Research Article Open Access
Absorption Spectral Analysis of Zn2+ or Cu2+ Coordination with Human Serum Albumin using Zincon
Koji Matsuura* and Ikuyo SugimotoResearch Core for Interdisciplinary Sciences, Okayama University, Okayama, Japan
- *Corresponding Author:
- Koji Matsuura
Cardiovascular Physiology
Graduate School of Medicine
Dentistry and Pharmaceutical Sciences
Okayama University, 3-1-1 Tsushima-naka
Kita, Okayama 700-8530, Japan
Tel: +81-86-251-8610
Fax: +81-86-251-8610
E-mail: kojimatu@md.okayama-u.ac.jp
Received date: August 29, 2014; Accepted date: September 25, 2014; Published date: September 30, 2014
Citation: Matsuura K and Sugimoto I (2014) Absorption Spectral Analysis of Zn2+ or Cu2+ Coordination with Human Serum Albumin using Zincon. J Anal Bioanal Tech 5:209 doi: 10.4172/2155-9872.1000209
Copyright: © 2014 Matsuura K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Human serum albumin (HSA) is used for mammalian embryo culture in assisted reproductive technology (ART) to adjust osmotic pressure and growth factor concentration in culture media. In human serum, transition metal ions, particularly Zn2+, bind to the coordination sites of HSA; thus analyzing the binding of these metal ions may help predict the possible harmful effects of these metals on embryo culture. In this study, we found that metal ions and zincon bound to HSA, and that a complex was formed with the HSA molecule. Using this method, the metals that bind to the multi-binding site of HSA, particularly Zn2+ can be evaluated by colorimetry in an ART laboratory. This analytical method is potentially applicable for metal detection in HSA in ART clinics and also for quick analysis of the coordination of 64Cu2+ with HSA as a probe chemical in positron emission tomography.
Keywords
Human serum albumin; Metal coordination; Zincon
Introduction
Human serum albumin (HSA) is a water-soluble monomeric protein comprising 585 amino acids, with a molecular mass of 66,500 Da [1]. The physiological roles of HSA are (a) maintaining osmotic pressures in serum; (b) transport of fatty acids, amino acids, and metal ions; and (c) scavenging oxidants and reductants [2]. This protein is used clinically to treat severe hypoalbuminemia and traumatic shock [3]. In assisted reproductive medicine (ART), recombinant HSA (rHSA) is used to supplement the embryo culture medium to adjust the osmotic pressure, and it is also considered to be a protein source during embryonic development [4,5]. Because simple instrumental equipment is preferred for molecular analysis in clinics and ART laboratories, non-timeconsuming analysis such as colorimetry based on absorption spectroscopy are preferable for evaluating the molecules that bind to HSA.
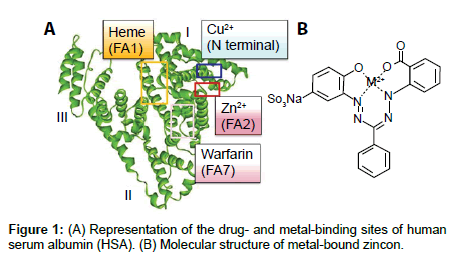
When HSA is used as a clinical supplement, the effects of transition metal binding need to be considered because HSA has multiple transition metal-binding sites (Figure 1A) [6,7]. Zn2+ and Cu2+ have similar coordination chemical characteristics because of their similar Lewis acidity; however, the addition of these metals induces different phenomena in developmental biology [8]. The physiological Zn2+ concentration in two-cell stage mouse embryos is approximately 1 μM and the dynamics of the zinc concentration are also important for metaphase II oocytes, because they regulate the promotion of oocyte maturation [9,10]. However, the Cu2+ concentration is less than 1/10 of that of zinc in two-cell stage mouse embryos [9]. O’Shea et al. [11] reported that a significantly higher incidence of gross anomalies was observed in mouse embryos (9 days after pregnancy) incubated with 25 μM of copper sulfate compared with the controls, in which the medium lacked copper sulfate [11]. The physiological roles of these metals differ during in vivo and in vitro cell proliferation; thus, a chemical assay should be developed for HSA-binding Zn2+ or Cu2+, which can be employed before the use of HSA in embryo culture or clinical treatments.
HSA can also bind organic ligands such as drugs, and assay systems have been reported for detecting ligand binding [12,13]. Ohyoshi et al. [12] evaluated the association constants of Zn2+ with albumin using an indicator ligand, 2-(5-bromo-2-pyridylazo)-5-[N-n-propyl-N- (3-sulfopropyl) amino] phenol (5-Br-PAPS), and optical absorption spectroscopy [12]. Li et al. [13] reported that the binding constant of baicalein to bovine serum albumin was decreased after the addition of Cu2+ and Fe3+, which was observed by spectroscopic analysis such as circular dichroism and two- and three-dimensional fluorescence spectroscopy [13]. Colorimetry or absorption spectroscopy is suitable for clinical use because of low initial costs. 5-Br-PAPS (80 USD/0.1 g) is slightly more expensive compared with 1-(2-hydroxycarbonylphenyl)- 5-(2-hydroxy-5-sulfophenyl)-3-phenylformazan (zincon, Figure 1B; 50 USD/1 g) [14]. Zincon can recognize the difference in the peak maxima of the Cu2+ (600 nm) and Zn2+ (620 nm) coordinating forms, and the protein-bound metal concentrations of Cu2+/Zn2+– metalloprotein superoxide dismutase (SOD) have been analyzed by photo-absorption spectroscopy using zincon and denaturating reagents [15]. Therefore, we developed a simple assay system for native HSA-binding metals using zincon as the chelating material.
The binding of Zn2+ and Cu2+ to HSA were analyzed on the basis of the spectral changes after the addition of zincon solution in order to evaluate the metal ions coordinated with the metal-binding site of HSA by recording the absorption spectrum. Some chromophores such as heme and/or biveldin bind strongly to HSA in human serum, and the absorption peak maxima of these molecules overlap with those of zincon [16,17]. Thus, we removed the heme- and biveldin-bound HSAs from human serum by the Cohn cold ethanol method [18,19], which allowed us to selectively remove the heme- and biveldin-bound HSA and to record absorption spectra of the zincon- and metal-bound HSA in the serum by removing the background derived from the chromophore peaks in the absorption spectrum. On the basis of these methods, Zn2+- and Cu2+-metal binding to recombinant HSA or HSA in human body fluids, including serum or plasma can be analyzed at a clinic.
Experimental
Purification of HSA from human serum
The use of human serum was approved by the institutional review board of Miyake clinic. HSA purification from human serum was performed by the Cohn cold ethanol fractionation method [18,19]. Ethanol was added to human serum at 4-5°C and the final concentration of ethanol was 35%. After mixing the suspension for several minutes, it was centrifuged at 15000 rpm for 1 h and the supernatant was diluted 15-20 times with 50 mM HEPES (pH 7.4) (Nacalai Tesque, Kyoto, Japan). The solution was then concentrated by centrifugation at 3000 rpm using the Amicon Ultra-15 3000 MWCO (Merck Millipore, Billerica, MA, USA). This process was repeated three times. According to the SDS-PAGE assay, low amounts of globulin (<10 %) were present in the purified HSA (pHSA).
Preparation of the metal-bound HSA
In a 1.5 mL tube, we mixed 20 mg/mL of HSA (Sigma–Aldrich, St. Louis, MO, USA) with 3 mM Zn2+ (zinc acetylacetonate, Wako Chemicals, Osaka, Japan) or Cu2+ (CuSO4 hydrate, Wako Chemicals) to adjust the concentration ratio to 1:10. HSA was adsorbed onto a negative-ion exchange resin (DEAE-650C; TOYOPEARL, Tosoh, Tokyo, Japan) and the excess metal that did not bind to HSA was removed. After discarding the supernatant, 50 mM HEPES (pH 7.4) was added and the suspension was centrifuged from the resin at 15000 rpm for 1 min. The supernatant was removed with a micropipette 10 times. After washing, the protein was eluted by adding 1 M NaCl solution (Wako Chemicals, Osaka, Japan) and centrifuged at 15000 rpm for 1 min to recover the supernatant. After diluting the supernatant 15-20 times with 50 mM HEPES (pH 7.4), the sample was centrifuged at 3000 rpm and concentrated using a spin column (Amicon Ultra-15 30000 MWCO). We repeated this process twice and obtained HSA samples bound with Zn2+ or Cu2+.
Recording the absorption spectra
The absorption spectra were recorded using a UV spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). All samples were diluted with 50 mM sodium tetraborate decahydrate (pH 9; Wako Chemicals, Osaka, Japan), and zincon (1.6 mM stock solution; Tokyou Kasei, Tokyo, Japan) was added to the solutions [20]. Human serum was diluted 20- fold with purified water. The purified HSA was diluted and the zincon concentration was adjusted to 80 μM.
Results and Discussion
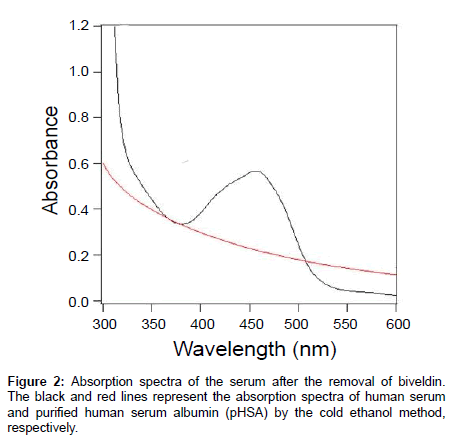
Figure 2 shows the absorption spectra of the serum and pHSA obtained after applying the cold ethanol method (pHSA) in black and red lines, respectively. The peak around 400 nm was assigned to the heme-bound HSA and was observed in the serum. The peaks at 460 nm and 480 nm in the serum were derived from the biveld in-bound HSA. These peaks were observed only in the serum and they disappeared completely after treatment with 35% ethanol (Figure 2, red line).
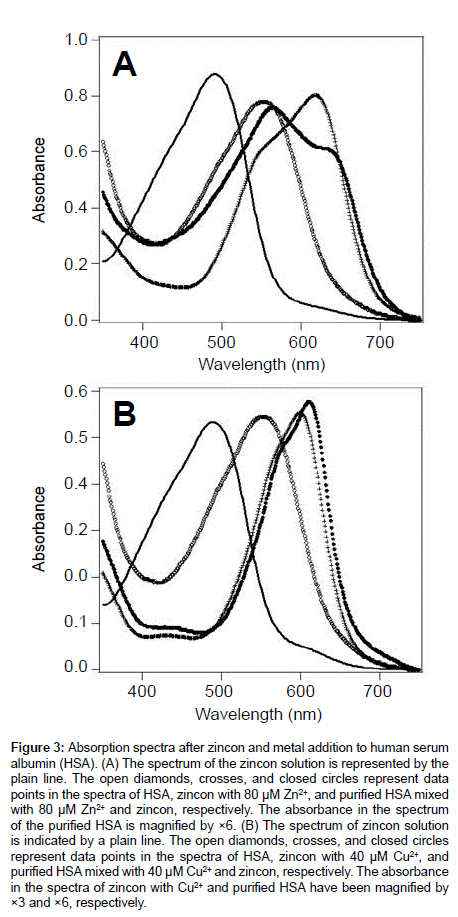
Figure 3 shows the absorption spectra of the metal-bound HSA after the addition of zincon solution. When Zn2+ or Cu2+ is not coordinated to zincon, the peak maximum of zincon was observed at 480 nm. After addition of these metal ions, this peak wavelength at 480 nm derived from zincon was red-shifted to 600-650 nm, which are different with the peak of HSA observed at 550 nm shown in open diamonds (Figure 3). The peak maxima at 650 nm and 620 nm were assigned to zincon coordinating with Zn2+ and Cu2+ in HSA, respectively. When the metalcoordinating zincon was bound to HSA, the zincon peaks were redshifted from those at 620 nm and 650 nm without HSA, respectively. The HSA-bound zincon or free zincon could be recognized on the basis of the peak shift of zincon. The peak maxima of this zincon metal-bound form were different from that reported in a previous study of SOD [15], because the perturbation of zincon from protein was reduced by the denaturation of SOD.
Figure 3: Absorption spectra after zincon and metal addition to human serum albumin (HSA). (A) The spectrum of the zincon solution is represented by the plain line. The open diamonds, crosses, and closed circles represent data points in the spectra of HSA, zincon with 80 μM Zn2+, and purified HSA mixed with 80 μM Zn2+ and zincon, respectively. The absorbance in the spectrum of the purified HSA is magnified by ×6. (B) The spectrum of zincon solution is indicated by a plain line. The open diamonds, crosses, and closed circles represent data points in the spectra of HSA, zincon with 40 μM Cu2+, and purified HSA mixed with 40 μM Cu2+ and zincon, respectively. The absorbance in the spectra of zincon with Cu2+ and purified HSA have been magnified by ×3 and ×6, respectively.
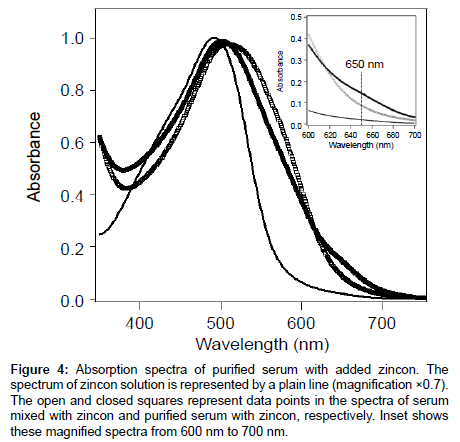
In the spectrum of pHSA as shown in Figure 4, the shoulder at 650 nm suggests that Zn2+ was bound to HSA from human serum (pHSA). Because of its high affinity for Zn2+, HSA is the major plasma carrier of Zn2+; thus, more than 98% of Zn2+ was bound to HSA [6,21-23]. The reported dissociation constant of Cu2+ for HSA formation is 6.7 × 10−17 (M); however, in plasma, only approximately 10% of the total Cu2+ is bound to HSA in physiological conditions [6]. Therefore, Zn2+ binding to HSA may occur in the human body and the absorption spectra reflected this phenomenon.
Figure 4: Absorption spectra of purified serum with added zincon. The spectrum of zincon solution is represented by a plain line (magnification ×0.7). The open and closed squares represent data points in the spectra of serum mixed with zincon and purified serum with zincon, respectively. Inset shows these magnified spectra from 600 nm to 700 nm.
Next, we consider the possible reason for the peak red-shifts in HSA and the metal-bound zincon form based on the molecular structure of HSA. Zn2+ binds strongly to MBS-A in the I/II interdomain contact region, which is surrounded by FA1, FA2, and FA7 (Figure 1) [6]. Crystal structures of HSA suggest that heme and warfarin bind to FA1 and FA7, respectively [24,25]. According to these crystallographic structures, the metal-bound zincon may be able to coordinate with FA1 and FA7 in HSA. Coordination of Zn2+ with zincon may occur in these binding sites of HSA, because both Zn2+ and zincon can interact with the amino acids in these sites. The N-terminal binding site is primarily specific for Cu2+, and MBS-A is the secondary Cu2+ binding site [6]. The red-shift in HSA with Cu2+-coordinating zincon suggests that the Cu2+-coordinating zincon may bind to both the N-terminal region and MBA-A in HSA. However, we could not detect the peak of Cu2+-coordinating zincon in pHSA because the Cu2+-binding content of HSA was too low to distinguish in the spectrum. Thus, the detailed coordination structure of the zincon-metal complex with HSA should be analyzed using ESR and/or vibrational spectroscopy (FT-IR or Raman spectroscopy). The binding site of this transitional metal could also be determined by mutational studies.
Our proposed method could be used for identifying metal-bound HSA samples, including rHSA. After purification by the cold ethanol method, the absorption spectra of HSA can be recorded to determine whether the metal binding may be harmful. If we check the metal coordination with HSA before using HSA in mammalian embryo culture, we can prevent the detrimental effect of Cu2+ coordination with HSA in the embryo culture. Furthermore, a single-chain antibody– albumin fusion protein conjugated with a metal chelator was used in a 64Cu2+-positron emission tomography (PET) imaging study [26]. HSA has multiple metal-binding sites, and thus 64Cu2+-bound HSA may be used as an imaging reagent. This analytical method can be employed for the successful preparation of metal-bound HSA as probe materials in research and clinical institutes. In the future, we aim to determine the concentrations of metals that bind to HSA, and optimize them for clinical use.
Conclusion
In this study, we developed an analytical method for evaluating metal coordination with HSA from human serum using an ethanol fractionation method and based on the absorption spectrum of zincon. The metal-coordinating zincon can bind to HSA and the unbound forms can be recognized by absorption spectroscopy. According to this analytical method, our results showed that the Zn2+ ion coordinated with serum HSA. By the peak shift or shoulder of zincon at 650 nm or 620 nm, the coordination of Zn2+ or Cu2+ with HSA, respectively, can be detected by absorption spectra in clinics without any requirement for elemental analysis. This method can be used to characterize metals that coordinate with HSA in plasma before clinical use, such as embryo culture in ART, as well as for analyzing Zn2+ or Cu2+ bound to HSA for use in PET imaging techniques.
Acknowledgements
This study was supported partly by a grant-in-aid for Scientific Research for Challenging Exploratory Research (No. 23650262 to K. M.) and Special Coordination Funds for Promoting Sciences and Technology from the Ministry of Education, Science, Sports and Culture of Japan (K. M.). We thank Osamu Okitsu (Miyake Clinic) for providing the human serum samples. The authors would like to thank Enago for the English language review.
References
- Chuang VT, Otagiri M (2006) Stereoselective binding of human serum albumin. Chirality 18: 159-166.
- Shimo T, Takahashi M, Takekawa R, Tanaka S, Hasui M, et al. (2010) Role of serum albumin as an antioxidant in idiopathic nephrotic syndrome. Japanese Journal of Pediatric Nephrology 23: 164-167.
- Kobayashi K (2006) Summary of recombinant human serum albumin development. Biologicals 34: 55-59.
- Bungum M, Humaidan P, Bungum L (2002) Recombinant human albumin as protein source in culture media used for IVF: a prospective randomized study. Reprod Biomed Online 4: 233-236.
- Thompson JG (2000) In vitro culture and embryo metabolism of cattle and sheep embryos - a decade of achievement. Anim Reprod Sci 60-61: 263-75.
- Fanali G di Masi A, Trezza V, Marino M, Fasano M, et al. (2012) Human serum albumin: from bench to bedside. Mol Aspects Med 33: 209-290.
- Bal W, Christodoulou J, Sadler PJ, Tucker A (1998) Multi-metal binding site of serum albumin. J Inorg Biochem 70: 33-39.
- Huheey JE, Keiter EA, Keiter RL (1993) Inorganic Chemistry: Principles of Structure and Reactivity. 4th edition Harper Collins College Publishers 70: A279.
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK (2010) Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 6: 674-681.
- Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TK (2012) A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 86: 114.
- O'Shea KS, Kaufman MH (1979) Influence of copper on the early post-implantation mouse embryo: an in vivo and in vitro study. Wilhelm Roux Arch Dev Biol 186: 297-308.
- Ohyoshi E, Hamada Y, Nakata K, Kohata S (1999) The interaction between human and bovine serum albumin and zinc studied by a competitive spectrophotometry. J Inorg Biochem 75: 213-218.
- Li D, Zhu M, Xu C, Ji B (2011) Characterization of the baicaleine-bovine serum albumin complex without or with Cu2+ or Fe3+ by spectroscopic approaches. Eur J Med Chem 46: 588-599.
- http://dominoweb.dojindo.co.jp/
- Säbel CE, Neureuther JM, Siemann S (2010) A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal Biochem 397: 218-226.
- Jacobsen J, Brodersen R (1983) Albumin-bilirubin binding mechanism. J Biol Chem 258: 6319-6326.
- Kocakulak M, Denizli A, Rad AY, PiÅŸkin E (1997) New sorbent for bilirubin removal from human plasma: Cibacron Blue F3GA-immobilized poly(EGDMA-HEMA) microbeads. J Chromatogr B Biomed Sci Appl 693: 271-276.
- Cohn EJ, Strong LE, Hughes WL, Mulford DJ, Ashworth JN, et al. (1946) Preparation and properties of serum and plasma proteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc 68: 459-475.
- Murozuka T, Takeda Y, Izumi H, Murai K, Shirakawa S, et al. (1999) Viral Safety of Plasma-Derived Blood Products III. Effects of Virus Inactivation by Pasteurization in Human Serum Albumin Concentrates. Journal of the Japan Society of Blood Transfusion 45: 362-365.
- Chuang JH, Kao YJ, Ruderman NB, Tung LC, Lin Y (2011) Optimal concentrations of N-decanoyl-N-methylglucamine and sodium dodecyl sulfate allow the extraction and analysis of membrane proteins. Anal Biochem 418: 298-300.
- Giroux EL, Henkin RI (1973) Macromolecular ligands of exchangeable copper, zinc, and cadmium in human serum. Bioinorg Chem 2: 125-133.
- Peters T (1995) All about Albumin: Biochemistry, Genetics and Medical Applications. Academic Press San Diego and London.
- Blindauer CA, Harvey I, Bunyan KE, Stewart AJ, Sleep D, et al. (2009) Structure, properties, and engineering of the major zinc binding site on human albumin. J Biol Chem 284: 23116-23124.
- Wardell M, Wang Z, Ho JX, Robert J, Ruker F, et al. (2002) The atomic structure of human methemalbumin at 1.9 A. Biochem Biophys Res Commun 291: 813-819.
- Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, et al. (2005) Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353: 38-52.
- Yazaki PJ, Kassa T, Cheung CW, Crow DM, Sherman MA, et al. (2008) Biodistribution and tumor imaging of an anti-CEA single-chain antibody-albumin fusion protein. Nucl Med Biol 35: 151-158.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18674
- [From(publication date):
November-2014 - Aug 11, 2025] - Breakdown by view type
- HTML page views : 13870
- PDF downloads : 4804