobes. |
UK |
| Radhakrishnan et al. [19] | 1984 | 32 M | 6 weeks | CSF cells: 23 WBCs/uL CSF protein 190mg/dL Serum VDRL titer 1:160 CSF-VDRL titer 1:80 FTA-ABS: positive at 1:320 HIV: UK |
MRI was unavailable. Head CT scan showed an area of decreased density involving left parietal cortex and slight ventricular dilation. |
One month later, there was improvement in speech but mood was highly labile, and the patient remained confused with profound loss of memory and receptive aphasia. |
| Yao et al. [20] | 2010 | 42 M | 9 months | CSF cells: 38 WBCs/uL (lymphocytes 70%) CSF protein 80 mg/dL CSF glucose was normal Gram stain was negative Syphilis TRUST titer 1:64 Serum TPHA: positive HIV serology was negative. |
Cerebral edema and hyperintensities involving the right parietal, occipital and temporal lobes |
1-year follow-up, the patient was deemed normal in terms of neurological examination. MRI showed that the hyperintensity had disappeared. |
| Saunderson [21] | 2012 | UK | UK | UK | Reported to have mesial temporal changes. | UK |
| Xiang et al. [22] | 2013 | 43 M 30 M 45 M 53 M 39 M 46 M |
5 days 7 days
4 years |
All 6 were tested positive for TPHA, RPR, and antibodies against syphilis in the serum and CSF. HIV: UK |
MRI in all the cases revealed T2-FLAIR hyperintensities of the unilateral or bilateral mesial temporal lobes, including the hippocampi. | Patient prognoses were good in 4 that received early anti- syphilis treatment, but 2 that received delayed treatment due to misdiagnoses did not see substantial symptomatic improvements. |
| Derouich [23] | 2013 | 50 M | UK | UK | Mesial temporal changes on MRI | UK |
| Hagiwara [24] | 2014 | 48 M | UK | Serum HIV serology was negative. |
||
| Scheid [25] | 2005 | 34 M | UK | CSF cells 22 WBCs/uL CSF protein 95 mg/dL Serum VDRL titer 1:8 CSF-VDRL titer 1:4 Serum TPHA titer 1:81920 CSF-TPHA titer 1:524288. HIV ½ Ab: negative. |
T2-FLAIR hyperintense signal alteration in the left medial temporal lobe. |
After 8 months, there was still cognitive Slowing and impaired a memory. Repeat MRI showed atrophy of left medial temporal lobe. |
| Lessig et al. [26] | 2006 | 57 M | 4 months | CSF 70 WBCs/uL | Bilateral FLAIR hyperintensities in the temporal lobes and frontal tips without gadolinium enhancement, and diffuse atrophy. | The patient required further treatments for relapse after 2, 6, and 10 months. Repeat MRI of the brain at 6 months showed no improvement. |
| (90% lymphocytes) | ||||||
| CSF protein 157 mg/dL | ||||||
| CSF glucose 44 mg/dL | ||||||
| CSF-VDRL titer 1:8 | ||||||
| Gram stain was negative | ||||||
| Serum RPR titer 1:128 | ||||||
| HIV serology was negative. |
Table 1: M: male; F: female; CSF: cerebrospinal fluid; UK: data unknown or unavailable; WBC: white blood cell; VDRL: Venereal Disease Research Laboratory; RPR: Rapid plasma reagin; TPHA: Treponema pallidum haemagglutination assay; microhemagglutination assay for ` Treponema pallidum antibodies (MHA-TP); FTA-ABS: fluorescent treponemal antibody-absorption; TRUST: Toluidine Red Untreated Serum Test.
In addition to serum and CSF laboratory testing, imaging features may help to differentiate between this rare presentation of neurosyphilis and the more common HSV encephalitis. Neurosyphilis may be associated more with atrophy of the medial temporal lobe. On the other hand, gyral enhancement, cortical and subcortical edema, hemorrhage, or areas of restricted diffusion are frequently described in HSV infection [5].
It is unknown what causes the T2 hyperintensity changes in the mesial temporal lobes of patients with this atypical form of neurosyphilis. It is thought that small-vessel ischemic changes and marked meningovascular inflammation cause vasogenic, cytotoxic, and interstitial edema to occur causing parenchymal damage [4]. Another opinion is that abnormal signals are probably related to proliferation of glial cells rather than cytotoxic edema (Wang X). Coinciding seizures may also play a role in causing signal changes of the mesial temporal lobes [25]. In a study examining brain MRI abnormalities in 15 patients with general paresis, abnormal high T2-FLAIR signals were found in nine cases, mainly in the bilateral temporal, insular, and frontal lobes and hippocampus accompanied by cerebral and/or hippocampus atrophy [27]. It is yet to be determined whether mesial temporal encephalitis is part of general paresis or its own distinct manifestation of neurosyphilis.
It is unlikely that the HIV co-infection was an innocent bystander in the case described. The association between syphilis and HIV infection is an important one because both can often present simultaneously [1,28,29]. The genito-ulcerative chancres seen in syphilis increase the risk for viral entry during unprotected sexual intercourse [1]. Also, the increased concentration of T cells at the site of the chancres augments the possibility of the HIV virus infecting and replicating in the host cell [1]. Studies of patients with syphilis co-infected with HIV have described persistent chancres, ulcerative skin lesions, rapid progression to gummatous disease with destructive lesions of the skin, bones or viscera, and a greater frequency of ocular involvement (eg, uveitis, keratitis, optic neuritis, optic atrophy, or chorioretinitis) [30].
Co-infection with HIV may increases the incidence of the neurological complications of syphilis. Neurosyphilis should be considered in the differential diagnosis of any patient with HIV [31]. It has been reported that the incidence of symptomatic neurosyphilis among HIV positive individuals with early syphilis was found to be 3–4 times higher (2.1% vs 0.6%) as compared with HIV-negative persons [32]. In another study, the incidence of neurosyphilis was shown to be 23.5% in HIV-positive patients with untreated syphilis as compared to 10% in HIV-negative patients with untreated syphilis [28].
More so, in HIV positive patients, neurosyphilis may progress in an aggressive and often atypical manner [1,28,29]. It is believed that co-infection with HIV may have potentiating effects on the syphilitic infection [28,29,33]. A more fulminant form of parenchymal disease, referred to as necrotizing neurosyphilis, has also been reported to occur in patients with HIV [34]. None of the cases reports listed in Table 1 were documented as being associated with a concomitant HIV infection. This is the first reported case of neurosyphilis limbic encephalitis in a patient with HIV. It is unknown whether HIV increases the risk of mesial temporal encephalitis in patients with neurosyphilis.
It is important to note that HIV positive patients with neurosyphilis are at least 2.5 times less likely to normalize the CSF-VDRL reactivity after penicillin treatment. This risk is even higher if the CD4+ T-cell count is <200 cells/uL [35]. Thus, the Centers for Disease Control and Prevention treatment guidelines recommend that HIV positive patients with neurosyphilis undergo routine CSF examination every 3–6 months until the cell count normalizes [33,36].
Prognosis of limbic encephalitis due to neurosyphilis varies. Among the 18 patients listed in Table 1 with follow-up data, 11 showed mild to moderate clinical and or radioraphical improvement following antibiotic therapy, 1 required retreatment for recurrent relapses, and 6 had no improvement or worsening of their disease. Early diagnosis and treatment has been shown to improve outcome [22].
Conclusion
Neurosyphilis continues to surprise us with atypical and new presentations. A review of the literature argues that it should be considered in the initial diagnostic workup of limbic encephalitis and not just a mere diagnosis of exclusion. Concomitant HIV infection is common and may change the presentation of neurosyphilis. Clinicians need to be aware of the different manifestations so that early treatment can potentially decrease morbidity in this subgroup of patients.
References
- Tyler KL, Martin JB (1993) Neurosyphilis. In: Tyler KL, Martin JB, ed. Infectious Diseases of the Central Nervous System. Chapter 11. Philadelphia: FA Davis; 250.
- Kent ME, Romanelli F (2008) Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother 42: 226-236.
- Smith MM, Anderson JC (2000) Neurosyphilis as a cause of facial and vestibulocochlear nerve dysfunction: MR imaging features. AJNR Am J Neuroradiol 21: 1673-1675.
- Bash S, Hathout GM, Cohen S (2001) Mesiotemporal T2-weighted hyperintensity: neurosyphilis mimicking herpes encephalitis. AJNR Am J Neuroradiol 22: 314-316.
- Elisa V, Ana FG, Rita R, Sofia R, Alice R, et al. (2012) Neurosyphilis versus Herpes Encephalitis in a Patient with Confusion, Memory Loss, and T2-Weighted MesiotemporalHyperintensity. Case reports in infectious diseases
- Denays R, Collier A, Rubinstein M, Atsama P (1999) A 51-year-old woman with disorientation and amnesia. Lancet 354: 1786.
- Angus F, Maysuria H, Bryan CS (1998) Neurosyphilis mimicking herpes simplex encephalitis. J S C Med Assoc 94: 315-317.
- Szilak I, Marty F, Helft J, Soeiro R (2001) Neurosyphilis presenting as herpes simplex encephalitis. Clin Infect Dis 32: 1108-1109.
- Bousende M, Cravo I, Lopes L, Gonçalves C, Valverde A, et al. (2012) [Mesial-temporal lesions in patients with neurosyphilis]. Acta Med Port 25 Suppl 1: 64-68.
- Omer TA, Fitzgerald DE, Sheehy N, Doherty CP (2012) Neurosyphilis presenting with unusual hippocampal abnormalities on magnetic resonance imaging and positron emission tomography scans: a case report.J Med Case Rep 6: 1-4
- Fadil H, Gonzalez-Toledo E, Kelley BJ, Kelley RE (2006) Neuroimaging findings in neurosyphilis. J Neuroimaging 16: 286-289.
- Agayeva N, Karli-Oguz K, Saka E (2013) Teaching NeuroImages: a neurosyphilis case presenting with atypical neuroradiologic findings. Neurology 80: e119.
- Gaud S, Sauvée M, Muresan M, Gospodaru N, Foscolo S, et al. (2011) [Left mesiotemporal lesions and anterograde memory impairement: a case of neurosyphilis]. Rev Neurol (Paris) 167: 833-836.
- Jeong YM, Hwang HY, Kim HS (2009) MRI of neurosyphilis presenting as mesiotemporal abnormalities: a case report. Korean J Radiol 10: 310-312.
- Hama K, Ishiguchi H, Tuji T, Miwa H, Kondo T (2008) Neurosyphilis with mesiotemporal magnetic resonance imaging abnormalities. Intern Med 47: 1813-1817.
- Chen CW, Chiang HC, Chen PL, Hsieh PF, Lee YC, et al. (2005) General paresis with reversible mesial temporal T2-weighted hyperintensity on magnetic resonance image: a case report. ActaNeurol Taiwan 14: 208-212.
- Vieira Santos A, Matias S, Saraiva P, Goulão A (2005) Differential diagnosis of mesiotemporal lesions: case report of neurosyphilis. Neuroradiology 47: 664-667.
- E Marano, F Briganti, F Tortora, A Elefante, A De Rosa,et al. (2004) Neurosyphilis with complex partial status epilepticus and mesiotemporal MRI abnormalities mimicking herpes simplex encephalitis. J NeurolNeurosurg Psychiatry 75: 833-833
- Radhakrishnan K, Ashok PP, Sridharan R, El-Mangoush MA (1984) Periodic EEG pattern in meningovascular syphilis. J NeurolNeurosurg Psychiatry 47: 1360-1361.
- Yao Y, Huang E, Xie B, Cheng Y (2012) Neurosyphilis presenting with psychotic symptoms and status epilepticus. NeurolSci 33: 99-102.
- Saunderson RB, Chan RC (2012) Mesiotemporal changes on magnetic resonance imaging in neurosyphilis. Intern Med J 42: 1057-1063.
- Xiang T, Li G, Xiao L, Chen S, Zeng H, et al. (2013) Neuroimaging of six neurosyphilis cases mimicking viral encephalitis. J NeurolSci 334: 164-166.
- Derouich I, Messouak O, Belahsen MF (2013) Syphilitic limbic encephalitis revealed by status epilepticus. BMJ Case Rep 2013.
- Yuta Hagiwara*, Takahiro Shimizu,NaoyaKunika, Hisanao Akiyama, Yasuhiro Hasegawa et al. (2014) Neurosyphilis Exhibiting MRI Abnormalities Mimicking Limbic Encephalitis.Austin Journal of Clinical Neurology
- Scheid R, Voltz R, Vetter T, Sabri O, von Cramon DY (2005) Neurosyphilis and paraneoplastic limbic encephalitis: important differential diagnoses. J Neurol 252: 1129-1132.
- Lessig S, Tecoma E (2006) Perils of the prozone reaction: neurosyphilis presenting as an RPR-negative subacute dementia. Neurology 66: 777.
- Wang X, Yang Y, Wang X, Li C (2014) MRI findings and early diagnosis of general paresis of the insane. Neurol Res 36: 137-142.
- Lynn WA, Lightman S (2004) Syphilis and HIV: a dangerous combination. Lancet Infect Dis 4: 456-466.
- Johns DR, Tierney M, Felsenstein D (1987) Alteration in the naturalhistory of neurosyphilis by concurrent infection with the human immunodeficiency virus. New England Journal of Medicine 316: 1569-1572
- Golden MR, Marra CM, Holmes KK (2003) Update on syphilis: resurgence of an old problem. JAMA 290: 1510-1514.
- Katz DA, Berger JR (1989) Neurosyphilis in acquired immunodeficiency syndrome. Arch Neurol 46: 895-898.
- Taylor MM, Aynalem G, Olea LM, He P, Smith LV, et al. (2008) A consequence of the syphilis epidemic among men who have sex with men (MSM): neurosyphilis in Los Angeles, 2001-2004. Sex Transm Dis 35: 430-434.
- Musher DM, Hamill RJ, Baughn RE (1990) Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med 113: 872-881.
- Morgello S, Laufer H (1989) Quaternary neurosyphilis in a Haitian man with human immunodeficiency virus infection. Hum Pathol 20: 808-811.
- Marra CM, Maxwell CL, Tantalo L, Eaton M, Rompalo AM, et al. (2004) Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin Infect Dis 38: 1001-1006.
- Centers for Disease Control and Prevention1, Workowski KA, Berman SM (2006) Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 55: 1-94.
Citation: Kader T Abdele Rahman, Goran Rakocevic (2014) Limbic Encephalitis Caused By Neurospyhilis in a HIV-Positive Male: Case Report and Review . J Neuroinfect Dis 5:160.
Copyright: © 2014 Kader T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 14007
- [From(publication date): 6-2014 - Jul 01, 2025]
- Breakdown by view type
- HTML page views: 9477
- PDF downloads: 4530