Metabolic Endoscopy with Intra-Gastric Balloon, Improves Obesity Related Hepatic Steatosis Indices, with Changes in Gut Microbiota
Received: 16-Jul-2022 / Manuscript No. JOWT-22-69376 / Editor assigned: 18-Jul-2022 / PreQC No. JOWT-22-69376 (PQ) / Reviewed: 01-Aug-2022 / QC No. JOWT-22-69376 / Revised: 06-Aug-2022 / Manuscript No. JOWT-22-69376 (R) / Published Date: 13-Aug-2022
Abstract
Background and Aims: Emerging evidence suggest that metabolic endoscopy with devices such as the intra-gastric balloon (IGB) may be valuable, besides bariatric surgery, in managing obesity and related non-alcoholic fatty liver disease (NAFLD). NAFLD ranges from hepatic steatosis through non-alcoholic steatohepatitis to fibrosis and cirrhosis. We sought to determine the efficacy of the IGB in obesity-related hepatic steatosis and related non-invasive indices along with changes in gut microbiota and nutritional patterns.
Materials and Methods: Thirty-three obese patients, body mass index (BMI) >30kg/m2, with hepatic steatosis were recruited for IGB treatment. Three patients withdrew early in the study. Of the remaining thirty, mean whole group BMI was 39.3±6kg/m2 and mean whole group weight was 110.5±18.5kg. Two patients failed to present for end-of-study assessments. On IGB removal at six months, paired baseline and end-of-study results were available for 28 patients. Anthropometric, nutritional data, blood and fecal samples were collected at baseline and at six months. Gut microbiota diversity was assessed by 16S RNA sequencing.
Results: On IGB removal, patients were sub-divided into those losing ≥10% of initial body weight (Group 1) and those losing <10% of initial bodyweight (Group 2). Group 1 had a significant reduction (p<0.05), in weight, BMI, waist circumference (WC), HOMA-IR, HbA1C, AST, GGT along with a non-significant reduction in ALT and NAFLD fibrosis score (p<0.08). Group 2 had a significant reduction in WC, p=0.02. Retrospective analyses between Group 1 and Group 2 showed no differences in baseline characteristics. At baseline, the mean estimated daily total energy intake (TEI) reported by the cohort was 6467.5±3413.6KJ, with estimated daily nutrient composition at approximately 51±52% carbohydrate (CHO), 19.3±5% protein, and 25.6±8.3 fat. Mean daily sugar intake was estimated at 97.8±135.7g. At final follow up, comparing Group 1 to Group 2, estimated daily TEI showed a non-significant reduction at 5550.9±2227.4 vs 8404.7± 1566.1 (p= 0.07) as did total fat intake(g) at 37.9±16.5 vs 67.5±61.7, p=0.08. There was between Group 1 and Group 2 however, a difference in estimated daily carbohydrate intake at 167±70.5 vs 248.3±141.6 (p=0.05) along with a difference in estimated daily sugar consumption, at 79.8 ±48g vs 137.3 ±88.6 (p=0.02). In Group 1, at final follow up, there was compared to baseline a significant reduction in CHO as a percentage of TEI, at 54.5±8.9 reduced to 49.1±6.4, p=0.04. The cohort bacterial community structure did not differ significantly at baseline but was mildly altered post-IGB and enriched with the genus Bacteroides. The microbiomes differed in the two groups post-IGB. Group 1 showed a decrease in Streptococcus, Rothia and Butyrivibrio, while Clostridium XI was enriched.
Conclusions: Metabolic endoscopy with IGB improves anthropometric indices in obese patients with hepatic steatosis. Indices associated with obesity-related hepatic steatosis were also reduced in patients losing ≥10% of initial body weight. These patients showed a significant reduction in carbohydrate consumption. The weight loss and lowered CHO consumption was accompanied by mild changes in the microbiome with enrichment of Clostridium XI in Group 1. The significance of these nutritional and microbiota changes is uncertain but warrants further investigation.
Keywords
Obesity; Non-alcoholic fatty liver disease (NAFLD); Hepatic steatosis; Metabolic endoscopy; Intra-gastric balloon; Microbiota
Abbreviations:
BMI: Body Mass Index; CAP: CRP (C-reactive protein); HDL: high density lipoprotein; IGB intra-gastric balloon; LDL: low density lipoprotein; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; VCTE: Vibration-Controlled Transient Elastography
Introduction
Obesity-related liver disease, non-alcoholic fatty liver disease (NAFLD), is now the leading cause of liver disease in affluent countries and many emerging economies and is expected to soon become the main indication for liver transplantation [1,2]. NAFLD is mostly linked with obesity and closely associated with other metabolic syndrome components, including impaired glucose tolerance (type 2 diabetes), hypertension, and dyslipidemia [3]. Emerging evidence suggests that intestinal microbiota may contribute to the development of obesity and NAFLD through processes that may include increased energy harvest, impaired intestinal permeability, metabolic endotoxemia, immune system modulation, and modification of satiety [4]. Cross-sectional studies have also examined the relationship between gut microbiota and NAFLD patients [5,6].
Lifestyle interventions remain the primary treatment proposal for NAFLD, with all guidelines suggesting weight loss of up to 10% [7]. This is, however, difficult for patients, and metabolic endoscopic devices such as the intra-gastric balloon (IGB) may provide an effective modality. IGBs may be useful because as compared to metabolic surgery, they are less invasive. Additionally, IGBs calm patients fears of the potential complications of metabolic surgery. Finally, IGBs reduce patients’ stigma of undergoing metabolic surgery and offer choice to patients who want to downsize but do not want to undergo metabolic surgery [8,9].
Abu Dayyeh, et al. [10], involving the Orbera intra-gastric balloon (IGB) as used here in our study, it had already been shown that at 6 months, at which point the IGB was removed, 71.8% of the patients who underwent IGB insertion and behavioural management program (BMP - control Group) achieved significant excess weight loss (EWL) compared to subjects in the BMP alone (control group), on an intention to treat analysis, p<0.001. In a subsequent met-analysis it was shown with 3 randomised controlled studies that the %EWL in patients who received the Orbera IGB (n = 131) compared with a control group (n = 95), the mean difference in %EWL in patients who received the Orbera IGB over controls was 26.9%, p ≤ 0.001 [11].
In a more recent American Gastroenterological Association technical review of the IGB in the management of obesity [12], seven randomised controlled trials met inclusion criteria, with outcome of weight loss at six months. The met-analysis comprised 628 individuals who underwent IGB therapy, and 551 individuals who were treated with control-standard of care (SOC). Mean weight loss in the IGB group differed significantly (p<0.05) from the SOC group with weight loss ranging from 7.1kg to 17.1kg (IGB Group) and in the SOC group ranging from 3.2kg to 6.4kg (mean difference 7.0kg; 95% CI, 4.7– 9.3kg). The point on the controlled efficacy of the IGB is well made. We ourselves have furthermore shown [13] that the IGB is efficacious in weight loss.
Mechanistically, it is believed that the IGB increases satiety through mechanical and neuroendocrine mechanisms [14,15]. However, the precise or putative mechanisms of the IGB in reducing obesity and indices associated with NAFLD remain unclear. Specifically, no studies have examined the effect of IGB treatment and weight loss on gut microbiota and the impact of nutritional patterns in the process. The purpose of our current study was therefore to determine whether metabolic endoscopy with the IGB, in obese patients with hepatic steatosis, induces weight loss and in so doing reduces indices associated with obesity and hepatic steatosis and whether the mechanism may involve changes in gut microbiota. Mechanistically, we then examined changes in metabolic parameters, gut microbiota and dietary patterns.
Materials and Methods
Study Design
This was a prospective study conducted at the Guy’s and St Thomas’ Hospital (King’s College, London) from August 2015 to April 2016. Included patients satisfied the following criteria: age ≥ 18 years; BMI ≥ 30 kg/m2. All patients had a diagnosis of obesity-related hepatic steatosis confirmed by abdominal ultrasound (n=20), or a raised CAP reading above 268 dB/m on VCTE, n=10 [16]; alcohol intake in all patients was ≤ 20 grams per day. A comprehensive liver screen to exclude the presence of other liver diseases was also performed.
Thirty-three obese patients, body mass index (BMI) >30kg/m2, with hepatic steatosis were recruited for IGB treatment. Three patients withdrew early in the study. Of the remaining thirty, mean whole group BMI was 39.3±6kg/m2 and mean whole group weight was 110.5±18.5kg. Two patients failed to present for end-of-study assessments. On IGB removal at six months, paired baseline and end-of-study results were available for 28 patients. Anthropometric, nutritional data, blood and fecal samples were collected at baseline and at six months. Gut microbiota diversity was assessed by 16S RNA sequencing.
The included patients therefore were all obese with hepatic steatosis. Patients agreed to insertion of intra-gastric balloon (IGB) as a management of obesity either as a bridge to metabolic surgery or as a stand-alone therapy. Exclusion criteria included: any exposure to antibiotics or probiotics in the previous six months; decompensated cirrhosis; chronic gastrointestinal diseases; previous surgery that had modified the gastrointestinal anatomy; severe gastritis/esophagitis, gastric ulceration; intolerance of IGB insertion, or the inability to maintain IGB therapy for >4 months, and current pregnancy.
A standard clinical workup prior to IGB insertion, including anthropometric assessment, fasting blood tests, and liver Fibroscan- CAP assessment. On the day of IGB insertion, patients provided a fecal sample for profiling the gut microbiota. The primary endpoint was weight loss at the time of IGB removal at six months from insertion, with repeat collection of biological samples and clinical assessment. The study was conducted according to the principles of the Declaration of Helsinki and the National Statement on Ethical Conduct in Research Involving Humans.
Intra-gastric Balloon Insertion
Patients underwent gastroscopy under conscious sedation, following which an IGB (Orbera, Apollo Endosurgery, Austin, Texas 78746, USA) was inserted orally into the stomach and filled with 500- 600mL of normal saline. At six months following insertion, each IGB was extracted as per the manufacturer’s instructions [13].
Dietary Intervention and Nutrient Surveyvv
Each patient received between 2-3 individualized, face-to-face, visits with a clinical dietician during the six months following IGB insertion. Patients were counseled on portion control to reduce total calorie intake without any specific macro- or micronutrient modification recommendations. Dietary intake assessment was carried out before IGB insertion and following IGB removal. Dietary composition and energy intake were ascertained through a self-reported three-day food diary, 24-hour recall, and a mini food frequency questionnaire. Nutritional intake data was calculated using Foodworks 8 (Xyris Software; Australia).
Anthropometrics, Biochemistry, and Liver Stiffness Assessment
Weight, height, blood pressure, heart rate, and waist circumference measurements were undertaken using standard protocols at baseline (just before IGB insertion) and following IGB removal [17]. Fasting blood samples were collected at baseline and at IGB removal for assessment of blood counts, liver function tests, renal function, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, glucose, insulin, HbA1c, vitamin D, CRP, and insulin resistance. Insulin resistance was calculated using the Homeostasis Model Assessment (HOMA)-IR score [18]. The Fib- 4 score was determined as a serological index of liver fibrosis, with a cut-off range of < 1.45 approximating with F0-F1 level of fibrosis, and scores > 3.25 approximating with F3-F4 [19]. The NAFLD fibrosis score was also calculated [19]. Vibration-Controlled Transient Elastography (VCTE) was measured using a FibroScan® device (Paris, France; Model 502 Touch]). Controlled attenuation parameter (CAP - a coefficient directly proportional to hepatic steatosis) was measured in conjunction with VCTE. Cut-off values > 268 dB/m correlated with steatosis (>5% liver fat) [20].
Gut Microbiota
Crude stool was collected from each patient either at the time of intragastric balloon insertion, or within 48 hours of IGB insertion. Fecal DNA extraction was carried out using the PowerSoil® DNA Isolation Kit (MO BIO Laboratories, USA). 16S RNA sequencing was performed on an Illumina MiSeq instrument (Illumina Inc, USA) [21]. The sequence reads were analyzed using the MiSeq SOP Pipeline of the Mothur bioinformatics toolbox, and sequence alignment was performed using the Silva bacterial database. Classification of sequences and operational taxonomic units (OTUs; phylum to genus) was undertaken using the Ribosomal Database Project (RDP) MultiClassifier script.
Statistical Analysis
Based on previous studies examining for significant metabolic (weight loss >5%) and histological changes following a lifestyle intervention in subjects with NAFLD, we estimated that the sample size required for a 90% confidence interval, with a 0.5 standard deviation, would be 26-30 patients [22,23]. At the study completion, all patients were divided into those losing ≥10% of initial body weight and those losing <10% initial body weight. This level of weight loss is accepted as required to cause a reduction in obesity-related liver disease through a reduction in insulin resistance [24]. Statistical analyses were performed using SPSS 24.0 (SPSS Inc, Chicago, USA) and R 3.6.3. Our data were tested by the Shapiro-Wilk’s test and found not to be normally distributed. Non-parametric continuous data were analyzed using the Mann Whitney test, while categorical data were analyzed using Chisquared tests or Fisher’s exact tests. When appropriate, Pearson or Spearman correlations were used to examine for correlations between total weight-loss amount, the two categories of weight loss (<10% versus ≥10%), and all changes in anthropometric, biochemical, and serological parameters between baseline and follow up. All significance tests were two-tailed, and differences were considered significant at p < 0.05.
As required, missing values involving clinical parameters were first scaled using StandardScaler and then imputed using k-Nearest Neighbors (KNNImputer). These parameters were then fed to a decision tree (DecisionTreeClassifier) to quantify their contribution to the target variable, which involving the two categories of weight loss. These three modules were available through the machine learning library scikitlearn package ver.0.24.1. Two-sided Wilcoxon signed-rank tests and permutational multivariate analyses of variance were used to assess for any statistically significant differences in taxonomic and functional measures of bacterial community composition. Spearman rank tests were used to assess for any correlations between significant changes in clinical parameters occurring between baseline and follow-up as required. All analyses were performed with corrections for multiple comparisons using the Benjamini–Hochberg procedure as we have previously published [25]. Non-metric multidimensional scaling (NMDS) using the Bray-Curtis dissimilarity measure was also used to check for the absence of any clear separation between pre- and post- IGB samples.
Results
Anthropometric and biochemical results
Thirty-three patients were recruited for the study with three withdrawing early in the study. Baseline anthropometric and biochemical results were therefore available for 30 patients. The mean whole cohort baseline weight was 110.5±18.5kg, BMI 39.3±6kg/ m2, waist circumference 125.1±13.7cm. All obese patients had their diagnosis of steatosis established by the presence of hepatic steatosis on liver ultrasound (n=20), or a raised CAP reading above 268 dB/m on VCTE (n=10) [16]. The mean weight of the whole group at baseline was 110.5±18.5kg, BMI 39.3±6kg/m2, waist circumference 125.1±13.7cm. Half of the patients had metabolic syndrome, and 30% had type 2 diabetes (T2DM). The median HOMA-IR score was 5.29 (1.67-8.90), and the mean HbA1c was 6.35%. Baseline fibrosis scores were: NAFLD fibrosis score −0.73 (-1.04 − -0.42), FIB-4 1.27 (1.03−1.52), and median liver stiffness on transient elastography 6Kpa. The mean baseline CAP reading was 281dB/m. Paired baseline and follow-up results were available for 28 patients, with 2 patients failing to present for end-ofstudy assessments. On IGB removal at six months, patients were subdivided into those losing ≥10% of initial body weight (Group 1, n=15; Table 1) and those losing <10% of initial bodyweight (Group 2, n=13; Table 2).
Compared to baseline, Group 1 had a significant reduction (p<0.05), in bodyweight, BMI, waist circumference (WC), HOMA-IR, HbA1C, AST, GGT along with a non-significant reduction in ALT and NAFLD fibrosis score (p<0.08). To elaborate, in Group 1, the mean weight loss was 17kg, approximately 15.25% lost compared to baseline with mean BMI reduction at 6.35 kg/m2. In Group 1, statistically significant reductions were also observed in mean waist circumference 125.9 ± 13.5 vs 110.5 ± 12.4 p<0.01), median HOMA-IR scores of 5.29 (1.85 – 7.73) vs. 2.24 (0.82 – 2.83), p=0.01); median % HbA1c of 6.45 (range 5.58 – 7.33) vs. 5.80 (5.50 – 6.10), p<0.01 and AST at 28.1 ± 9.5 to 22.8 ± 5.2, p<0.05) as compared to baseline (Table 1). In Group 2, mean weight loss was 2.1kg, at approximately 2.78% from baseline, BMI reduction of 0.73 kg/m2 (all non-significant compared to their baseline). Group 2 only had a significant reduction in WC, p=0.02. Retrospective analyses between Group 1 and Group 2 showed no differences in anthropometric baseline characteristics between these 2 Groups (Table 3).
| Group 1: Weight loss ≥ 10% (n=15) |
|||
|---|---|---|---|
| Clinical parameter | Pre-IGB therapy | Post-IGB therapy | p-value |
| Weight (kg), mean ± SD | 110.5 ± 18.5 | 93.5 ± 15.5 | <0.01 |
| BMI (kg/m2), mean ± SD | 40.2 ± 6.7 | 33.9 ± 5.4 | <0.01 |
| Waist circumference (cm), mean ± SD | 125.9 ± 13.5 | 110.5 ± 12.4 | <0.01 |
| Systolic blood pressure (mmHg), mean ± SD | 132 ± 27 | 131 ± 23 | 0.91 |
| Diastolic blood pressure (mmHg), mean ± SD | 79 ± 15 | 81 ± 14 | 0.50 |
| HOMA-IR, median (range*) | 5.29 (1.85 – 7.73) | 2.24 ± (0.82 – 2.83) | 0.01 |
| HbA1c (%), median (range*) | 6.45 (5.58 – 7.33) | 5.80 ± (5.50 – 6.10) | <0.01 |
| Fasting cholesterol (mmol/L), mean ± SD | 4.46 ± 1.10 | 4.32 ± 1.58 | 0.85 |
| Fasting LDL (mmol/L), mean ± SD | 2.71 ± 1.24 | 2.38 ± 1.13 | 0.50 |
| Fasting triglycerides (mmol/L), mean ± SD | 1.54 ± 0.89 | 1.53 ± 1.64 | 0.99 |
| Fasting HDL (mmol/L), mean ± SD | 1.22 ± 0.35 | 1.31 ± 0.35 | 0.13 |
| ALT (U/L), mean ± SD | 28.9 ± 14.3 | 21.1 ± 8.34 | 0.08 |
| AST (U/L), mean ± SD | 28.1 ± 9.5 | 22.8 ± 5.2 | 0.04 |
| GGT (U/L), mean ± SD | 46.4 ± 29.1 | 30.0 ± 15.4 | 0.03 |
| Ferritin (μg/L), mean ± SD | 107.8 ± 164.5 | 105.8 ±103.8 | 0.22 |
| 25-hydroxy vitamin D (nmol/L), mean ± SD | 41.1 ± 17.8 | 58.3 ± 13.1 | 0.43 |
| Fib4 score, median (range*) | 1.27 (1.03 – 1.52) | 1.17 (0.51 – 1.40) | 0.43 |
| NAFLD fibrosis score, median (range*) | -0.73 (-1.04 – -0.42) | -1.22 (-1.67 – -0.80) | 0.08 |
| Transient elastography (median stiffness, kPa), median (range*) [n=9] | 6.01 (4.9 – 7.1) | 5.32 (4.71 – 7.4) | 0.31 |
| CAP (dB/m), mean ± SD | 276.4 ± 92 | 248.1 ± 78 | 0.68 |
Table 1: Changes in clinical parameters following IGB therapy. Weight loss at IGB removal ≥10%, Group 1.
At six months, the 28 patients were divided into those losing ≥10% of body weight from baseline, p<0.01 (Group 1, n=15, Table 1) and those losing <10% from baseline (Group 2, n=13, Table 2). In Group 1, with weight reduction of ≥10%, there was a parallel significant reduction of BMI, waist circumference, HOMA-IR, HbA1C, AST, and GGT. ALT was also reduced but did not reach significance, p=0.08. Retrospective analysis between Group 1 and Group 2 showed no differences between them at baseline (Table 3).
| Group 2: Weight loss <10% (n=13) |
|||
|---|---|---|---|
| Clinical parameter | Pre-IGB therapy | Post-IGB therapy | p-value |
| Weight (kg), mean ± SD | 110.4 ± 18.2 | 108.3 ± 19.4 | 0.12 |
| BMI (kg/m2), mean ± SD | 38.4 ± 5.4 | 37.7 ± 6.2 | 0.15 |
| Waist circumference (cm), mean ± SD | 124.2 ± 12.2 | 119.4 ± 14.4 | 0.02 |
| Systolic blood pressure (mmHg), mean ± SD | 120 ± 16 | 121 ± 24 | 0.78 |
| Diastolic blood pressure (mmHg), mean ± SD | 79 ± 19 | 79 ± 17 | 0.99 |
| HOMA-IR, median (range*) | 3.22 ± (1.33 – 5.10) | 2.05 ± (1.02 – 3.67) | 0.11 |
| HbA1c (%), median (range*) | 6.25 ± (5.67 – 6.83) | 6.17 ± (4.89 – 6.78) | 0.33 |
| Fasting cholesterol (mmol/L), mean ± SD | 4.27 ± 1.53 | 4.59 ± 1.10 | 0.29 |
| Fasting LDL (mmol/L), mean ± SD | 2.55 ± 1.14 | 2.59 ± 1.15 | 0.50 |
| Fasting triglycerides (mmol/L), mean ± SD | 1.64 ± 0.99 | 1.53 ± 1.64 | 0.81 |
| Fasting HDL (mmol/L), mean ± SD | 1.20 ± 0.33 | 1.29 ± 0.41 | 0.77 |
| ALT (U/L), mean ± SD | 21.5 ± 7.9 | 20.5 ± 8.00 | 0.12 |
| AST (U/L), mean ± SD | 24.2 ± 5.6 | 25.1 ± 5.5 | 0.60 |
| GGT (U/L), mean ± SD | 35.3 ± 25.0 | 35.8 ± 21.3 | 0.89 |
| Ferritin (μg/L), mean ± SD | 144.7 ±143.7 | 129.4 ±160.0 | 0.88 |
| 25-hydroxy vitamin D (nmol/L), mean ± SD | 45.2 ± 13.1 | 47.0 ± 16.4 | 0.43 |
| Fib4 score, median (range*) | 1.10 (0.61 – 1.60) | 1.13 (0.37 – 1.46) | 0.42 |
| NAFLD fibrosis score, median (range*) | -1.10 (-1.79 – -0.41) | -0.81 (-1.67 – -1.10) | 0.72 |
| Transient elastography (median stiffness, kPa), median (range*) [n=7] | 6.68 (4.5 – 10.0) | 4.53 (4.34 – 6.4) | 0.60 |
| CAP (dB/m), mean ± SD | 286.4 ± 74 | 294.6 ± 38 | 0.61 |
| * 25-75% interquartile ranges expressed. | |||
Table 2: Changes in clinical parameters following IGB therapy. Weight loss at IGB removal ≥10%, Group 2.
In Group 2 weight reduction at 6 months was <10% of initial body weight. In Group 2 Waist circumference was significantly reduced from baseline, p=0.02, but no other clinical parameters were reduced. Retrospective analysis between Group 1 and Group 2 showed no differences between them at baseline (Table 3).
| Baseline characteristic | Group 1: Weight loss ≥ 10% (n=15) |
Group 2: Weight loss < 10% (n=13) |
p-value |
|---|---|---|---|
| Age (years), mean ± SD | 48.5 ± 10.1 | 44.5 ± 11.0 | 0.33 |
| Gender (M/F) | 3/12 (20%/80%) | 5/8 (38%/62%) | 0.26 |
Ethnicity
|
7 (47%) 6 (40%) 2 (13%) |
8 (62%) 3 (23%) 2 (15%) |
0.52 |
| Type 2 diabetes | 5/15 (33%) | 4/13 (31%) | 0.60 |
| Metabolic syndrome | 7/15 (47%) | 8/13 (62%) | 0.59 |
| Weight (kg), mean ± SD | 110.5 ± 18.5 | 110.4 ± 18.2 | 0.99 |
| BMI (kg/m2), mean ± SD | 40.2 ± 6.7 | 38.4 ± 5.4 | 0.45 |
| Waist circumference (cm), mean ± SD | 125.9 ± 13.5 | 124.2 ± 12.7 | 0.74 |
| Systolic blood pressure (mmHg), mean ± SD | 132 ± 27 | 120 ± 16 | 0.15 |
| Diastolic blood pressure (mmHg), mean ± SD | 79 ± 15 | 79 ± 19 | 0.97 |
| Fasting glucose (mmol/L), mean ± SD | 5.99 ± 2.78 | 5.41 ± 1.19 | 0.49 |
| Fasting insulin (pmol/L), mean ± SD | 123.2 ± 79.0 | 88.8 ± 66.1 | 0.24 |
| HOMA-IR, median (range*) | 5.29 (1.67 – 8.90) | 3.22 ± (1.33 – 5.10) | 0.32 |
| HbA1c (%), median (range*) | 6.45 (5.58 – 7.33) | 6.25 ± (5.67 – 6.83) | 0.68 |
| Fasting cholesterol (mmol/L), mean ± SD | 4.46 ± 1.10 | 4.27 ± 1.53 | 0.71 |
| Fasting LDL (mmol/L), mean ± SD | 2.71 ± 1.24 | 2.55 ± 1.14 | 0.72 |
| Fasting triglycerides (mmol/L), mean ± SD | 1.54 ± 0.89 | 1.64 ± 0.99 | 0.78 |
| Fasting HDL (mmol/L), mean ± SD | 1.22 ± 0.35 | 1.20 ± 0.33 | 0.91 |
| ALT (U/L), mean ± SD | 28.9 ± 14.3 | 21.5 ± 7.9 | 0.11 |
| AST (U/L), mean ± SD | 28.1 ± 9.5 | 24.2 ± 5.6 | 0.20 |
| ALP (U/L), mean ± SD | 79.0 ± 23.4 | 82.0 ± 39.8 | 0.56 |
| GGT (U/L), mean ± SD | 46.4 ± 29.1 | 35.3 ± 25.0 | 0.31 |
| Ferritin (μg/L), mean ± SD | 102.6 ± 159.2 | 144.7 ±143.7 | 0.48 |
| 25-hydroxy vitamin D (nmol/L), mean ± SD | 41.1 ± 18.3 | 45.2 ± 13.1 | 0.43 |
| Fib4 score, median (range*) | 1.27 (1.03 – 1.52) | 1.10 (0.61 – 1.60) | 0.50 |
| NAFLD fibrosis score, median (range*) | -0.73 (-1.04 – -0.42) | -1.10 (-1.79 – -0.41) | 0.28 |
| Transient elastography (median stiffness, kpa), median (range*) [n=16] | 6.01 (4.9 – 7.1) | 6.68 (4.5 – 10.0) | 0.71 |
| CAP (dB/m), mean ± SD | 276.4 ± 92 | 286.4 ± 74 | 0.80 |
| * 25-75% interquartile ranges expressed. | |||
Table 3 : Retrospective analysis and comparison of the anthropometric characteristics between the 2 weight loss Groups at baseline.
Nutritional Modifications
At baseline, the mean estimated daily total energy intake (TEI) reported by the cohort was 6467.5±3413.6KJ, with estimated daily nutrient composition at approximately 51±52% carbohydrate (CHO), 19.3±5% protein, and 25.6±8.3 fat (Table 4). Mean daily sugar intake was estimated at 97.8±135.7g. Changes in nutrient parameters following IGB therapy in Groups 1 and 2 are shown in Tables 5 and 6, respectively. In Group 1, at final follow up, there was compared to baseline a significant reduction in CHO as a percentage of TEI, at 54.5±8.9 reduced to 49.1±6.4, p=0.04 (Table 5). In Group 2, there was an increase, not significant, in TEI at 6 months (6741.8 KJ to 8404.7 KJ,p=0.16), but a significantly higher intake of total sugar (77.8 g to 137.3g, p=0.02) and iodine, p<0.05 (Table 6). At final follow up, comparing Group 1 to Group 2, estimated daily TEI showed a non-significant reduction at 5550.9±2227.4 vs 8404.7±1566.1 (p= 0.07) as did total fat intake(g) at 37.9±16.5 vs 67.5±61.7 (p=0.08) (Table 7). There was between Group 1 and Group 2 however, a difference in estimated daily carbohydrate intake at 167±70.5 vs 248.3±141.6 (p=0.05) along with a difference in estimated daily sugar consumption, at 79.8 ±48g vs 137.3 ±88.6 (p=0.02) (Table 7), plus iodine, retinol, and riboflavin (p,0.05).
| Nutrient parameter | Mean (SD) | 25th Percentile | Median | 75th Percentile |
|---|---|---|---|---|
| Total Kilojoules | 6467.5 (3413.6) | 4406.90 | 5533.62 | 7383.28 |
| Vitamin E (mg) | 12.4 (9.7) | 3.99 | 9.84 | 18.54 |
| Omega-3 (grams) | 0.30 (0.65) | 0.04 | 0.11 | 0.24 |
| Iodine (μg) | 153.4 (94.2) | 89.17 | 137.46 | 192.95 |
| Selenium (μg) | 66.4 (35.0) | 48.21 | 61.78 | 76.84 |
| Zinc (mg) | 9.6 (6.8) | 5.58 | 7.90 | 12.83 |
| Iron (mg) | 10.5 (8.5) | 4.82 | 7.70 | 12.59 |
| Phosphorus (mg) | 1210.6 (584.0) | 810.91 | 1023.97 | 1505.98 |
| Calcium (mg) | 727.3 (402.9) | 352.93 | 710.80 | 1002.51 |
| Magnesium (mg) | 326.4 (167.9) | 191.39 | 303.61 | 400.06 |
| Potassium (mg) | 2570.7 (1062.8) | 1901.03 | 2374.34 | 3054.56 |
| Sodium (mg) | 1626.4 (965.2) | 962.73 | 1405.53 | 2205.86 |
| B-Carotene (μg) | 2384.9 (2399.1) | 700.06 | 1806.20 | 2395.12 |
| Retinol (μg) | 386.9 (405.9) | 103.91 | 194.10 | 805.09 |
| Vitamin A (μg) | 648.2 (532.0) | 286.32 | 500.91 | 680.34 |
| Folate (μg) | 368.3 (240.8) | 220.30 | 339.13 | 416.79 |
| Vitamin C (mg) | 173.5 (190.7) | 57.63 | 103.09 | 192.21 |
| Niacin Eq (mg) | 29.7 (13.2) | 22.50 | 25.16 | 34.69 |
| Niacin (mg) | 16.0 (7.1) | 11.44 | 14.23 | 17.79 |
| Riboflavin (mg) | 2.1 (1.3) | 0.88 | 1.92 | 2.90 |
| Thiamine (mg) | 1.31 (0.9) | 0.59 | 1.05 | 1.82 |
| Fibre (grams) | 19.4 (8.1) | 14.28 | 20.18 | 21.68 |
| Water (grams) | 2195.9 (1403.7) | 1171.53 | 1522.84 | 2943.20 |
| Starch (grams) | 104.3 (47.7) | 72.24 | 105.23 | 126.50 |
| Total Sugars (grams) | 97.8 (135.7) | 44.50 | 73.96 | 83.16 |
| Carbohydrate (% KJ) | 51.3 (52.0) | 44.15 | 50.70 | 54.83 |
| Carbohydrate (grams) | 202.7 (139.1) | 138.83 | 178.23 | 209.32 |
| Cholesterol (mg) | 193.1 (172.4) | 99.24 | 128.00 | 209.03 |
| Monounsaturated fat (grams) | 17.9 (15.5) | 10.23 | 17.17 | 22.40 |
| Fat as polyunsaturated fat (% KJ) | 18.0 (5.0) | 14.33 | 18.01 | 21.87 |
| Fat as monounsaturated fat (% KJ) | 43.9 (5.9) | 40.43 | 44.34 | 47.34 |
| Fat as saturated fats (% KJ) | 38.1 (8.5) | 31.72 | 38.13 | 42.43 |
| Polyunsaturated Fat (grams) | 7.2 (4.9) | 4.48 | 6.43 | 10.02 |
| Saturated Fat (% KJ) | 8.8 (3.7) | 6.58 | 8.52 | 11.51 |
| Saturated Fat (g) | 16.3 (12.0) | 7.74 | 12.61 | 23.09 |
| Total Fats (%) | 25.6 (8.3) | 22.07 | 25.70 | 32.99 |
| Total Fats (grams) | 45.8 (28.9) | 24.17 | 43.27 | 57.68 |
| Protein (% KJ) | 19.3 (5.0) | 16.18 | 18.77 | 22.35 |
| Protein (grams) | 70.7 (39.7) | 48.94 | 59.05 | 82.59 |
| Fructose (grams) | 23.2 (39.5) | 0.10 | 14.0 | 213.3 |
Table 4: Baseline nutrient parameters for the entire cohort.
At baseline, the mean estimated daily total energy intake reported by the cohort was 6467.5±3413.6KJ, with estimated daily nutrient composition at approximately 51±52% carbohydrate (CHO), 19.3±5% protein, and 25.6±8.3 fat.
| Nutrient parameter | Pre-IGB | Post-IGB | p-value |
|---|---|---|---|
| Total Kilojuoles | 6605.0 (3852.8) | 5550.9 (2227.4) | 0.35 |
| Vitamin E (mg) | 12.2 (8.7) | 12.5 (8.7) | 0.89 |
| Omega-3 (grams) | 0.2 (0.2) | 0.5 (0.8) | 0.20 |
| Iodine (μg) | 159.1 (92.2) | 167.8 (88.6) | 0.62 |
| Selenium (μg) | 59.3 (20.3) | 63.0 (21.8) | 0.66 |
| Zinc (mg) | 8.9 (4.3) | 8.4 (3.2) | 0.54 |
| Iron (mg) | 10.0 (5.6) | 8.9 (4.4) | 0.55 |
| Phosphorus (mg) | 1118.4 (433.7) | 1164.1 (550.6) | 0.75 |
| Calcium (mg) | 709.0 (391) | 826.9 (515.4) | 0.40 |
| Magnesium (mg) | 306.4 (114.3) | 339.4 (131.7) | 0.17 |
| Potassium (mg) | 2595.9 (931.8) | 2597.2 (1324.8) | 1.00 |
| Sodium (mg) | 1566.2 (928.5) | 1564.3 (716.9) | 1.00 |
| B-Carotene (μg) | 1999.7 (1481.2) | 1747.4 (1422.3) | 0.62 |
| Retinol (μg) | 422.1 (430.2) | 375.6 (364.7) | 0.54 |
| Vitamin A (μg) | 567.1 (387.3) | 533.0 (317.8) | 0.78 |
| Folate (μg) | 414.2 (282.4) | 417.5 (241.9) | 0.97 |
| Vitamin C (mg) | 224.6 (239.9) | 134.9 (107.5) | 0.18 |
| Niacin Eq (mg) | 26.8 (9.8) | 28.9 (12.9) | 0.59 |
| Niacin (mg) | 14.3 (6.1) | 15.9 (8.2) | 0.49 |
| Riboflavin (mg) | 2.1 (1.2) | 2.1 (1.1) | 0.86 |
| Thiamine (mg) | 1.4 (0.8) | 1.5 (0.9) | 0.60 |
| Fibre (grams) | 19.0 (4.5) | 18.6 (7.1) | 0.81 |
| Water (grams) | 1972.0 (1321.6) | 2581.3 (1473.7) | 0.18 |
| Starch (grams) | 107.4 (36.6) | 86.1 (43.3) | 0.07 |
| Total Sugars (grams) | 119.1 (187.1) | 79.8 (48.9) | 0.38 |
| Carbohydrate (% KJ) | 54.5 (8.9) | 49.1 (6.4) | 0.04 |
| Carbohydrate (grams) | 226.7 (181.6) | 167.0 (70.5) | 0.23 |
| Cholesterol (mg) | 169.7 (91.2) | 181.9 (133.4) | 0.73 |
| Monounsaturated fat (grams) | 17.2 (10.4) | 14.9 (6.4) | 0.46 |
| Fat as polyunsaturated fat (% KJ) | 16.7 (4.7) | 18.9 (7.3) | 0.35 |
| Fat as monounsaturated fat (% KJ) | 45.6 (4.8) | 43.9 (6.5) | 0.46 |
| Fat as saturated fats (% KJ) | 37.7 (7.4) | 37.2 (11.5) | 0.88 |
| Polyunsaturated Fat (grams) | 5.9 (3.0) | 6.5 (3.4) | 0.61 |
| Saturated Fat (% KJ) | 7.9 (2.8) | 8.3 (2.5) | 0.57 |
| Saturated Fat (g) | 15.0 (10.9) | 12.6 (7.0) | 0.48 |
| Total Fats (%) | 23.5 (7.0) | 25.7 (5.9) | 0.28 |
| Total Fats (grams) | 42.2 (24.8) | 37.9 (16.5) | 0.58 |
| Protein (% KJ) | 18.1 (5.2) | 20.4 (2.6) | 0.13 |
| Protein (grams) | 63.8 (22.6) | 66.5 (27.9) | 0.75 |
| Fructose (grams) | 29.9 (53.4) | 28.6 (54.1) | 0.79 |
| *All values expressed as mean ± SD | |||
Table 5: Changes in nutrient parameters following IGB therapy in Group 1.
In Group 1, at final follow up, there was compared to baseline a significant reduction in CHO as a percentage of total energy intake, at 54.5±8.9 reduced to 49.1±6.4, p=0.04.
| Nutrient parameter | Pre-IGB | Post-IGB | p-value |
|---|---|---|---|
| Total Kilojuoles | 6741.8 (3045.4) | 8404.7.9 (5425.3) | 0.16 |
| Vitamin E (mg) | 13.8 (11.4) | 20.8 (16.5) | 0.10 |
| Omega-3 (grams) | 0.5 (1.00) | 0.4 (0.6) | 0.43 |
| Iodine (μg) | 162.2 (99.9) | 224.9 (146.2) | 0.05 |
| Selenium (μg) | 79.8 (46.8) | 101.1 (74.0) | 0.35 |
| Zinc (mg) | 11.5 (9.1) | 13.4 (8.0) | 0.35 |
| Iron (mg) | 12.3 (11.5) | 14.2 (9.7) | 0.26 |
| Phosphorus (mg) | 1392.4 (742.7) | 1649.2 (1013.0) | 0.31 |
| Calcium (mg) | 814.8 (427.0) | 1114.8 (795.5) | 0.12 |
| Magnesium (mg) | 380.6 (213.9) | 453.9 (235.0) | 0.17 |
| Potassium (mg) | 2671.4 (1256.5) | 3229.8 (1881.3) | 0.18 |
| Sodium (mg) | 1779.6 (1073.0) | 1861.4 (1492.5) | 0.74 |
| B-Carotene (μg) | 3224.9 (3168.9) | 4280.1 (4736.5) | 0.24 |
| Retinol (μg) | 394.4 (405.2) | 639.1 (466.4) | 0.02 |
| Vitamin A (μg) | 836.7 (657.3) | 1135.8 (1086.8) | 0.20 |
| Folate (μg) | 350.1 (180.5) | 433.9 (231.1) | 0.07 |
| Vitamin C (mg) | 121.2 (102.3) | 152.9 (100.1) | 0.12 |
| Niacin Eq (mg) | 34.4 (16.6) | 36.0 (20.4) | 0.78 |
| Niacin (mg) | 18.6 (8.3) | 19.0 (11.0) | 0.88 |
| Riboflavin (mg) | 2.1 (1.3) | 3.0 (1.6) | 0.01 |
| Thiamine (mg) | 1.4 (1.0) | 1.7 (1.1) | 0.21 |
| Fibre (grams) | 23.9 (12.1) | 23.9 (12.1) | 0.33 |
| Water (grams) | 2667.4 (1497.1) | 3185.5 (1560.2) | 0.26 |
| Starch (grams) | 108.5 (60.2) | 110.0 (72.5) | 0.92 |
| Total Sugars (grams) | 77.8 (31.3) | 137.3 (88.6) | 0.02 |
| Carbohydrate (% KJ) | 47.6 (10.9) | 50.9 (9.5) | 0.32 |
| Carbohydrate (grams) | 187.3 (68.6) | 248.3 (141.6) | 0.09 |
| Cholesterol (mg) | 236.5 (246.9) | 240.4 (194.7) | 1.00 |
| Monounsaturated fat (grams) | 19.5 (13.4) | 24.7 (23.1) | 0.21 |
| Fat as polyunsaturated fat (% KJ) | 19.1 (5.4) | 18.1 (7.7) | 0.86 |
| Fat as monounsaturated fat (% KJ) | 40.5 (5.4) | 40.1 (4.9) | 0.86 |
| Fat as saturated fats (% KJ) | 40.5 (9.2) | 41.8 (9.2) | 0.67 |
| Polyunsaturated Fat (grams) | 9.2 (6.5) | 11.3 (11.2) | 0.42 |
| Saturated Fat (% KJ) | 10.4 (4.5) | 9.5 (4.3) | 0.46 |
| Saturated Fat (g) | 19.6 (10.9) | 24.9 (26.9) | 0.30 |
| Total Fats (%) | 27.9 (9.9) | 26.1 (10.0) | 0.45 |
| Total Fats (grams) | 53.3 (34.6) | 67.5 (61.7) | 0.24 |
| Protein (% KJ) | 20.4 (5.0) | 19.4 (4.0) | 0.49 |
| Protein (grams) | 83.5 (54.7) | 91.4 (51.9) | 0.58 |
| Fructose (grams) | 15.5 (8.7) | 25.4 (21.9) | 0.18 |
| *All values expressed as mean ± SD | |||
Table 6: Changes in nutrient parameters following IGB therapy in Group 2.
In Group 2, there was an increase, not significant, in total energy intake at 6 months (6741.8 kJ to 8404.7 kJ, p=0.16), but a significantly higher intake of total sugar (77.8 g to 137.3g, p=0.02) and iodine, p<0.05.
| Nutrient parameter | Group 1: Weight loss ≥10% (n=15) |
Group 2: Weight loss < 10% (n=13) |
p-value |
|---|---|---|---|
| name="_Hlk85994561">Total Kilojoules | 5550.9 (2227.4) | 8404.7 (1566.1) | 0.07 |
| Carbohydrate (grams) | 167.0 (70.5) | 248.3 (141.6) | 0.05 |
| Carbohydrate (% KJ) | 49.1 (6.4) | 50.9 (9.5) | 0.21 |
| Starch (grams) | 86.1 (43.3) | 110.0 (72.5) | 0.86 |
| Fibre (grams) | 18.6 (7.1) | 23.9 (12.1) | 0.86 |
| Total Sugar (grams) | 79.8 (48.9) | 137.3 (88.6) | 0.04 |
| Fat (grams) | 37.9 (16.5) | 67.5 (61.7) | 0.08 |
| Total Fats (%) | 25.7 (5.9) | 26.1 (10.0) | 0.89 |
| Total Fats (grams) | 37.9 (16.5) | 67.5 (61.7) | 0.20 |
| Monounsaturated fat (grams) | 14.9 (6.4) | 24.7 (23.1) | 0.62 |
| Fat as polyunsaturated fat (% KJ) | 18.9 (7.3) | 18.1 (7.7) | 0.66 |
| Fat as monounsaturated fat (% KJ) | 43.9 (6.5) | 40.1 (4.9) | 0.54 |
| Fat as saturated fats (% KJ) | 37.2 (11.5) | 41.8 (9.2) | 0.55 |
| Polyunsaturated Fat (grams) | 6.5 (3.4) | 11.3 (11.2) | 0.75 |
| Saturated Fat (% KJ) | 8.3 (2.5) | 9.5 (4.3) | 0.40 |
| Saturated Fat (g) | 12.6 (7.0) | 24.9 (26.9) | 0.17 |
| Cholesterol (mg) | 181.9 (133.4) | 240.4 (194.7) | 0.78 |
| Protein (% KJ) | 20.4 (2.6) | 19.4 (4.0) | 0.48 |
| Protein (grams) | 66.5 (27.9) | 91.4 (51.9) | 0.62 |
| Fructose (grams) | 28.6 (54.1) | 25.4 (21.9) | 0.54 |
| Vitamin E (mg) | 12.5 (8.7) | 20.8 (16.5) | 0.78 |
| Omega-3 (grams) | 0.5 (0.8) | 0.4 (0.6) | 0.97 |
| Iodine (μg) | 167.8 (88.6) | 224.9 (146.2) | 0.04 |
| Selenium (μg) | 63.0 (21.8) | 101.1 (74.0) | 0.59 |
| Zinc (mg) | 8.4 (3.2) | 13.4 (8.0) | 0.49 |
| Iron (mg) | 8.9 (4.4) | 14.2 (9.7) | 0.86 |
| Phosphorus (mg) | 1164.1 (550.6) | 1649.2 (1013.0) | 0.60 |
| Calcium (mg) | 826.9 (515.4) | 1114.8 (795.5) | 0.81 |
| Magnesium (mg) | 339.4 (131.7) | 453.9 (235.0) | 0.18 |
| Potassium (mg) | 2597.2 (1324.8) | 3229.8 (1881.3) | 0.07 |
| Sodium (mg) | 1564.3 (716.9) | 1861.4 (1492.5) | 0.38 |
| B-Carotene (μg) | 1747.4 (1422.3) | 4280.1 (4736.5) | 0.35 |
| Retinol (μg) | 375.6 (364.7) | 639.1 (466.4) | 0.04 |
| Vitamin A (μg) | 533.0 (317.8) | 1135.8 (1086.8) | 0.26 |
| Folate (μg) | 417.5 (241.9) | 433.9 (231.1) | 0.31 |
| Vitamin C (mg) | 134.9 (107.5) | 152.9 (100.1) | 0.12 |
| Niacin Eq (mg) | 28.9 (12.9) | 36.0 (20.4) | 0.17 |
| Niacin (mg) | 15.9 (8.2) | 19.0 (11.0) | 0.18 |
| Riboflavin (mg) | 2.1 (1.1) | 3.0 (1.6) | 0.02 |
| Thiamine (mg) | 1.5 (0.9) | 1.7 (1.1) | 0.24 |
| Water (grams) | 2581.3 (1473.7) | 3185.5 (1560.2) | 0.65 |
| *All values expressed as mean ± SD | |||
Table 7: Differences in nutrient intake between the two groups at final follow-up.
At final follow up, comparing Group 1 to Group 2, estimated daily total energy intake showed a non-significant reduction at 5550.9±2227.4 vs 8404.7±1566.1 (p= 0.07) as did total fat intake(g) at 37.9±16.5 vs 67.5±61.7 (p=0.08). There was between Group 1 and Group 2 however, a difference in estimated daily carbohydrate intake at 167±70.5 vs 248.3±141.6 (p=0.05) along with significant differences in estimated daily sugar consumption, at 79.8 ±48g vs 137.3 ±88.6 (p=0.04), plus increases in iodine, retinol and riboflavin, p<0.05.
Gut Microbiota Changes
The cohort bacterial community structure did not differ significantly at baseline (Figure 1). It was however, mildly altered post-IGB removal and enriched with the genus Bacteroides: Group 1 showed a decrease in Streptococcus, Rothia and Butyrivibrio, while Clostridium XI was enriched.
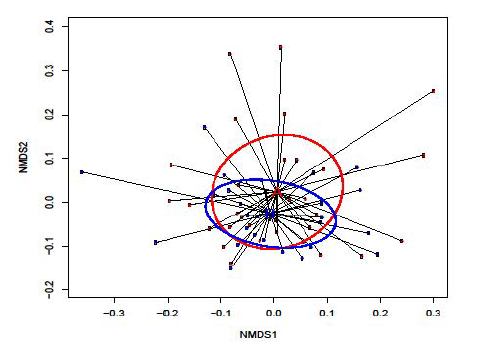
Figure 1: Following IGB removal, retrospective analysis showed no significant difference in the overall α-diversity of gut microbiota at baseline and at the end of treatment for the whole cohort. This is demonstrated by non-metric multidimensional scaling (NMDS) using the Bray-Curtis dissimilarity measure where the absence of any clear separation between pre- and post-IGB samples is indicative of non-significant clustering in the gut microbiota before and after the intervention. A (red samples) = pre-IGB; and B (blue samples) = post-IGB. Nonmetric Multi-Dimensional Scaling (NMDS) indicates no significant change in gut microbiota diversity before and after IGB intervention for the whole cohort.
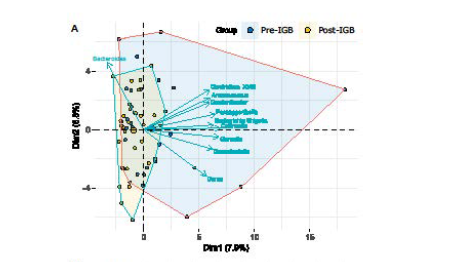
Figure 2A: Principal component analysis of 16S metagenomic data of bacterial genera in the faeces of obese patients with hepatic steatosis, in a 2D dimensional space made by the two most varying principal components, pre- and post-intragastric balloon (IGB) treatment. Arrows identify genera that contributed more significantly than others to the overall variability of data. Variation in microbiota genera pre-IGB genera is indicated in blue, and variation in microbiota genera post-IGB is indicated in yellow. These data support a reduction in bacterial genera in the post-IGB microbiome which was enriched by the genus Bacteroides.
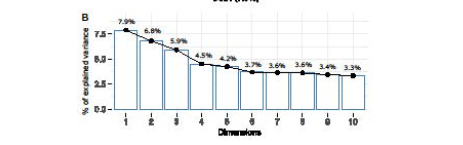
Figure 2B: Quantification of the variance of data as explained by the first ten principal components. Analyzing the intra-group bacterial abundance, we found that Group 1 and Group 2 differed for Finegoldia (p=0.047) and Ruminococcus2 (p=0.05) in pre-IGB and for a few other lower-level operational taxonomic units (OTUs), namely Butyrivibrio (p=0.038), Clostridium XI (p=0.034), Rothia (p=0.05), and Streptococcus (p=0.041) in the Post-IGB group as shown in Figure 3.
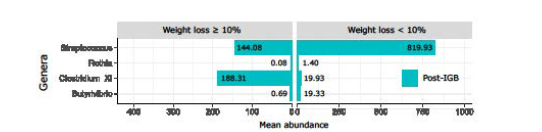
Figure 3: Back-to-back bar-plot representing the differentially abundant bacteria in Group 1 (≥10% weight loss) and Group 2 (<10% weight loss). Bar height depends on the mean abundance of genus in each group. As compared to Group 2, the microbiomes of patients in Group 1 had a reduced abundance of streptococcus, Rothia, and Butyrivibrio which may be carbohydrate fermenting genera. There was however enrichment of the genus Clostrdium XI which is thought to enhance fermentation of dietary fibre to short chain fatty acids. The role of a change in microbiota in weight loss and how to achieve that change remains to be determined.
The bacterial community structure at the taxonomic genus level differed between the pre and post-IGB groups, with genus Bacteroides forming the largest bacterial populations in the post-IGB group (Figure 2A). The following genera were reduced in the post-IGB group compared to the pre-IGB group: Clostridium XVIII, Anaerococcus, Gordonibacter, Paraeggerthella, Escherichia Shigella, Collinsella, Gemella, Granulicatella and Dorea (Figure 2A). However, groups Pre-IGB and Post-IGB did not differ macroscopically, with the first ten principal components accounting for only 47% of the total data variability (Figure 2B). Analyzing the intra-group bacterial species abundance post-IGB, Group 1 (weight loss ≥ 10%) and Group 2 (weight loss < 10%) significantly differed for the genera Streptococcus (p=0.04), Rothia (p=0.05) and Butyrivibrio (p=0.04) that were more abundant in Group 2, while Clostridium XI (p=0.03) was nearly ten times more abundant in Group 1 (Figure 3). Changes in bacterial Operational taxonomic units (OTUs) in Group 1 and Group 2 at IGB removal are shown in Table 8 and Table 9, showing that the actual changes in the OTUs are significant but small and as such the functional relevance remains uncertain.
| Group 1: Weight loss ≥ 10% (n=15) |
|||
|---|---|---|---|
| Taxonomic information [Phylum, Class, Order, Family, Genus] |
Pre-IGB | Post-IGB | Corrected p-values |
| Actinobacteria; Actinobacteria; Coriobacteriales; Coriobacteriaceae; Paraeggerthella | 0.003 (0.01) | 0.00 (0.00) | 0.04 |
| Firmicutes; Clostridia; Clostridiales; Clostridiales_Incertae_Sedis_XIII; Mogibacterium | 0.01 (0.05) | 0.00 (0.00) | 0.03 |
| Firmicutes; Negativicutes; Selenomonadales; Veillonellaceae; Negativicoccus | 0.002 (0.01) | 0.01 (0.00) | 0.04 |
| *All values are expressed as mean relative frequencies (%) ± SD, with Benjamin-Hochberg corrections applied to all. | |||
Table 8: Changes in bacterial Operational taxonomic units in Group 1, at IGB removal.
Changes in bacterial Operational taxonomic units (OTUs) in Group 1 and Group 2 at IGB removal are shown in Table 8 and 9, showing that the actual changes in the OTUs are significant but small and as such the functional relevance remains uncertain.
| Group 2: Weight loss <10% (n=13) |
|||
|---|---|---|---|
| Taxonomic information [Phylum, Class, Order, Family, Genus] |
Pre-IGB | Post-IGB | Corrected p-values |
| Firmicutes; Clostridia; Clostridiales; Lachnospirareae; Dorea | 0.84 (0.81) | 0.28 (0.22) | <0.01 |
| *All values are expressed as mean relative frequencies (%) ± SD, with Benjamin-Hochberg corrections applied to all. | |||
Table 9: Changes in bacterial Operational taxonomic units in Group 2, at IGB removal.
Changes in bacterial Operational taxonomic units (OTUs) in Group 1 and Group 2 at IGB removal are shown in Table 8 and 9, showing that the actual changes in the OTUs are significant but small and as such the functional relevance remains uncertain.
Discussion
Non-alcoholic fatty liver disease (NAFLD) is a liver disease ranging from hepatic steatosis through non-alcoholic steatohepatitis to fibrosis and cirrhosis; NAFLD is mostly related to obesity [3,7]. Alarmingly, the rates are also rising in adolescents and young adults [26,27]. The rising rates of obesity and NAFLD, coupled with limited treatment options, have led to a growing interest in metabolic endoscopic therapy. The IGB is an emerging metabolic endoscopic treatment of obesity and NAFLD. In this study, we sought to determine the efficacy of the IGB in obesity-related hepatic steatosis and related non-invasive indices along with changes in gut microbiota and nutritional patterns. Thirtythree obese patients were initially recruited but three left the study early. Thirty obese patients were then treated with the IGB. With two patients failing to show for final follow-up we had data for 28 patients at baseline and at six months. We then sought to determine the impact of IGB treatment on weight loss, nutritional patterns and gut microbiota upon IGB removal at six months. Anthropometric, nutritional data, blood and fecal samples were collected at baseline and at six months. Gut microbiota diversity was assessed by 16S RNA sequencing.
On IGB removal patients were sub-divided into those losing ≥10% of initial body weight (Group 1) and those losing ≤10% of initial bodyweight (Group 2). Group 1 had a significant reduction (p<0.05), in weight, BMI, waist circumference (WC), HOMA-IR, HbA1C, AST, GGT along with a non-significant reduction in ALT and NAFLD fibrosis score (p<0.08). Group 2 had only a significant reduction in WC, p=0.02. Retrospective analyses between Group 1 and Group 2 showed no differences in baseline characteristics. The mean estimated daily total energy intake (TEI) in KJ, reported by the whole cohort at baseline was 6467.5±3413.6, with estimated daily nutrient composition of 51±52% carbohydrate (CHO), 19.5±5% protein, and 27±25.6% fat. Mean daily sugar intake was estimated at 97.8±135.7g. At final follow up, comparing Group 1 to Group 2, estimated daily TEI showed a nonsignificant reduction at 5550.9±2227.4 vs 8404.7± 1566.1 (p= 0.07) as did total fat intake(g) at 37.9±16.5 vs 67.5±61.7, p=0.08. There was between the 2 groups however, a significant reduction in estimated daily carbohydrate intake at 167±70.5 vs 248.3±141.6 (p=0.05) along with a reduction in estimated daily sugar consumption, at 79.8 ±48g vs 137.3 ±88.6 (p=0.02). In Group 1, at final follow up, there was compared to baseline a significant reduction in CHO as a percentage of TEI, at 54.5±8.9 reduced to 49.1±6.4, p=0.04.
This data show that as previous, there is a reduction in weight induced by the IGB [10,28,13,12]. The latter paper was a technical review of the IGB in the management of obesity by the American Gastroenterological Association. It compromised seven randomised controlled trials, with outcome of weight loss at six months. The metanalysis comprised 628 individuals who underwent IGB therapy, and 551 individuals who were treated with control-standard of care (SOC). Mean weight loss in the IGB group differed significantly (p<0.05) from the SOC group with weight loss ranging from 7.1kg to 17.1kg (IGB Group) and in the SOC group ranging from 3.2kg to 6.4kg (mean difference 7.0kg; 95% CI, 4.7–9.3kg). With our previous publication [13] of 135 patients our weight loss was of the order in the me-analysis.
We and others, having made the point that the IGB can induce reasonable weight loss and that the IGB may be useful for patients who do not want metabolic surgery or may act as bridge to metabolic surgery, the aim here was mechanistic.
In our analysis here, we found that gut bacterial microbiomes (inferred from 16S metagenomic data in faeces) did change with weight reductions, with some improvements in anthropometrics plus metabolic and hepatic health. Our design here did not include a liver biopsy and patients were stratified as having hepatic steatosis through abdominal ultrasound or a high CAP score. As such the patients could not be said to have NAFLD but to have obesity related hepatic steatosis.
To the best of our knowledge, this research is the first to report on the effects of IGB therapy on the gut microbiota, whereas changes in gut microbiota due to gastric bypass surgery and associated weight loss have been documented [29,30]. While previous reports have suggested a specific microbial signature pertaining to the obese or non-obese NAFLD phenotype [31,32], we highlight the overwhelming effect of dietary change in influencing the gut microbial environment, as opposed to any changes in weight or anthropometrics per se. Indeed, the absence of dietary constituent analysis in previous cross-sectional studies may explain the often-discrepant findings [33,34]. There was at final follow-up differences between Group 1 and Group 2 in iodine, retinol and riboflavin, p<0.05, the significance of which is uncertain.
Our data showed that there was a significant ~5% reduction in total CHO intake in patients losing more than 10% of their body weight. Our patients were free-living subjects without any supervised dietary prescriptions, which enabled us to examine the nutrient constituents that contributed most significantly to gut microbial changes. Our findings are in line with those of a recent trial examining the effectiveness of a low-carbohydrate diet compared with a calorie-restricted diet in combination with IGB therapy [35]. Therefore, a lower -carbohydrate intake could induce a greater loss with the with IGB therapy, and it is associated with gut microbiome changes. This hypothesis is supported by published data suggesting that weight change is unlikely to drive independently significant alterations in the gut microflora [36-38].
There were differences between the microbiomes, at the taxonomic level of genus, in the pre-IGB compared to post-IGB groups, with the exception of Genus Bacteroides forming the largest bacterial populations in the post-IGB group (Figure 2). High levels of Bacteroides were previously correlated with weight loss [39]. Clostridium is a predominant cluster of species in the human gut. In this study we observed that the abundance of Clostrdium XI significantly increased in Group 1 post-IGB (low carbohydrate intake and > 10% weight loss). Species forming the Clostridium XI cluster have been demonstrated to ferment dietary fibres to produce short chain fatty acids (SCFAs) as metabolic by-products. SCFAs may be related to gut microbiota, protect against obesity by increasing energy expenditure and appetite control, as recently reviewed [26]. At the smallest taxonomic levels, we found that the most varying bacteria belonged to the following species: Clostridium XVIII, Anaerococcus, Gordonibacter, Paraeggerthella, Escherichia Shigella, Collinsella, Gemella, Granulicatella and Dorea.
It is worth noting that a shift in numbers, which, although not statistically significant, may still have a functional relevance. i.e., metabolic function may not be density dependent. Relating microbiome community structure to clinical observation is challenging as changes in population density, if sustained over time, may have a long-term impact [40,41], particularly in the presence of other independent variables such as diet. It is also worth pointing out that the gut is known to harbour microbial niches that differ in both composition, host factor susceptibility, their richness and diversity [42,43]. In this, our primary study investigating this area, we could not aim to study these gut microbiota niches without knowing whether IGB induced-weight loss was related to any overall changes in Gut microbiota. This is an area that might be fruitful for future studies.
In conclusion, the gut microbiota of obese patients with hepatic steatosis exhibits subtle changes following weight loss with IGB therapy. The importance of these changes remain to be determined. Additionally, dietary modifications appear to be independent factors in weight loss with the IGB, especially as regards lowered carbohydrate intake. Manipulation of specific macronutrients could potentially be involved in inducing weight loss to complement with the IGB which may be of consequent clinical benefits in hepa
References
- Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M (2019) From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 16(7): 411-428.
- Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, et al. (2021) Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol.
- Tomic D, Kemp WW, Roberts SK (2018) Nonalcoholic fatty liver disease: current concepts, epidemiology and management strategies. Eur J Gastroenterol Hepatol 30(10): 1103-1115.
- Sinclair P, Brennan DJ, le Roux CW (2018) Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat Rev Gastroenterol Hepatol 15(10): 606-624.
- Boursier J, Mueller O, Barret M, Machado M, Fizanne L, et al. (2016) The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63(3): 764-775.
- Loomba R, Seguritan V, Li W, Long T, Klitgord N, et al. (2019) Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab 30(3): 607.
- Ando Y, Jou JH (2021) Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin Liver Dis (Hoboken) 17(1): 23-28.
- Chandan S, Mohan BP, Khan SR, Facciorusso A, Ramai D, et al. (2021) Efficacy and Safety of Intragastric Balloon (IGB) in Non-alcoholic Fatty Liver Disease (NAFLD): a Comprehensive Review and Meta-analysis. Obes Surg 31(3): 1271-1279.
- Lari E, Burhamah W, Lari A, Alsaeed T, Al-Yaqout K, et al. (2021) Intra-gastric balloons - The past, present and future. Ann Med Surg (Lond) 63: 102138.
- Abu Dayyeh BK, Eaton LL, Woodman G, Fusco M, Shayani V, et al. (2015) 444 A Randomized, Multi-Center Study to Evaluate the Safety and Effectiveness of an Intragastric Balloon As an Adjunct to a Behavioral Modification Program, in Comparison With a Behavioral Modification Program Alone in the Weight Management of Obese Subjects. Gastrointest Endosc 5.
- shAbu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, et al. (2015) ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 82(3): 425-438 e425.
- Shah R, Davitkov P, Abu Dayyeh BK, Saumoy M, Murad MH (2021) AGA Technical Review on Intragastric Balloons in the Management of Obesity. Gastroenterology 160(5): 1811-1830.
- Nguyen V, Li J, Gan J, Cordero P, Ray S, et al. (2017) Outcomes following Serial Intragastric Balloon Therapy for Obesity and Nonalcoholic Fatty Liver Disease in a Single Centre. Can J Gastroenterol Hepatol 2017: 4697194.
- Jirapinyo P, Thompson CC (2017) Endoscopic Bariatric and Metabolic Therapies: Surgical Analogues and Mechanisms of Action. Clin Gastroenterol Hepatol 15(5): 619-630.
- Mathus-Vliegen E, Spangeus A, Walter S, Ericson AC (2021) Weight loss with or without intragastric balloon causes divergent effects on ghrelin cell expression. Obes Sci Pract 7(2): 199-207.
- Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, et al. (2012) Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 32(6): 902-910.
- Marfell-Jones MJ, Stewart AD, de Ridder JH (2012) International standards for anthropometric assessment. International Society for the Advancement of Kinanthropometry. Wellington, New Zealand.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7): 412-419.
- Demir M, Lang S, Nierhoff D, Drebber U, Hardt A, et al. (2013) Stepwise combination of simple noninvasive fibrosis scoring systems increases diagnostic accuracy in nonalcoholic fatty liver disease. J Clin Gastroenterol 47(8): 719-726.
- Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, et al. (2017) Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 66(5): 1022-1030.
- Pindjakova J, Sartini C, Lo Re O, Rappa F, Coupe B, et al. (2017) Gut Dysbiosis and Adaptive Immune Response in Diet-induced Obesity vs. Systemic Inflammation. Front Microbiol 8: 1157.
- Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, et al. (2010) Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51(1): 121-129.
- St George A, Bauman A, Johnston A, Farrell G, Chey T, et al. (2009) Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol 24(3): 399-407.
- Conlon BA, Beasley JM, Aebersold K, Jhangiani SS, Wylie-Rosett J (2013) Nutritional management of insulin resistance in nonalcoholic fatty liver disease (NAFLD). Nutrients 5(10): 4093-4114.
- Kindinger LM, MacIntyre DA, Lee YS, Marchesi JR, Smith A, et al. (2016) Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. Sci Transl Med 8(350): 350ra102.
- Kim KN, Yao Y, Ju SY (2019) Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 11(10).
- Temple JL, Cordero P, Li J, Nguyen V, Oben JA (2016) A Guide to Non-Alcoholic Fatty Liver Disease in Childhood and Adolescence. Int J Mol Sci 17(6).
- Force ABET, Committee AT, Abu Dayyeh BK, Kumar N, Edmundowicz SA, et al. (2015) ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 82(3): 425-438 e425.
- Lee CJ, Florea L, Sears CL, Maruthur N, Potter JJ, et al. (2019) Changes in Gut Microbiome after Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial. Obes Surg 29(10): 3239-3245.
- Popov VB, Thompson CC, Kumar N, Ciarleglio MM, Deng Y, et al. (2016) Effect of Intragastric Balloons on Liver Enzymes: A Systematic Review and Meta-Analysis. Dig Dis Sci 61(9): 2477-2487.
- Lee G, You HJ, Bajaj JS, Joo SK, Yu J, et al. (2020) Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun 11: 4982.
- Loomba R, Seguritan V, Li W, Long T, Klitgord N, et al. (2017) Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab 25(5): 1054-1062 e1055.
- Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, et al. (2013) Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 58(1): 120-127.
- Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, et al. (2013) Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 11(7): 868-875 e861-863.
- Maekawa S, Niizawa M, Harada M (2020) A Comparison of the Weight Loss Effect between a Low-carbohydrate Diet and a Calorie-restricted Diet in Combination with Intragastric Balloon Therapy. Intern Med 59(9): 1133-1139.
- Finucane MM, Sharpton TJ, Laurent TJ, Pollard KS (2014) A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One 9(1): e84689.
- Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, et al. (2018) An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab 27(3): 559-571 e555.
- Pataky Z, Genton L, Spahr L, Lazarevic V, Terraz S, et al. (2016) Impact of Hypocaloric Hyperproteic Diet on Gut Microbiota in Overweight or Obese Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Dig Dis Sci 61(9): 2721-2731.
- Dao MC, Everard A, Clement K, Cani PD (2016) Losing weight for a better health: Role for the gut microbiota. Clin Nutr Exp 6: 39-58.
- Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, et al. (2018) The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr 57(Suppl 1): 1-14.
- Saffouri GB, Shields-Cutler RR, Chen J, Yang Y, Lekatz HR, et al. (2019) Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun 10(1): 2012.
- Borgo F, Garbossa S, Riva A, Severgnini M, Luigiano C, et al. (2018) Body Mass Index and Sex Affect Diverse Microbial Niches within the Gut. Front Microbiol 9: 213.
- Belizario JE, Faintuch J, Garay-Malpartida M (2018) Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm 2037838.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Nguyen V, Mandour MO, Bianco SD, Blumenthal C, Male V, et al. (2022)Metabolic Endoscopy with Intra-Gastric Balloon, Improves Obesity Related HepaticSteatosis Indices, with Changes in Gut Microbiota. J Obes Weight Loss Ther 12: 507.
Copyright: © 2022 Nguyen V, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2603
- [From(publication date): 0-2022 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 2201
- PDF downloads: 402