Research Article Open Access
Maternal Demographic and Placental Risk Factors in Term Low Birth Weight in Ghana
Aleksenko Larysa1,2, Tettey Yao2, Gyasi Richard2, Obed Samuel3, Farnell Damian Joseph John4 and Quaye Isaac Kweku5*1University of Namibia, School of Medicine, Department of Pathology, Windhoek, Namibia
2Department of Pathology, Faculty of Health Science, School of Biomedical and Allied Health Sciences, University of Ghana, Accra, Ghana
3Department of Obstetrics and Gynaecology, Faculty of Health Science, School of Biomedical and Allied Health Sciences, University of Ghana, Accra, Ghana
4School of Dentistry, Cardiff University, Cardiff CF14 4XY, United Kingdom
5University of Namibia School of Medicine, Department of Biochemistry and Microbiology, Windhoek, Namibia
- *Corresponding Author:
- Isaac K Quaye
Department of Biochemistry and Microbiology
University of Namibia, School of Medicine
P/Bag 13301, Windhoek, Namibia
Tel: 264612065044
E-mail: dr.quaye@gmail.com
Received date: May 11, 2017; Accepted date: May 14, 2017; Published date: May 19, 2017
Citation:Larysa A, Yao T, Richard G, Samuel O, John FDJ, et al. (2017) Maternal Demographic and Placental Risk Factors in Term Low Birth Weight in Ghana. J Preg Child Health 4:325. doi:10.4172/2376-127X.1000325
Copyright: ©2017 Larysa A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Several studies report on factors that associate preterm birth and intrauterine growth restriction with low birth weight (LBW). However, few studies discuss risk factors that associate with LBW for full-term births. No such studies exist that involve a population from Ghana. Method: We used a nested case-control study approach to examine maternal socio-demographic and placental factors that contribute significantly to term LBW in Ghana. We assessed also the incidence of LBW in general at a major teaching hospital facility in Ghana. Results: Univariate and multivariate logistic regression analysis were used to investigate maternal sociodemographic and placental factors that associate with LBW. Following the preliminary univariate analysis, a stepwise logistic regression analysis showed that unstable income source, single motherhood, combined effect of pre-eclampsia and anaemia; ORs of 5.366 (95% CI: 1.986 to 14.497), 21.390 (95% CI: 3.610 to 126.734) and 3.246 (95% CI: 1.074 to 9.814), respectively, and placental weight and irregular insertion of the umbilical cord (variables scaled by a factor of 10-2 to aid interpretation) ORs 0.28 (95% CI: 0.115 to 0.683), 0.010 (95% CI: 0.001 to 0.173 respectively) on the chorionic plate, were risk factors for LBW. The socio-demographic and placental factors reveal a core role of maternal and infant nutritional deficiencies in term LBW in Ghana. The general prevalence of LBW in the Hospital facility was 6.2%. Conclusion: We conclude that poor maternal and infant nutrient supply is key factors in term LBW in Ghana. These factors are amenable to appropriate nutritional and educational interventions.
Keywords
Term low birth weight; Maternal nutrition; Fetal nutrition
Introduction
Low birth weight (LBW) is defined as birth weights that are less than 2500 g at birth, and it is the single most important risk factor in neonatal and infant health [1-3] . LBW occurs in 15.5 % of all live births or about 20.5 million infants per year worldwide [2,3] . LBW occurs in preterm babies (less than 37 completed weeks of gestation), infants with intrauterine growth restriction (IUGR), and full-term infants (greater or equal to 37-41 completed weeks of gestation) [4,5]. Although many sources in the literature consider risk factors specifically for preterm and/or IUGR on LBW infants [1,6,7], few studies consider those factors that associate with LBW in full-term births or “term LBW”. Term LBW is the focus of the present study [8-10].
LBW babies are in general at a higher risk of long-term adverse health consequences, including cardiovascular, renal, metabolic disorders, and learning disabilities [11-13] . Two primary factors are known to correlate with LBW, namely: Maternal nutrient availability; and, placental structure and health [14,15]. The developing fetus is obligatorily dependent on the mother for nutrient supply, and so maternal adequate nutrition is critical for the provision of the required amounts of nutrients (carbohydrates, proteins and lipids) for the developing fetus [15]. On the other hand, the placenta must be well developed and healthy to ensure that the available nutrients from the mother are delivered to the fetus [16,17]. Comparative studies of maternal socio-demographic factors in various countries and in sub- Saharan Africa show that young age (and/or maternal age greater than 35), a single motherhood and lifestyle behaviours (e.g., physical activities and smoking) are high risk factors to poor fetal growth and pregnancy outcomes [18,19]. Maternal occupations that require prolonged standing, walking or vigorous physical exertion, and psychological factors such as stress and anxiety may also contribute to LBW. However, these effects vary between populations [20,21]. It has been reported that placental factors alone contribute to neonatal growth retardation in 36% of LBW cases [22,12]. Placental factors include changes in placental morphology and insults to the placenta such as infarcts, chronic villous inflammation, and vascular vessel thrombosis. Placental morphological changes include the length, breadth, weight, and any shifts from central insertion of the umbilical cord on the chorionic plate to an irregular velamentous insertion [23,24]. Such changes affect the placental efficiency with regards to adequate development of the vasculature to meet the nutrient and oxygen supply to the fetus [25,26]. The placental weight serves as a marker of the available surface area for maternal-fetal nutrient exchange [27,28]. In our study, we examined firstly the incidence rate of LBW in general at the Korle-Bu Teaching Hospital, Accra, Ghana, retrospectively. Secondly, we investigated the socio-demographic and placental factors of prospective mothers that associate with term LBW in a nested case-control study at the hospital. We provide evidence that maternal socio-demographic and placental factors that impact on nutrient supply to the fetus are important contributors to low birth weight in term infants.
Materials and Methods
The study site
The study was conducted at the Department of Pathology and Maternity Wards of the Obstetrics and Gynecology Department of the Korle-Bu Teaching Hospital (KBTH), Accra, Ghana. KBTH is a leading tertiary referral hospital that serves the city of Accra, the surrounding urban population and the Southern part of Ghana.
Extraction of incidence data and Case-control study
The records of all deliveries and weights of babies occurring at the KBTH between May 2000 and January 2006 were documented. As part of the nested case-control study (i.e., a case-control study “nested” within a cohort study), prospective mothers were recruited at the antenatal clinic of the Obstetrics and Gynaecology wards during their normal visit after 12 weeks of gestation. At recruitment, the study objective was first explained to each prospective mother, after which a consent form was signed if consent to participate was given. Subsequently, a structured questionnaire assessing socio-demographic characteristics, maternal diseases and past obstetric outcome(s) was completed. A total of 200 expectant mothers were recruited consecutively within a 1.7 year period from May 2004 to January 2006. These mothers were followed until delivery, at which time babies were classified as either cases or controls based on their birth weight. We defined pre-eclampsia as blood pressure greater or equal to 140/90 mmHg with proteinuria of at least 1 plus on dipstick in two samples taken 6 h apart. Anaemia in pregnancy is defined as haemoglobin concentration less than 10 g/dl after 8 to 10 weeks of gestation. Finally, term birth refers to those births that occurred during or after 37 weeks of gestation. This study was approved by the Protocol and Ethical Review Committee of the University of Ghana Medical School.
Collection of placental samples at delivery
The neonates were weighed during the first hour after delivery using manually calibrated scales by nurses of the maternity wards. All placentas were collected and placed in labelled containers, and then they were transported to the Pathology Department, KBTH. The placentas were washed with water and cleaned of blood clots in the laboratory.
Placental measurements (see below for details) of both low birth weight (<2500 g) neonates (designated as “cases”) and of normal birth weight neonates (i.e., in the range: 2500 g to<4300 g) (designated as “controls”). Samples were included for analysis if the mother had a singleton, delivery of a live neonate birth without major gross abnormalities at or after 37 weeks of gestation. Out of the 200 subjects that were enrolled, 56 delivered outside of the hospital. Thus, 144 subjects were used in this analysis, consisting of 72 “cases” and 72 “controls”.
Examination and processing of placenta
Gross examination of the placentas
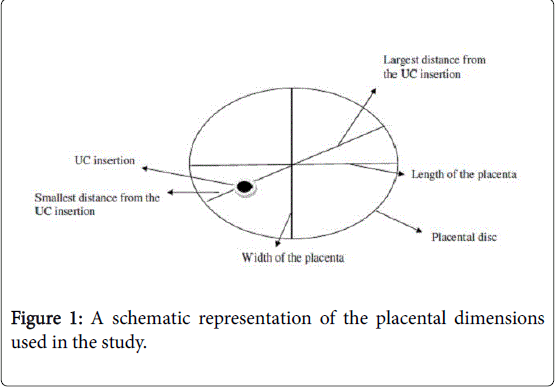
All fresh and cleaned placentas were examined immediately or kept in a refrigerator (1-3°C) and examined within 24-48 h after delivery. Morphometric data was documented covering the weight of the placenta, length, width, thickness and the largest or smallest distance from the point of insertion of the umbilical cord to the edge of the placental disc, as is shown schematically in Figure 1. The dimensions of the placenta were taken as follows: horizontal and vertical lines serving as perimeter markers were drawn and the largest horizontal line taken as the length while the vertical line was taken as the breadth., The largest and smallest distances from the point of the insertion of the umbilical cord to the perimeter were measured, as shown in Figure 1. The placenta (excluding the umbilical cord and membranes) was weighed. After a detailed gross examination, the placenta was cut in serial slices ½ cm to 1 cm thick parallel to a line through the insertion of the umbilical cord. Samples of tissue were taken for histology as follows: (a) from the site of insertion of the umbilical cord (UC); (b) from each margin of the central slice including both placental tissue and chorionic membrane; and, (c) from all representative lesions or random slices throughout the placental tissue if no lesion could be identified grossly.
Histological evaluation
The tissue samples were soaked in 50-60 ml of 10% neutral buffered formalin for 2-3 days and they were embedded in paraffin wax after tissue fixation. Sections of tissue of 5 microns thickness were made, and these tissues were then stained with hematoxylin, eosin and Giemsa stain for identification of chronic intervillositis.
The histological slides were examined by light microscopy under ×40, ×100, ×400.
Magnification with Leica DML type 020-518.500 DM/LS light microscope at the Department of Pathology, KBTH.
Statistical analysis
All calculations were carried out using either MS EXCEL (2013), SPSS V20, or STATA V13. Low birth weight was explored as a function of categorical (nominal) factors such as demographics and maternal characteristics by contingency tables. Associations between low birth weight and these factors were examined by chi-squared tests of the contingency tables. For those cases where sample sizes were low in a given class, and where this also meant that more than 20% of expected values had values less than 5), Monte Carlo simulation was used in SPSS V20 to establish accurate measures of probabilities (i.e., the pvalues and their associated 99% confidence intervals) (Table 1).
| Low birth weight N=172 |
Controls N=72 |
|
|---|---|---|
| Occupation | ||
| Unemployed | 13 | 3 |
| Small scale trader | 41 | 30 |
| Employed (self or receiving salary) | 18 | 39 |
| χ2=15.691, dof=2, P (1-tailed)=0.0004; OR (unemployed compared to small scale trader)=3.171 (95% CI: 0.830 to 12.119); OR (unemployed compared to employed)=9.389 (95% CI: 2.376 to 37.098) | ||
| Parity | ||
| 0 | 38 | 28 |
| 01-Jun | 34 | 44 |
| χ2 (continuity correction)=2.266, dof=1, P (1-tailed)=0.1323; OR (0 compared to 1-6)=1.756 (95% CI: 0.906 to 3.406) | ||
| Age (years) | ||
| <21 | 32 | 11 |
| 21-30 | 28 | 40 |
| ≥ 31 | 12 | 21 |
| χ2=14.828, dof=2, P (1-tailed)=0.0006; OR (<21 compared to 21-30)=4.156 (95% CI: 1.797 to 9.609); OR (21-30 compared to ≥ 31)=5.091 (95% CI: 1.899 to 13.647). | ||
| Marital status | ||
| Single | 12 | 1 |
| Married | 60 | 71 |
| χ2 (continuity correction)=8.456, dof=1, P (1-tailed)=0.0036; OR (Single compared to Married)=14.2 (95% CI: 1.794 to 112.396) | ||
| Planned versus unplanned pregnancy | ||
| Unplanned | 16 | 1 |
| Planned | 56 | 71 |
| χ2 (continuity correction)=13.073, dof=1, P (1-tailed)=0.0003; OR (Unplanned compared to Planned)=20.286 (95% CI: 2.610 to 157.651) | ||
| Income | ||
| Stable | 17 | 39 |
| Unstable | 55 | 33 |
| χ2 (continuity correction)=12.886, dof=1, P (1-tailed)=0.0003; OR (stable compared to unstable)=3.824 (95% CI: 1.871 to 7.813) | ||
Table 1: Maternal socio-demographic and reproductive health characteristics between cases and controls (univariate analysis).
Odds ratios and their associated 95% confidence intervals were also determined for appropriate group-wise comparisons (described in the main text) using appropriate formula in MS EXCEL (also checked using univariate binary logistic regression in STATA V13). Low birth weight was also explored as a function of continuous factors such as placental weight, length, width, thickness and distances from the placental disc of the umbilical cord insertions (Table 2).
| Complications | Low birth weight (N=72) | Controls (N=72) |
|---|---|---|
| Antenatal | ||
| No complications | 49 | 59 |
| Pre-eclampsia | 6 | 0 |
| Anemia | 17 | 13 |
| χ2 = 7.459, dof = 2, P (via Monte Carlo; 1-tailed)=0.021 (99% CI: P=0.020 to 0.021); OR (No complications compared to pre-eclampsia or anemia)=2.130 (95% CI: 0.978 to 4.640). | ||
| Placental abnormalities | ||
| No gross abnormality | 40 | 50 |
| Chronic infarctions (< 25%) | 26 | 21 |
| Chronic infarctions (25 – 50%) | 4 | 0 |
| Acute infarctions (< 25%) | 2 | 1 |
| χ2=5.976, dof=3, P (via Monte Carlo; 1-tailed=0.100), (99% CI: P=0.099 to 0.101); OR (No gross abnormality compared to chronic or acute infarctions=1.818), (95% CI: 0.918 to 3.602). | ||
| Vascular tree morphology | ||
| Magistral vasculature | 27 | 26 |
| Disperse vasculature | 45 | 46 |
| χ2 (continuity correction=0), dof =1, P (1-tailed)>0.999; OR: magistral compared to disperse=1.062 (95% CI: 0.539 to 2.090). | ||
| Umbilical cord insertion | ||
| Normal | 56 | 60 |
| Velamentous | 5 | 1 |
| Furcate | 5 | 6 |
| Other abnormal insertions | 6 | 5 |
| χ2=2.986, dof=3, P (via Monte Carlo; 1-tailed)=0.446 (99% CI: P=0.445 to 0.448); OR (normal compared to velamentous or furcate or other)=1.429 (95% CI: 0.621 to 3.284). | ||
Table 2: Antenatal and placental complications in LBW and controls.
Data was found to be slightly skewed only (due to a small number of outliers), and unpaired two-sample t-tests were used to detect differences in these continuous variables for the low birth weight group compared to the control group. As an additional and independent check, (non-parametric) Mann-Whitney tests were carried out and the results of these tests (not quoted here) were found to agree strongly with those results of the t-tests (i.e., in terms of detecting differences between the groups) (Table 3).
| Gross measurements | Low birth weight N=72 | Controls N=72 | t-test: P (2-sided)= |
|---|---|---|---|
| Weight (g) | 327.8 ± 61.2 | 429.5 ± 105.6 | 0 |
| Length (mm) | 187.8 ± 44.1 | 200.9 ± 28.6 | 0.037 |
| Width (mm) | 154.8 ± 21.7 | 171.1 ± 19.0 | 0 |
| Thickness (mm) | 11.5 ± 4.7 | 11.2 ± 3.9 | 0.602 |
| Largest distance (mm) | 111.1 ± 17.1 | 123.1 ± 21.5 | 0 |
| Smallest distance (mm) | 56.1 ± 18.2 | 65.0 ± 21.0 | 0.015 |
Table 3: Gross placental dimensions (mean ± standard deviation) in cases and controls.
Stepwise (multivariate) binary logistic regression was used to explore the data further and to account for any potentially confounding effects of the variables on each other. It was found that standardized residuals were generally small (i.e., less than 2) in all binary logistic regression calculations. Nominal factors were treated as binary variables (e.g., complications: yes or no) in order to reduce any potential problems of small sample sizes in specific groups. Furthermore, continuous variables (i.e., placental weight, length, age, etc.) were treated as (continuous) covariates. Note that odds ratios are determined with respect to a unit increase of these continuous variables, albeit scaled by a factor of 10-2 to make odds ratios easier to interpret in Table 4. As there were a small number of outliers in these continuous variables, robust regression was used in multivariate logistic regression in STATA V13 in order to account for any possible model misspecification. However, any differences in final results for robust methods compared to non-robust (i.e., standard) methods were minimal. All measures of model fit (e.g., pseudo R-squared values) were found to increase very strongly for the multivariate model compared to equivalent univariate calculations, as expected.
| Unadjusted (univariate) | Adjusted (multivariate) | ||||
|---|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI | Wald: P= |
| Complications (none to pre-eclampsia or anaemia) | 2.13 | 0.978 to 4.640 | 3.246 | 1.074 to 9.814 | 0 |
| Stable income (unstable to stable) | 3.824 | 1.871 to 7.813 | 5.366 | 1.986 to 14.497 | 0 |
| Marital status (unmarried to married) | 14.2 | 1.794 to 112.396 | 21.389 | 3.610 to 126.734 | 0 |
| Placental weight (scaled by a factor of 10-2) | 0.178 | 0.092 to 0.342 | 0.28 | 0.115 to 0.683 | 0 |
| Largest distance (scaled by a factor of 10-2) | 0.032 | 0.004 to 0.277 | 0.01 | 0.001 to 0.173 | 0 |
| Smallest distance (scaled by a factor of 10-2) | 0.122 | 0.020 to 0.742 | 0.04 | 0.004 to 0.445 | 0 |
Table 4: Odds ratios from univariate (unadjusted) analyses and multivariate (adjusted) analyses via stepwise logistic multivariate regression (via“robust” methods) of maternal demographics, placental complications and gross placental dimensions.
Results
The incidence of LBW within the period from May 2000 to January 2006, at the KBTH was 6.2% (50,574 term live births with 3109 weighing <2500 g). In a univariate analysis of the maternal demographic and reproductive health characteristics that impact LBW (Tables 1 and 2), unstable source of income, age band, single motherhood, unplanned pregnancy and antenatal complications, were significantly associated with low birth weight (P=0.0003, 0.0006, 0.0036, 0.0003 and 0.021, respectively).
All continuous variables except “thickness” (i.e., placental weight, length, width and largest and smallest distances) were found to be significantly different for the low birth weight group compared to the control group in a univariate analysis.
After adjusting for confounding effects in a stepwise (multivariate) logistic regression analysis, unstable income, single motherhood and antenatal complications (pre-eclampsia and anaemia) were the only maternal demographic and reproductive health variables that remained significantly associated with LBW (P=0.001, 0.001 and 0.037, respectively) with an ORs of 5.366 (95% CI: 1.986 to 14.497), 21.390 (95% CI: 3.610 to 126.734), and 3.246 (95% CI: 1.074-9.814), respectively. Interestingly, vessel tree abnormalities had no significant effect on LBW. All the gross placental measurements were significantly lower in LBW compared to controls in a univariate analysis. However, only the placental weight, and the largest and smallest distances from the point of insertion of the umbilical cord were highly associated with LBW in a stepwise logistic regression analysis adjusting for the effects of all other variables (P=0.005, 0.002, 0.001, respectively; ORs 0.28 (95% CI: 0.115 to 0.683), 0.010 (0.001 to 0.173 and 0.04 (95% CI 0.004 to 0.445), respectively).
Discussion
Much evidence exists in the literature relating to factors that associate with LBW in preterm births. By contrast, fewer studies consider risks factors relating to LBW in full-term births, and still fewer studies (or reviews) concentrate on a diverse range of subject populations [1,6,7]. These factors make it imperative for such data to be collated. Our study shows that, proxy factors on nutrition, particularly unstable income source and single motherhood, serve as high-risk factor for term LBW in term infants in Ghana. Studies in Pakistan and China showed that maternal low primary education, anaemia, hypertension and socio-economic factors were strong factors that influenced term LBW [8,9]. Intrauterine malnutrition as a result of poor maternal nutrition can lead to restricted growth in utero and it can serve as a proxy to LBW [29,30]. A single mother in Ghana, especially in the low income range, will have difficulty obtaining adequate income to afford enough, well-balanced food to eat. Clearly, this will in turn affect her nutritional supply and concomitantly neonatal nutrition. It is well established that placental nutrient transport efficiency is a core maternal factor that associates with LBW. Furthermore, antenatal complications also associate with LBW [28,31].
It has been reported that the placenta may act as a nutrient sensor that adjust maternal nutrient supply to fetal growth [27,30]. In our study, the combined effect of pre-eclampsia and anaemia were the respective placental and antenatal complications seen associating strongly with term LBW. Recent reports have compelling evidence of the role of nutritional deficiencies and inadequacies in pre-eclampsia particularly micronutrients, iron supplementation and inadequate diversity in food sources [32-34]. Anaemia is a common maternal nutritional disorder that has also recently been shown to lead to fetal anaemia, poor growth and LBW [35-37]. Interestingly, there was little evidence in our study that vascular tree structure associated with LBW suggesting that the structure may not strongly impact the volume or available surface area for nutrient exchange in the study population. The vascular tree has been suggested to be the sole source of oxygen and nutrient to the fetus, thereby defining placental efficiency [23,24]. Thus, it appears that nutrient delivery to the fetus may be minimally affected if placental efficiency in nutrient delivery is not unduly compromised by the vascular tree morphology. This hypothesis is partially supported from the very strong association of placental weight with LBW in the present population. The placental weight is a proxy for the available surface area for materno-fetal nutrient exchange, and so the larger the weight the greater the nutrient exchange (and vice versa) [28,31].
Previous reports have suggested that a shift in the centrality of the umbilical cord insertion on the chorionic plate reflects the level of vasculature available for nutrient supply [17,23]. Thus, a greater shift leads to a lower level of vascular distribution available for nutrient exchange (and vice versa) [24]. The largest distance from the point of centrality is a high risk factor to term LBW in our population. Our results suggest that such a shift may indeed affect nutrient supply to the fetus, probably from changes in the vascular tree surface area required for nutrient transport to the fetus [23]. However, further research is necessary to establish if this observation is seen in other study populations. The overall LBW incidence found in this study was 6.2% for our sample of those mothers attending the Maternity Wards of the Obstetrics and Gynaecology Department of the Korle-Bu Teaching Hospital (KBTH), Accra, Ghana. This result contrasts a previous study in the community that reported a prevalence of 11% [38]. The difference could be due to early mortality rates within the Health Facility that precluded inclusion in the data capture. The overall prevalence in sub-Saharan Africa is 14%, which is ranked second globally after South-East Asia with a prevalence of 26% [3].
Finally, we conclude that maternal nutrition is a core problem in term LBW in Ghana. LBW prevention or reduction in incidence can be partially amenable to improved maternal nutrition and education during pregnancy.
Acknowledgement
The authors wish to acknowledge the support of the nurses at the Obstetrics and Gynecology Department of the Korle-Bu Teaching Hospital, Accra, Ghana, and the technicians of the Department of Pathology, Faculty of Health Sciences, School of Allied Health, and University of Ghana.
Author Contribution
AL conceived the study. All authors contributed to the design of the study. AL carried out the work. AL, DFJJ and QIK wrote the paper.
References
- De-Bernabe JV, Soriano T, Albaladejo R, Juarranz M, Calle ME, et al. (2004) Risk factors for low birth weight: A review. Eur J Obstet Gynecol Reprod Biol 116: 3-15.
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, et al. (2010) Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet 375: 1969-1987.
- Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, et al. (2013) National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 1: e26-e36.
- National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010.
- McCormick MC (1985) The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 312: 82-90.
- Blanc AK, Wardlaw T (2005) Monitoring low birth weight: an evaluation of international estimates and an updated estimation procedure. Bull World Health Organ 83: 178-185.
- Metgud CS, Naik VA, Mallapur MD (2012) Factors affecting birth weight of a new-born – A community based study in rural Karnataka, India. PLoS ONE 7: e40040.
- Koullali B, Oudijk MA, Nijman TA, Mol BW, Pajkrt E (2016) Risk assessment and management to prevent preterm birth. Semin Fetal Neonatal Med 21: 80-88.
- Mavalankar DV, Gray RH, Trivedi CR (1992) Risk factors for preterm and term low birth weight in Ahmedabad, India. Int J Epidemiol 21: 263-272.
- Bian Y, Zhang Z, Liu Q, Wu D, Wang S (2013) Maternal risk factors for low birth weight for term births in a developed region in China: A hospital-based study of 55,633 pregnancies. J Biomed Res 27: 14-22.
- Hack M, Klein NK, Taylor HG (1995) Long-term developmental outcomes of low birth weight infants. Future Child 5: 176-196.
- Sharma SR, Giri S, Timalsina U, Bhandari SS, Basyal B, et al. (2015) Low birth weight at term and its determinants in a tertiary hospital of Nepal: Acase-control study. PLoS ONE 10: e0123962.
- Hediger ML, Overpeck MD, Ruan WJ, Troendle JF (2002) Birth weight and gestational age effects on motor and social development. Paediatr Perinat Epidemiol 16: 33-46.
- King TF, Bergin DA, Kent EM, Manning F, Reeves EP, et al. (2013) Endothelial progenitor cells in mothers of low-birth weight infants: A link between defective placental vascularization and increased cardiovascular risk? J Clin Endocrinol Metab 98: 33-39.
- Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB (2014) Maternal-fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int J Mol Sci 15: 16153-16185.
- Jansson T, Powell TL (2006) IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: Does the placenta function as a nutrient sensor? A review. Placenta 27 Suppl A: 91-97.
- McMinn J, Wei M, Schupf N, Cusmai J, Johnson EB, et al. (2006) Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta 27: 540-549.
- Watson-Jones D, Weiss HA, Changalucha JM, Todd J, Gumodoka B, et al. (2007) Adverse birth outcomes in United Republic of Tanzania-impact and prevention of maternal risk factors. Bull World Health Organ 85: 9-18.
- Salafia CM, Zhang J, Charles AK, Bresnahan M, Shrout P, et al. (2008) Placental characteristics and birth weight. Paediatr Perinat Epidemiol 22: 229-239.
- Golding J, Shenton T (1990) Low birth-weight and pre-term delivery in South-east Asia. The WHO international collaborative study of hypertensive disorders of pregnancy. Soc Sci Med 30: 497-502.
- Elshibly EM, Schmalisch G (2008) The effect of maternal anthropometric characteristics and social factors on gestational age and birth weight in Sudanese new-born infants. BMC Public Health 8: 244-245.
- Darling RD, Atav AS (2012) Risk factors for low birth weight in New York state counties. Policy Polit Nurs Pract 13: 17-26.
- Kent EM, Breathnach FM, Gillan JE, McAuliffe FM, Geary MP, et al. (2012) Placental pathology, birth weight discordance and growth restriction in twin pregnancy: Results of the ESPRiT Study. Am J Obstet Gynecol 207: 220 -225.
- Yampolsky M, Salafia CM, Shlakhter O, Haas D, Eucker B, et al. (2009) Centrality of the umbilical cord insertion in a human placenta influences the placental efficiency. Placenta 30: 1058-1064.
- Salafia CM, Yampolsky M, Misra DP, Shlakhter O, Haas D, et al. (2010) Placental surface shape, function and effects of maternal and fetal vascular pathology. Placenta 31: 958-962.
- Godfrey KM (2002) The role of the placenta in fetal programming - A review. Placenta 23: 20-27.
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, et al. (2006) Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 576: 935-946.
- Jansson T, Powell TL (2007) Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin Sci 113: 1-13.
- Roland MC, Friis CM, Godang K, Bollerslev J, Haugen G, et al. (2014) Maternal factors associated with fetal growth and birth weight are independent determinants of placental weight and exhibit differential effects by fetal sex. PLoS ONE 9: 87303.
- Winder NR, Krishnaveni GV, Veena SR, Hill JC, Karat CL, et al. (2011) Mother's lifetime nutrition and the size, shape and efficiency of the placenta. Placenta 32: 806-810.
- Diaz P, Powell TL, Jansson T (2014) The role of placental nutrient sensing in maternal-fetal resource allocation. Biol Reprod 91: 82.
- Wallace JM, Horgan GW, Bhattacharya S (2012) Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta 33: 611-618.
- Christian P (2014) Fetal growth restriction and preterm as determinants of child growth in the first two years and potential interventions. Nestle Nutr Inst Workshop Ser78: 81-91.
- Torheim LE, Barikmo I, Parr CL, Hatloy A, Ouattara F, et al. (2003) Validation of food variety as an indicator of diet quality assessed with a food frequency questionnaire for Western Mali. Eur J Clin Nutr 57: 1283-1291.
- Xu H, Shatenstein B, Luo ZC, Wei S, Fraser W (2009) Role of nutrition in the risk of preeclampsia. Nutr Rev 67: 639-657.
- Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, et al. (2016) Maternal anemia and risk of adverse birth and health outcomes in low and middle-income countries: Systematic review and meta-analysis. Am J Clin Nutr 103: 495-504.
- Khan A, Nasrullah FD, Jaleel R (2016) Frequency and risk factors of low birth weight in term pregnancy. Pak J Med Sci 32: 138-142.
- Mei VJ, Volmer M, Boersma ER (2000) Growth and survival of low birth weight infants from 0 to 9 years in a rural area of Ghana. Comparison of moderately low (1,501-2,000 g) and very low birth weight (1,000-1,500 g) infants and a local reference population. Trop Med Int Health 5: 571-577.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 4851
- [From(publication date):
June-2017 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 3807
- PDF downloads : 1044