Case Report Open Access
Long-term Survival of Large Cell Neuroendocrine Lung Carcinoma with Bony Metastases: A Case of Immunoprotectivity?
Deborah Paul, Sarah Lander, Anna R Cooper* and Wakenda K TylerDepartment of Orthopaedics and Rehabilitation, University of Rochester Medical Center, 601 Elmwood Ave, Box 665 Rochester, NY 14642, USA
- *Corresponding Author:
- Anna Cooper
Department of Orthopaedics and Rehabilitation
University of Rochester Medical Center
601 Elmwood Ave, Box 665, Rochester
NY14642, USA
Tel: 5852755168
E-mail: anna_cooper@urmc.rochester.edu
Received Date: April 06, 2016; Accepted Date: April 14, 2016; Published Date: April 21, 2016
Citation: Paul D, Lander S, Cooper AR, Tyler WK (2016) Long-term Survival of Large Cell Neuroendocrine Lung Carcinoma with Bony Metastases: A Case of Immunoprotectivity? J Orthop Oncol 2: 110. doi:10.4172/2472-016X.1000110
Copyright: © 2016 Paul D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Orthopedic Oncology
Abstract
Large cell neuroendocrine carcinoma (LCNEC) is a rare, highly malignant neoplasm with a dismal prognosis. The majority of patients will present with metastatic disease with a median overall survival of 6 months for this group. We present a case of metastatic LCNEC to the pelvis with a 10 year survival after tumor resection, radiation, and chemotherapy. We hypothesize that his survival and cancer stability are the result of an immune response brought on by a sub-acute turned chronic wound infection. After adjuvant therapies, he remained disease-free for 4 years until a recurrence in his lung and new metastases to his spine, which were treated with radiation. He remained disease-free for an additional 6 years, during which time, he discovered to have a chronic infection of his right femur with Staphylococcus Lugdunensis. To the best of our knowledge, this is the first long-term survivor of LCNEC with bony metastases.
Keywords
Lung carcinoma; Bone metastasis; Immunoprotection; Infection; Survival
Case Report
The patient, a fifty-five year old male, initially presented in 2003 with complaints of several months of worsening activity-related right hip pain that improved with rest. Initial plain radiographs of the hip and pelvis were read as negative and the patient was treated for presumed muscle strain. When symptoms persistently worsened, the patient presented to the emergency department in March of 2003 and was noted at that time to have an abnormality on plain film and CT scan. The patient’s social history was remarkable for 50-100 pack per year smoking history, 2 drinks of alcohol daily, and employment as a life-long laborer. The patient had no significant family history of cancer but a significant family history of coronary artery disease and diabetes.
Plain radiographs of the pelvis obtained in the emergency department revealed a destructive lytic lesion involving the right hemiplevis and acetabulum. Further work-up included serum laboratory tests, a bone scan, and chest, abdomen and pelvis CT. An elevated serum alkaline phosphatase at 149 U/L was the only significantly abnormal serum laboratory value. The bone scan revealed sites of abnormal uptake at the 5th rib, left scapula, right sacrum, left proximal femur and the right hemipelvis, which were read as concerning for metastatic disease. The CT scan revealed a 2.5 cm by 1.5 cm lung mass. His ECOG score was determined to 2 due to decreased mobility from right hip pain.
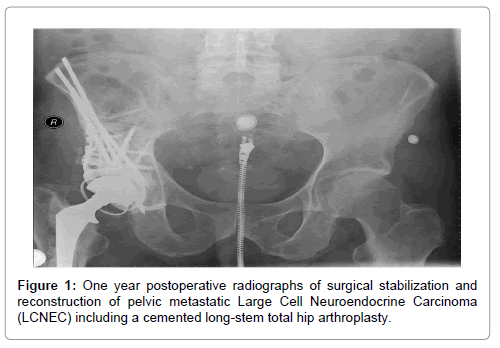
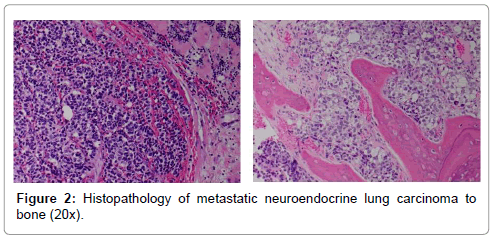
In April of 2003, the patient underwent a non-diagnostic needle biopsy of the lung mass. An image-guided needle biopsy of the pelvis was then performed. Findings revealed adenocarcinoma with immunostaining positive for cytokeratin-7, negative for cytokeratin-20 and positive for TTF-1 and were deemed consistent with metastatic non-small cell lung cancer. In May of 2003, the patient was taking to the operating room for surgical stabilization and treatment of the pelvic lesion, which included a modified Harrington pelvic and acetabular reconstruction (Figure 1). The patient received 10 liters of crystalloid and had an estimated blood loss of 4500 milliliters for the approximate 6 hour surgery. Specimen from this procedure stained positive for synaptophysin and chromogranin. Final pathologic diagnosis was metastatic large cell neuroendocrine carcinoma (Figure 2).
The patient’s post-operative course was complicated by persistent wound drainage with an 1 cm wound dehiscence at the inferior aspect of the incision. He also developed post-operative pneumonia requiring a readmission and IV antibiotics 3 weeks after his initial surgery. The wound drainage continued for approximately 3 months and then closed by secondary intent. The patient was cleared at that point for radiation and chemotherapy. He underwent an 5 day course of fractionated external beam radiation therapy with dosing of 40 Gy to the right femur and hemiplevis. He was also started on monthly intravenous zolandronic acid (4 mg) and on a carboplatinin and taxol based chemotherapy in September of 2003 for 4 full cycles. He received no further chemotherapy after these initial 4 cycles.
The patient had a remarkable course from this point forward as he returned to work full time and had minimal disease progression over the next several years. He reported occasional right hip and leg pain, but his functional status was unaffected. He discontinued the zolandronic acid treatment in 2005. In August of 2007 (4 years after diagnosis) he received 55 Gy of radiation to the right upper lobe lung mass because there was evidence of growth on follow-up chest CTs. He also developed a symptomatic lesion at the 3rd thoracic vertebra and received 30 Gy of radiation to this area, with resolution of his back pain. He was then followed annually with repeat pelvic and femur radiographs as well as chest, abdomen and pelvis CT scans until October of 2011.
In October of 2011, the patient experienced abrupt onset of severe right hip pain for approximately 4 days. The patient reported no fevers or chills at that time. Laboratory values were drawn showing a white blood cell count of 9.5, an ESR of 75 and a CRP 225. No recent prior baseline laboratory values were available for comparison. Based on this presentation, the patient was taken urgently to the operating room for washout, cultures and polyethylene exchange. The remaining implants were left in place. Intraoperative gram-stain was positive for gram positive cocci in pairs and clusters. Five out of five intra-operative cultures grew out Staphylococcus lugdunensis, which was sensitive to all antibiotics, including penicillin. He underwent 8 weeks of intravenous antibiotics (Ceftriaxone 2 g daily) and oral rifampin (300 mg twice daily), followed by another 4 months of oral Rifampin and initially responded well, remaining symptom-free for 18 months. His followup CRPs remained slightly elevated around 10-30 mg/L throughout this time. In 2012, he was diagnosed with insulin resistant diabetes and treated medically for this condition.
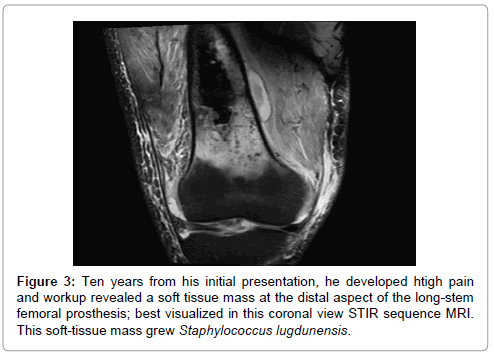
In early 2013, he developed dull, achy, persistent moderate right hip and leg pain. Image-guided hip aspiration was negative. CRP showed a slight increase to 35 mg/L. A decision was made again for close observation. In December of 2013, the patient presented with significant right thigh pain, leukocytosis and CRP of 106 mg/L. Imaging revealed a soft tissue mass at the distal end of the prosthesis, which was aspirated and grew out Staphylococcus lugdunensis. A washout of the hip with a polyethylene exchange was again performed. The distal thigh was also washed out at this time. A decision was made to again retain the hardware present due to the concern regarding loss of bone during the removal of implant, the lack of ideal reconstructive options later, and the anticipated decline in function expected from such a surgery. The patient was treated with 3 months of IV antibiotics, followed by permanent suppressive oral antibiotic therapy (Keflex PO four times daily). As of August 2015, the patient remains stable with reports of intermittent right leg pain that is manageable with anti-inflammatories and the occasional need for oxycodone. He continues to ambulate without an assistive device and remains fully employed.
Follow-up whole body technetium-99 bone scan in June of 2015, continues to show several skeletal sites of metastasis, including the lumbar spine, bilateral ribs and scapula, which have remained stable over the last 10 years (Figure 3). His pelvis and femur also show increased uptake, but this is thought to be due to underlying chronic infection as opposed to active cancer in these regions. His lung mass also remains stable in size with only slight interval increase since 2007. He currently receives no systemic treatment for his cancer and has not received radiation therapy since 2007. He follows up with his orthopedic surgeon every 6 months and his radiation oncologist and primary medical doctor on a yearly basis and receives a whole body bone scan and chest CT yearly.
Discussion
This case of metastatic large cell neuroendocrine carcinoma has several remarkable features. This patient’s survival and pathologic stability over 10 years are two areas of significance. Moreover, the potential interplay between his chronic infection and cancer stability contributes to the intriguing interplay between infection, immunity, and cancer.
Large cell neuroendocrine carcinoma (LCNEC) is a rare, accounting for only 2.87% of all lung cancer cases, highly malignant neoplasm with a miserable prognosis. Both genetics and smoking have been linked to LCNEC. Multiple series have reported 85-98% of patients who had a surgical resection of LCNEC had a history of habitual cigarette smoking [1,2]. Genetic risk factors include mutation of the epidermal growth factor receptor (EGFR) gene or tyrosine kinase domain (TKD) of the neurotrophic tyrosine receptor kinase (NTRK) gene [3,4]. LCNEC can be a challenge to diagnose since patients rarely present with pulmonary symptoms and <20% present with cough or hemoptysis [2,5].
On a molecular level, LCNEC has a high cellular proliferative rate, highly positive staining for p21waf1/cip1, increased microvessel density, and both regulation of apoptosis and angiogenesis are intimately involved in this disease process. Patients who had greater than 3.5% staining for p21waf1/cip1 and a microvessel density greater than 3.0% have been found to have a less favorable prognosis for survival [6]. LCNEC cytomorphologically reveals basaloid palisading, organoid nesting rosettes with necrosis [7]. As compared with other large cell carcinomas, LCNEC has high expression rates of Bcl-2 and Ki-67. The expression of Ki-67, p53, and Rb distinguish LCNEC from SCC or other carcinoid tumors while the expression of K-ras-w and C-raf-1 are genetically similar to SCC. This molecular profile may be responsible for the high local disease recurrence rate of these tumors found to be 52.4% [8].
LCNEC has a poor prognosis with a reported five year survival rate of 15–57% [9]. In a study by Veronesi et al, the overall five year survival rate for stage I was 52%, stage II 59%, stage III 20%. The rate of distant metastases is 65% with documented greatest propensity to metastasize to brain, liver, bone, and adrenal gland in descending order respectively [10,11]. Patients with stage IV LCNEC have a life expectancy of approximately six months [12].
Management of LCNEC is controversial [13]. Current treatment for LCNEC is a hybrid of the regimen for NSCLC and SCLC. All non-metastatic stages of LCNEC undergo surgical resection, like NSCLC, and receive adjuvant chemotherapy as per a SCLC regimen [14]. Additional adjuvant treatment options are currently under investigation. A study by Sun et al revealed a response rate of LCNEC to a platinum-based chemotherapy was 60% as compared to 11% when treated with a non-platinum based chemotherapy. A cisplatin-based chemotherapy to treat LCNEC has been reported to have comparable response rates to that of small-cell carcinomas. Unfortunately, LCNEC differs from other lung cancers as it has been shown to have higher rates of recurrent lung cancer/relapse rates (52.4%) as well as higher resistance to the available treatment options [15].
There have been a few cases reported in the literature of long-term disease free survivors with metastatic LCNEC. In one case report, a patient diagnosed with LCNEC and brain metastases relapse free for two years due to a novel therapeutic approach [16]. An abysmal prognosis was reported in a case study of a patient with LCNEC with a multitude of locations including the lumbar spine [17]. There are very few documented case reports of LCNEC with bony metastasis and we found no case reports in the literature of stage IV LCNEC and distant metastasis with survival greater than 2 years; this highlights the exceptional nature of our case.
We believe this patient’s long-term survival and cancer stability relates to his chronic infection. Both infections and cellular neoplasms elicit activation of the immune system. This activation varies to address the responsible pathogens through the activation of the general innate system and specific adaptive system. During an infection, the inflammatory response is usually immediate and characterized by the presence of phagocytic cells and other host derived protective factors to the site of infection [18]. By contrast, some neoplasms are able to evade the immune system early on during their development and delay recognition and attack by the host immune system by suppressing the inflammatory response [19]. It is not until the later stages when a stable tumor microenvironment has been established that these neoplasms cause an inflammatory response to be recognized by the host immune system to produce the robust immune response seen in the early stages of infections in the immunocompetent host [20]. After the tumor becomes established in the host organism with the expression of various growth and angiogenic critical factors, inflammation is recognized and the immune system initiates a cascade of events to try to respond to the tumor microenvironment.
This suggests that the ability of some neoplastic cells to delay an inflammatory response is protective and allows it to grow malignant and initially evade the host immune response compared to the initial immediate immune response seen during an acute infection [18]. Current research focuses on this possible relationship between the immune response and cancer through the use of immunotherapy agents as possible effective treatment options in the management of cancer. Immunotherapy centers on the T cell mediated response of the adaptive immune system that is often suppressed by protective factors produced by neoplastic cells during their establishment of the tumor microenvironment. This role of the immune system can be elucidated by further investigating the role of the immune response through chronic infections seen in the setting of cancer. While there are no current cases to better examine this relationship, we can look at the role of immunotherapy as a rapidly emerging alternative in cancer treatment.
Cancer immunotherapy focuses on magnifying the ability of the host’s immune system to respond to neoplastic cells through T cell recognition and activation. Antigen presenting cells present various foreign peptides to their respective major histocompatibility complex that will connect with a T cell for activation and stimulate a generation and expansion of a specific T cell repertoire. This process requires various checkpoints and cancer cells are able to evade these checkpoints to bypass destruction by T cells [21]. Immunotherapy agents such as Ipilimumab, have been shown to be effective in blocking these checkpoints by acting on cytotoxic T-lymphocyte antigen-4 (CTLA- 4) in various cancers including lung cancer [22]. The successful use of immunotherapy in the treatment of cancer patients show that the immune system is capable of eliciting a response to neoplastic cells if it can identify the cells as foreign and generate specifically differentiated T-cells to target those cells. The ability to manipulate and activate the immune system to recognize these foreign cells merits further research. Studying cancer in the setting of a chronic infection provides this opportunity.
After an extensive review of literature, we found no cases where a chronic infection has provided an immunoprotective effect in the setting of cancer. This is possibly due to the reality that opportunistic infections are a significant part of the morbidity and mortality of cancer patients. There are several historical and recent reports suggesting a link between improved survival and local infection in patients treated with surgery for sarcomas [23]. However, there have also been several more recent reports of retrospective data refuting this claim and it appears that conclusive data is still lacking on this question [24]. The possibility that a local infection may incite an immune response that in turn suppresses tumor cell activity is an enticing prospect. Studying cancer in the setting of an infection poses a significant challenge; a potential area of focus are patients with chronic infections early on in their cancer diagnosis with particular attention for those who are able to recover from multiple relapses of infections. Our case is one such opportunity to explore the possible protective role of a chronic infection in the setting of cancer over a 12-year period and to continue the discussion of the immune system’s role in cancer.
References
- Paci M, Cavazza A, Annessi V, Putrino I, Ferrari G, et al. (2004) Large cell neuroendocrine carcinoma of the lung: a 10-year clinicopathologic retrospective study.Ann Thorac Surg 77: 1163-1167.
- Takei H, Asamura H, Maeshima A, Suzuki K, Kondo H, et al. (2002) Large cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighty-seven cases.J Thorac Cardiovasc Surg 124: 285-292.
- Sakai Y, Yamasaki T, Kusakabe Y, Kasai D, Kotani Y,et al. (2013) Large-cell neuroendocrine carcinoma of lung with epidermal growth factor receptor (EGFR) gene mutation and co-expression of adenocarcinoma markers: a case report and review of the literature. Multidiscip Respir Med 8: 8-47.
- Marchetti A, Felicioni L, Pelosi G, Del Grammastro M, Fumagalli C, et al. (2008) Frequent mutations in the neurotrophic tyrosine receptor kinase gene family in large cell neuroendocrine carcinoma of the lung.Hum Mutat 29: 609-616.
- Zacharias J, Nicholson AG, Ladas GP, Goldstraw P (2003) Large cell neuroendocrine carcinoma and large cell carcinomas with neuroendocrine morphology of the lung: prognosis after complete resection and systematic nodal dissection.Ann Thorac Surg 75: 348-352.
- Ab' Saber AM, Massoni Neto LM, Bianchi CP, Ctenas BB, Parra ER, et al. (2004) Neuroendocrine and biologic features of primary tumors and tissue in pulmonary large cell carcinomas.Ann Thorac Surg 77: 1883-1890.
- Iyoda A, Baba M, Hiroshima K, Saitoh H, Moriya Y, et al. (2004) Imprint cytologic features of pulmonary large cell neuroendocrine carcinoma: Comparison with classic large cell carcinoma. Oncol Rep11: 285-288.
- Iyoda A, Jiang SX, Travis WD, Kurouzu N, Ogawa F, et al. (2013) Clinicopathological features and the impact of the new TNM classification of malignant tumors in patients with pulmonary large cell neuroendocrine carcinoma. Mol Clin Oncol 1: 437-443.
- Veronesi G, Morandi U, Alloisio M, Terzi A, Cardillo G, et al. (2006) Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144 surgical cases.Lung Cancer 53: 111-115.
- Swarts DR, Ramaekers FC, Speel EJ (2012) Molecular and cellular biology of neuroendocrine lung tumors: evidence for separate biological entities.Biochim Biophys Acta 1826: 255-271.
- Rossi G, Cavazza A, Marchioni A, Longo L, Migaldi M, et al. (2005) Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung.J Clin Oncol 23: 8774-8785.
- Bhamidipati PK, Ribbeck A, Liaghati-Nasseri G, Kumar R, Paidipaty B, et a l. (2011) An Atypical Presentation with Diagnostic Challenge of a Large Cell Neuroendocrine Cancer of Lung: A Case Report and Review of the Literature.Lung Cancer Int 2011: 912098.
- Sun JM, Ahn MJ, Ahn JS, Um SW, Kim H, et al. (2012) Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 77: 365-370.
- Iyoda A, Makino T, Koezuka S, Otsuka H, Hata Y (2014) Treatment options for patients with large cell neuroendocrine carcinoma of the lung.Gen Thorac Cardiovasc Surg 62: 351-356.
- Niho S, Kenmotsu H, Sekine I, Ishii G, Ishikawa Y, et al. (2013) Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: a multicenter phase II study. J Thorac Oncol 8: 980-984.
- Ryuge S, Jiang SX, Wada M, Katono K, Iwasaki M, et al. (2009) Long-term disease-free survivor of metastatic large-cell neuroendocrine carcinoma of the lung treated with amrubicin and irinotecan.Drug Des Devel Ther 3: 213-217.
- Yuan C, Keating B, Farricielli LA, Zhang K (2014) Large-cell neuroendocrine carcinoma (LCNEC) without pulmonary symptoms diagnosed in a cutaneous metastasis. Am J Case Rep 15: 97-102.
- Parham P, Janeway C (2009) The immune system. London; New York: Garland Science.
- Goldszmid RS, Dzutsev A, Trinchieri G (2014) Host immune response to infection and cancer: unexpected commonalities.Cell Host Microbe 15: 295-305.
- Trinchieri G (2015) Cancer Immunity: Lessons From Infectious Diseases. J Infect Dis 212: S67-S73.
- Li X, Hu W (2015) Emerging immune checkpoints for cancer therapy.Acta Oncol 54: 1706-1713.
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, et al. (2012) Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study.J Clin Oncol 30: 2046-2054.
- Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A (2007) Post operative infection and increased survival in osteosarcoma patients: are they associated?Ann Surg Oncol 14: 2887-2895.
- Lee JA, Kim MS, Kim DH, Lim JS, Park KD, et al. (2009) Postoperative infection and survival in osteosarcoma patients.Ann Surg Oncol 16: 147-151.
Relevant Topics
- 3D Printing in Limb-Sparing Surgery
- Adamantinoma
- Aneurysmal Bone Cysts
- Chondrosarcoma
- Chordomas
- Cryosurgery
- Enchondroma
- Ewing’s Sarcoma
- Fibrous Dysplasia
- Giant Cell Tumor of Bone
- Immunotherapy for Osteosarcoma
- Liquid Biopsy in Orthopedic Oncology
- Malignant Osteoid
- Metastatic Bone Cancer
- Molecular Profiling of Bone Tumors
- Multilobular Tumour of Bone
- Orthopaedic Oncology
- Osteocartilaginous Exostosis
- Osteochondrodysplasia
- Osteoma
- Osteonecrosis
- Osteosarcoma
- Primary Bone Tumors
- Sarcoma
- Secondary Bone Tumours
- Targeted Therapy in Bone Sarcomas
- Tumours of Bone
Recommended Journals
Article Tools
Article Usage
- Total views: 18097
- [From(publication date):
June-2016 - Aug 19, 2025] - Breakdown by view type
- HTML page views : 17068
- PDF downloads : 1029