MCP-1 Mediated Wound Injury Repair and Activation of Endothelium
Received: 01-Sep-2022 / Manuscript No. asoa-22-77592 / Editor assigned: 03-Sep-2022 / PreQC No. asoa-22-77592 (PR) / Reviewed: 17-Sep-2022 / QC No. asoa-22-77592 / Revised: 20-Sep-2022 / Manuscript No. asoa-22-77592 (R) / Accepted Date: 23-Sep-2022 / Published Date: 27-Sep-2022
Abstract
Reverse transcription- polymerase chain reaction, RNase protection, Western blot, and flow cytometric analysis showed that human umbilical vein endothelial cells express mRNA and surface protein of the monocyte chemotactic protein-1 (MCP-1) receptor CCR2, which was upregulated by inflammatory cytokines. MCP-1 induced migration of endothelial cells in a transwell assay, which was inhibited by the 9-76 MCP-1 receptor antagonists. Increased secretion of MCP-1 or interleukin-8, but not RANTES, on endothelial injury suggested a functional role of CCR2 in wound repair as measured by ELISA. After mechanical injury to endothelial monolayers, which spontaneously closed within 24 hours, wound repair was delayed by the 9-76 antagonists and by a blocking monoclonal antibody to MCP-1, but not to interleukin-8, and was improved by exogenous MCP-1. This was confirmed by quantification of cell migration into the wound area, whereas proliferation and viability were unaltered by MCP-1 or its analogue. Notably, immunohistochemistry of inflamed tissue revealed CCR2 staining on arterial, venous, and venular endothelium affected by cellular infiltration. This is the first demonstration of endothelial CCR2 expression ex vivo, inferring its involvement in inflammatory conditions. Thus endothelial cells express functional CCR2 that may have important implications for endothelial wound repair and inflammatory reactions.
Keywords
Atherosclerosis; Chemokine receptors; Migration; Chemokines; Endothelial cells
Introduction
Endothelial wound repair models have revealed a complex response involving migration and spreading of endothelial cells at the wound edge in the first 24 hours and proliferation distal to the wound after 36 hours. Although the regulation of endothe lial migration is known to be influenced by well characterized factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), endothelial cells can also respond to chemokines. For example, endothelial cell proliferation can be inhibited by the cysteine-x amino acid-cysteine (CXC) chemokines platelet factor 4 (PF-4), interferoninducible protein of 10 kd (IP-10), or growth- related oncogene (GRO-β) and, in contrast, induced by interleukin-8 (IL-8).
Chemokines are a family of homologous 8-10 kd proteins that direct leukocyte trafficking into areas of inflammation. The branches of this family are classified according to the position of the N-terminal cysteine residues. Cysteine-cysteine (CC) chemokines predominantly act on monocytes, T lymphocytes, natural killer cells, basophils, and eosinophils, whereas CXC chemokines act mainly on neutrophils and some lymphocyte subsets. Chemokines interact with G protein-coupled heptahelical receptors on the target cells, which may be regulated by the state of cell differentiation or by activation with various inflammatory mediators.
To date, a role for CC chemokines in endothelial cell proliferation or function has not been demonstrated. The CC chemokine MCP-1 has been found in atherosclerotic plaques and areas of endothelial denudation where it may direct mononuclear infiltration. The infusion of MCP-1 has been reported to increase the conductance after femoral arterial occlusion via vessel growth, implicating its involve- ment in angiogenesis. Furthermore, CCR2, a receptor for MCP-1, has recently been identified on vascular smooth muscle cells where it may influence proliferation and migration [1-3]. Hence, we studied the expression and function of CCR2 on endothelial cells. Our data show that endothelial cells express CCR2, which mediates endothelial cell migra tion to MCP-1, may contribute to the repair of endothelial wounds, and can be up regulated during inflammatory activation.
Surface Expression of CCR2 Protein as a Functional Receptor on HUVEC
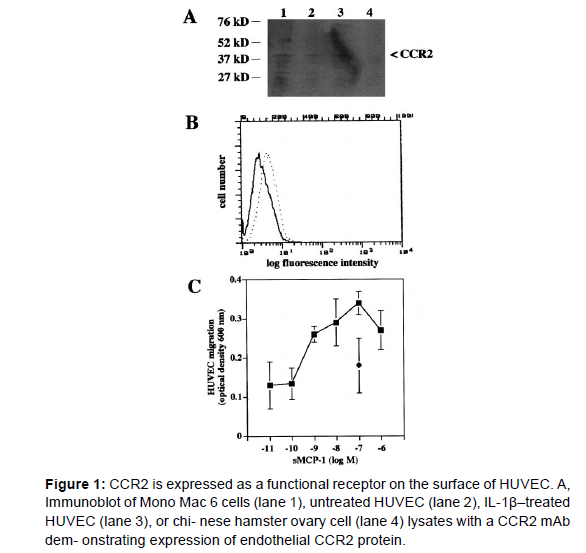
We next studied whether CCR2 protein was present in endothelial cells. Immunoblotting of HUVEC lysates revealed a band migrating at 38kDa consistent with the expression of CCR2 protein in endothelial cells, whereas Mono Mac 6 cell lysates were used as a positive and Chinese hamster ovary cell lysates as a negative control. The findings are consistent with similar bands observed for immunoblotting of CCR2 in B cells or Mono Mac 6 cells [4,5]. To test whether CCR2 is detectable on the endothelial surface, we performed flow cytometry analysis. HUVEC showed a distinct surface expression of CCR2, which was lower than that found on freshly isolated blood monocytes (not shown).32 Consistent with mRNA analysis, CCR2 protein and surface expression was up regulated by activation of HUVEC with inflammatory cytokines, eg, IL-1β (5 ng/mL) or TNF-α (50 U/mL) for 24 hours, as indicated by immunoblotting or flow cytometry (Table 1). These data show that CCR2 protein is expressed and regulated on the endothelial surface (Figure 1).
To study whether CCR2 surface expression was associated with functional responses of endothelial cells to MCP-1, we analyzed transmigration of HUVEC in a gelatin coated Trans well filter assays. CCR2 mediates MCP-1-induced signaling and transmigration in leukocytes, eg, in mononuclear cells, which characteristically occur in a bell-shaped dose- response curve and for monocytes, is optimal at 1 nmol/L of MCP-1 [6-8]. Addition of MCP-1 to the bottom chamber only (i.e., presence of a MCP-1 gradient) elicited HUVEC transmigration with a maximum index (ratio of stimulated migration/ spontaneous migration) of 2.84±0.68 (mean±SD, n=3) at 100 nmol/L; however, a bell-shaped dose-response curve was difficult to demonstrate. Transmigration induced by MCP-1 was blocked by the 9-76 MCP-1 analogue receptor antagonists, inferring a dependence on CCR2 (Figure 2). The presence of MCP-1 (100 nmol/L) in both top and bottom chamber (i.e., absence of a MCP-1 gradient) induced transmigration of HUVEC with an index (1.97±0.46, mean ± SD) that was lower than that in the presence of a gradient (i.e., MCP-1 in the bottom chamber only). This indicates that migration induced by MCP- 1 was both chemotactic (directed) and chemokinetic (nondirected). Thus endothelial CCR2 is a functional receptor for MCP-1[9,10].
Discussion
We found that endothelial cells express mRNA and surface protein for the MCP-1 receptor CCR2. This was associated with a migratory response to MCP-1 that may play a role in directing cell migration during endothelial wound injury. To our knowledge, this is also the first demonstration of CCR2 expression on inflamed endothelium in vivo. Although effects of CC chemokines and their receptors on endothelial cells have not yet been elucidated, various CXC chemokines have been shown to modulate endothelial cell proliferation.7-12 For example, PF-4 and IP-10 can suppress angiogenesis, possibly by competing with known growth factors, such as bFGF and TGF-β, for heparan sulfate proteoglycan binding sites and inhibiting the proliferative effects of these growth factors.
Endothelial cells express CXCR1 and CXCR4, which can mediate effects of the CXC chemokines IL-8 and stromal cell- derived factor-1 (SDF-1), respectively.13,38 - 40 The abil- ity of IL-8 to induce angiogenesis in association with the presence of CXCR1 on endothelial cells was paralleled and expanded by our findings that MCP-1 and endothelial CCR2 participate in direct receptor-mediated functions.
Our results show that endothelial cells expressed low levels of CCR2 mRNA and to a lesser extent, CCR1 mRNA. Previously, the expression of CXCR1 but not CXCR2 mRNA has been described in HUVEC. In a recent report, generation of a cDNA plasmid library from HUVEC mRNA using consensus region PCR primers for chemokine receptors and sequencing of random clones revealed the presence of mRNA for CXCR4 and CCR3 as well as for CXCR1, CCR1, and CCR2. Northern blot analysis confirmed the abundance of CXCR4 mRNA; however, it failed to detect mRNA for CCR1 or CCR2. A more recent study did not reveal CCR2 mRNA in HUVEC using only RTPCR or MCP-1-induced chemotaxis of HUVEC. In contrast, our data clearly demonstrate the expression of CCR2 mRNA in endothelial cells. Immunoblotting, surface staining, and migration assays further confirmed the expression of functional CCR2 on HUVEC. Similarly, flow cytometric analysis and migration assays indicated expression of functional CXCR4, but endothelial responses to MCP-1 or IL-8 were not seen in contrast to this and other reports. Contrasting findings may be due to differences in the sensitivity of RT-PCR, as well as the migration assays used or due to the heterogeneity of chemo- kine receptor expression in distinct HUVEC preparations or under culture conditions with different media.
The CCR2 mRNA and surface protein expression could be upregulated by activation of HUVEC with inflammatory cytokines, such as IL-1β or TNF-α. This is in accordance with findings that IL- 1β or TNF-α cause a biphasic regula- tion of endothelial CXCR4 mRNA expression with an initial inhibition and subsequent prolonged induction. In contrast, IL-1β or TNF-α downregulated CCR2 expression and MCP-1 mediated transmigration in monocytes, whereas IFN-γ decreased CXCR4 mRNA stability in HUVEC but did not affect CCR2 in monocytes. Moreover, IL-1α did not alter CXCR1 expression in HUVEC, and TNF-α did not regulate CCR1 and CCR2 in smooth muscle cells. The differential regulation of chemokine receptors by various cytokines in distinct cellular systems may indicate a cell specific involvement in various pathophysiological conditions. Our results suggest that regulation of endothelial CCR2 by inflammatory cytokines, which induce endothelial secretion of MCP-1, may promote endothelial migration under conditions such as inflammatory reactions or wound injury repair after angioplasty.
We found that MCP-1 induced a maximum index of HUVEC migration at 100 nmol/L, whereas leukocyte chemotaxis to MCP- 1 is optimal at 1 nmol/L. Inhibition with the 9-76 MCP-1 receptor antagonist confirmed that MCP-1- induced migration of endothelial cells was dependent on CCR2, as seen in monocytes. Endothelial cell migration and Ca2+ mobilization in response to the CXC chemokines IL-8 and SDF-1 also occurred more effectively at higher concentrations than those required for leukocyte responses. In addition, Ca2+ mobilization in smooth muscle cells was only induced by MCP-1 or MIP-1α at concentrations of 500 nmol/L,24 inferring cell type specific differences in chemokine responsiveness. Flow cytometry revealed that surface expression of CCR2 on HUVEC was lower than on monocytes. Moreover, specific binding sites for IL-8 on HUVEC have been suggested to be of lower affinity and density than on neutrophils. Thus migratory responses may vary with differences in chemokine receptor characteristics. A bell shaped dose response curve was difficult to demon- strate for HUVEC migration to SDF-1. Moreover, transmigration of HUVEC was induced by a MCP-1 gradient and, to a lesser extent, in the presence of nongradient MCP-1, showing that it was both chemotactic (directed) and chemo- kinetic (nondirected), as has been shown with transferrin. This suggests that MCP-1 increases overall movement of HUVEC (Figure 2).
We have used a previously described HUVEC wound injury model,29 which reproducibly exhibited migration of a comparable number of endothelial cells into the wound area and closure of the wound path within 24 hours to 36 hours, thus representing a suitable model to study wound healing and repair of vascular endothelium in vitro. The presence of 9-76 MCP-1 receptor antagonist or neutralizing MCP-1 mAb impaired the ability of HUVEC to migrate and close the wound, whereas addition of MCP-1 appeared to facilitate the repair, suggesting a role for MCP-1-induced migration in endothelial wound repair. Moreover, endothelial MCP-1 se- cretion was increased by multiple wound injury. This clearly extends findings that MCP-1 mRNA expression was in- creased by endothelial denudation after balloon angioplasty, particularly evident at the migrating cell front, and that higher levels of MCP-1 mRNA were found in migrating sub confluent than quiescent confluent endothelial cells, which was attributed to effects of endothelial bFGF. Secretion of chemokines and their immobilization to matrix proteins being established by the endothelial cell front may create a naturally occurring gradient to direct migration. Notably, exogenous MCP-1 resulted in a more efficient wound injury repair despite a more haphazard appearance of the monolayer. This may be due to a immobilization of MCP-1 to continuously generated matrix components at high concentrations. Endothelial cells have been shown to respond by migrating and spreading within 24 hours after wound injury, whereas proliferation becomes prominent only after 36 hours. Unlike the CXC chemokines IL-8 and PF-4, we observed that MCP-1 or its receptor antagonist did not affect HUVEC proliferation. Moreover, inhibition by the MCP-1 receptor antagonist was obvious within the first 12 hours. This suggests that MCP-1 predominantly acts by inducing migration during endothelial wound injury.
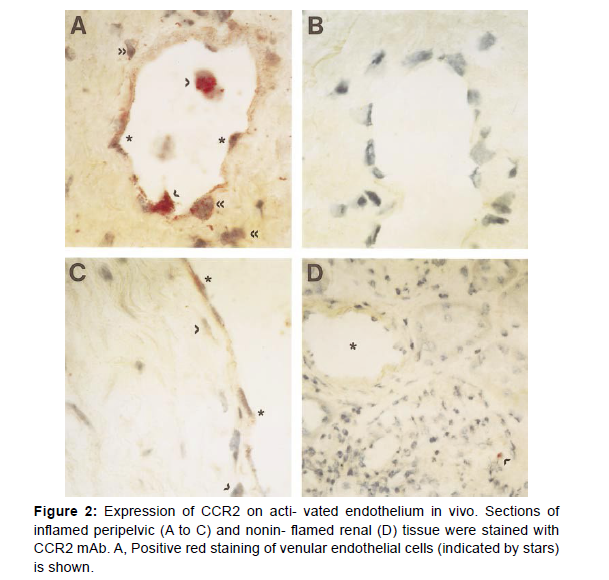
Our results expand findings that MCP-1 and MIP-1α are critical in wound repair by mediating macrophage recruitment and that MCP-1 may facilitate angiogenesis, implying that these processes are in part due to endothelial mechanisms. Notably, immunohistochemistry revealed CCR2 expression on activated endothelium of venules, veins, and arteries in chronically inflamed tissue, demonstrating for the first time endothelial expression of a CC chemokine receptor in vivo. The most prominent CCR2 staining on endothelial cells appeared to occur in the proximity of mononuclear infiltra- tion. This may suggest an upregulation of endothelial CCR2 in association with secretion of inflammatory cytokines by mononuclear cells, whereas CCR2 staining on subendothelial mononuclear cells appeared to be less prominent than in cells before extravasation, consistent with the differential regulation of CCR2 by cytokines in distinct cell types. A chronic inflammatory irritation may result in a disturbed endothelial integrity, which may require endothelial CCR2 expression to maintain vascular hemostasis under conditions of inflamma- tory activation. Conversely, excessive endothelial CCR2 levels may contribute to vascular damage. Vascular injury, eg, by angioplasty, can also trigger inflammatory reactions leading to dysregulated wound repair and ultimately restenosis. Our data may thus have important implications for many inflammatory reactions in vivo.
Our findings exemplify the diverse functions of chemo- kines that appear to play an increasingly evident role in vascular pathology. The identification of CCR2 in endothelial cells shown here and in vascular smooth muscle cells indicates that MCP-1 may not only mediate monocyte infiltration but may participate in endothelial or smooth muscle cell migration and proliferation during processes, such as atherosclerosis, restenosis, and chronic stages of inflammatory diseases. Intimal hyperplasia of vein grafts, which involves an exaggerated vascular wound healing response, has been associated with increased MCP-1 levels that may stimulate monocyte infiltration as well as promote endothelial migration. The advent of chemokine peptide antagonists, eg, the 9-76 MCP-1 analogue, which has already been tested in a murine arthritis model, may offer encouraging novel approaches in the treatment of inflammatory conditions, restenosis, or angiogenic tumors.
References
- Traill LW, Lim LMM, Sodhi NS, Bradshaw CJA (2010) Mechanisms driving change: altered species interactions and ecosystem function through global warming. J Anim Ecol 79: 937-947.
- Jabbar A, Abbas T, Sandhu ZUD, Saddiqi HA, Qamar MF, et al. (2015) Tick-borne diseases of bovines in Pakistan: major scope for future research and improved control. Parasit Vector 8: 283.
- Skagen FM, Aasheim ET (2020) Health personnel must combat global warming. Tidsskr Nor Laegeforen 14: 1-14.
- Eygelaar D, Jori F, Mokopasetso M, Sibeko KP, Collins N, et al. (2015) Tick-borne haemoparasites in African buffalo (Syncerus caffer) from two wildlife areas in Northern Botswana. Parasites & vectors 8: 1-11.
- Nejash A (2016) Review of Important Cattle Tick and Its Control in Ethiopia.Vector Biol J 3: 1-11.
- Hamsho A, Tesfamary G, Megersa G, Megersa M (2015) A Cross-Sectional Study of Bovine Babesiosis in Teltele District, Borena Zone, Southern Ethiopia. J Veterinar Sci Technolo 6: 1-4.
- Frölicher TL, Fischer E M, Gruber N (2018) Marine heatwaves under global warming. Nature 560: 360-364.
- Simuunza MC (2009) Differential Diagnosis of Tick-borne diseases and population genetic analysis of Babesia bovis and Babesia bigemina. Bio Med J 13: 36.
- Klopper A (2021) Delayed global warming could reduce human exposure to cyclones. Nature 98: 35.
- Kay JE (2020) Early climate models successfully predicted global warming. Nature 578: 45-46.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Weber K, Nelson P, Grone HJ, Weber C (2022) MCP-1 Mediated Wound Injury Repair and Activation of Endothelium. Atheroscler Open Access 7: 183.
Copyright: © 2022 Weber K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 1875
- [From(publication date): 0-2022 - Nov 11, 2025]
- Breakdown by view type
- HTML page views: 1414
- PDF downloads: 461