Mitigative Effects of Koenidine in Reducing the Toxicity Induced by Heavy Metal Lead in Human Neuroblastoma Cells
Received: 11-Nov-2016 / Accepted Date: 21-Nov-2016 / Published Date: 29-Nov-2016 DOI: 10.4172/2572-0406.1000113
Abstract
Lead is a heavy metal been widely used in the manufacturing of glass, pigments, makeup, pipes, wine, cooking, electronic components and batteries. It is a multimedia environmental pollutant, and its exposure via different routes is found to affect metabolic functions. Primarily Lead (Pb) plays an important role in inducing oxidative stress one of the important pathologies and can affect the functioning of antioxidant defense system. The cell growth and proliferation is severely affected with the exposure of lead in designated quantities. There is no specific therapy available to treat the toxicity being generated by Pb and the efforts are on to find suitable natural biologically active components that can effectively combat the toxicity. Koenidine (KND) one of the active ingredients from the plant Murraya koenigii was considered to test its efficacy in mitigating the toxicity elicited by Pb. Human SH-SY5Y neuroblastoma cells were exposed to different concentrations (0.01-10 μM) of Pb for 48 h and the cell viability was determined. The IC50 was observed at 5 μM and this particular concentration used for further investigations. The cells were also pre-treated with KND with a range of concentration from 0-100 μM for 48 h, no change in the cell viability was observed. KND was treated (50 μM) to the cells to verify its protective effects against the pre-treatment of Pb at a concentration of 5 μM 48 h, resulted in the significant increase in the cell viability at least by 29.4% from 12.1% when compared with Pb alone treated group. Intracellular glutathione, caspase-3 and prostaglandin E2 levels were quantified to find the protective effects of koenidine. Some significant results (but less than 18%) were observed in decreasing the oxidative stress, combating apoptosis and lessening the inflammation. This clearly indicates that koenidine can be effectively used to mitigate the toxicity provoked by Pb indicating the nature of koenidine as an effective antioxidant, anti-apoptotic, and anti-inflammatory agent.
Keywords: Lead; Koenidine ; Neuroblastoma cells; Glutathione; Caspase-3; Prostaglandin E2
9820Introduction
Exposure of Lead (Pb) is a major public health concern, has been found to alter the brain function effectively. This can lead to the changes in the learning and psychological function of children behavior [1-3]. The primary target for the lead induced poisoning is the developing nervous system [4,5]. Continued exposure to Pb leads to intellectual impairment and growth retardation [6-8]. The chronic exposure to brain is a multi-factorial event and is executed at intra cellular level [9,10]. Pb can affect the cell functioning by altering the structure and also neurochemically it substitutes calcium that results in altered calcium channel ultimately leading to changes in metabolism resulting in the generation of oxidative stress causing damage in the developing brain [11-13]. Literatures indicates that, Pb induces oxidative stress by generating reactive oxygen species in turn reducing the antioxidant defense system by depleting glutathione [14,15]. This will interfere with essential components that are required for the activation of antioxidant enzyme activities [15]. Toxic assault on the cells is an indication of oxidative damage and during these circumstances antioxidants play an important role in the chemical toxicity being generated by Pb associated with pathological changes [12,16]. Medical cases against Pb induced poisoning has been increased in the past decade and there is a need to find alternate options for the treatment which are very scantly [17,18].
Murraya koenigii (L.) Spreng , commonly recognized as “meethi neem” or “curry patta”, a small aromatic tree belongs to the family Rutaceae , a very common plant widely available in India and is a tropical to sub-tropical in nature. Murraya koenigii is very popular because of battery of medicinal properties. Leaves are a major part of Indian culinary practices with characteristics such as slight pungent odor with bitter taste regularly used for seasoning dishes [19]. Mostly roots, bark and leaves are having active bio-ingredients having antidiabetic [20], anticancer [21], antioxidant [22], hepatoprotective [23], and antilipase activities [24]. Reported important constituents are carbazole alkaloids, coumarins and flavonoids [25]. Koenidine (KND), 3,11-Dihydro-8,9-dimethoxy-3,3,5-trimethylpyrano[3,2-a]carbazole is an active ingredient said to have antioxidant [26-28], antiinflammatory [29], immune-modulatory [30] and apoptotic effects [31-33]. Therefore, the present report was focused to elucidate the mitigative properties of KND against Pb-induced toxicity in Human neuroblastoma cells.
Materials and Methods
RPMI-1640 medium, OPI were obtained from GIBCO Life Technologies. Fetal Bovine Serum, Penicillin and Streptomycin and Phosphate Buffered Saline were procured from CELLGRO, Mediatech. The kits were used for the assays of MTT cell proliferation (ATCC, Manassas, VA, USA), Glutathione (Dojindo Molecular Technologies, Gaithersburg, MA,, USA), Prostaglandin E2 (Amersham Biosciences Piscataway, NJ, USA) and Caspase-3 (Biovision, Mountain View, CA, USA). All other chemicals were of analytical grade and procured from Sigma including Koenidine (3,11-Dihydro-8,9-dimethoxy-3,3,5- trimethylpyrano[3,2-a]carbazole).
Cell culture
Human SH-SY5Y neuroblastoma cells were grown in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum, 50 μg/ml penicillin-streptomycin and OPI (150 μg/ml oxaloacetate, 50 μg/ml pyruvate and 0.2 U/ml insulin) in a humidified air/5% CO2 chamber at 37°C. Medium was changed every three days and were passed once they reached approximately 80% confluence.
Cell viability
Cell viability among the attached cell population was assessed by trypan blue exclusion technique. Trypan blue isotonic solution was added to the culture and number of deeply stained cells, representing dead cells was counted using Neubauer hemocytometer as described by Black and Berenbaum [34]. The number of stained cells was subtracted from the total count in order to determine the percentage of viable cells.
Exposure of Cells to Pb acetate and Koenidine (KND)
Human SH-SY5Y neuroblastoma cells were seeded at 2 × 104 cells per well in a 96 well plate. The cells were allowed to attach and grow for two days prior to the treatment. The cells were treated with varying concentrations of lead acetate (0.01-10 μM) in order to determine IC50 value. The cells were pre-treated with KND (0-100 μM) to determine the effect on cell growth. Further, the cells were exposed to 5 μM (IC50) of Pb in the presence or absence of KND for 48 h and the cell viability was determined by MTT reduction assay.
MTT reduction assay
Cell viability was also assessed by MTT [3-(4,5 dimethyl-2- thazolyl)-2,5 diphenyl-2H tetrazolium bromide] reduction assay as per the manufacturer’s instructions. In brief, the cells 2 × 105 per well were seeded into 96 well culture plates and allowed to attach. The medium containing varying concentrations of Pb in the presence or absence of KND was added to the cells and incubated for 48 h. Then, 10 μl of MTT reagent was added to the culture and incubated in the dark for four h at 37°C followed by cell lysis by the addition of 100 μl Detergent reagent provided with the kit. Then plate was left at room temperature in the dark for 2 h and the relative amount of MTT reduction was determined based on the absorbance measured at 570 nm using a plate reader.
Determination of glutathione
In brief, 5 × 105 cells were centrifuged and the suspension was washed with the Phosphate Buffered Saline. Suspended cells were lysed with 10 mM HCl and treated with 5% sulphosalycilic acid. The cells were centrifuged at 8,000 rpm for 10 min and to the supernatant 20 μl of enzyme working solution, 140 μl of coenzyme working solution and 20 μl of either one of the standard solution or the sample solution (cells treated with Pb in the presence or absence of KND) were added. Then the plate was incubated at 37°C for 10 min and 20 μl of substrate,5,5’- Dithiobis(2-nitrobenzoic acid) [DTNB] working solution was added. The plate was incubated at room temperature for 10 min and the optical density was measured at 415 nm using a micro plate reader and the concentration of GSH was calculated using a standard calibration curve. The reagents used throughout the experiment were prepared according to the manufacturer’s instructions.
Caspase-3 activity assay
Briefly, the assay is based on the spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labelled substrate DEVD-pNA. After the Pb-treatment of the cells in the presence or absence of KND, 3-5 × 106 cells were collected, resuspended in the 50 μl of chilled cell lysis buffer included in the kit and incubated for 10 min on ice and then centrifuged at 10,000 × g for 1 min. To the supernatant, 50 μl of the reaction buffer containing 10 mM dithiothreitol and 5 μl of the 4 mM DEVD-pNA substrate was added and incubated at 37°C and the optical density was measured at 405 nm after 2 h. The relative fold increase in caspase-3 activity was determined by comparing with the levels of the untreated control.
Determination of prostaglandin E2
The reagents used in the procedure were included in the kit contents. SH-SY5Y neuroblastoma cells 104 to 105 cells/well were placed in a standard 96 well microplate. The plate was incubated in a humidified air/5% CO2 chamber at 37°C and allowed the cells to attach. The cells were treated with Pb in the presence or absence of KND and continued the incubation for 48 h. Then, 20 μl of buffer A (2.5% Dodecyltrimethylammonium Bromide in 0.1 M Phosphate buffer pH 7.5). The cells were lysed by simple agitation and 50 μl of lysate was transferred to goat anti-mouse IgG coated plate (included in the kit) and 50 μl of lysis reagent containing Dodecyltrimethylammonium Bromide (prepared as described with 0.1 M Phosphate buffer pH 7.5 containing 0.9% Bovine Serum Albumin and 0.5% Kathon). Further, 50 μl of diluted PGE2 antibody and 50 μl of diluted conjugate (PGE2 conjugated to Horseradish Peroxidase) were added to the plate and incubated at room temperature for one hour. The cells were washed and added 150 μl of enzyme substrate (3,3’, 5,5’ Tetramethylbenzidine) readily provided with the kit. The contents were mixed thoroughly exactly for 30 min and the reaction was stopped by the addition of 100 μl of 1 M sulfuric acid. The optical density was measured at 450 nm within 30 min and concentration of total cellular PGE2 was calculated using appropriate standards.
Data analysis
Data were expressed as mean ± SD of at least four determinations from each group, repeated at least three times in different occasions. Statistical analysis was performed using one-way analysis of variance (ANOVA) and the statistical significance was assumed at p<0.05.
Results
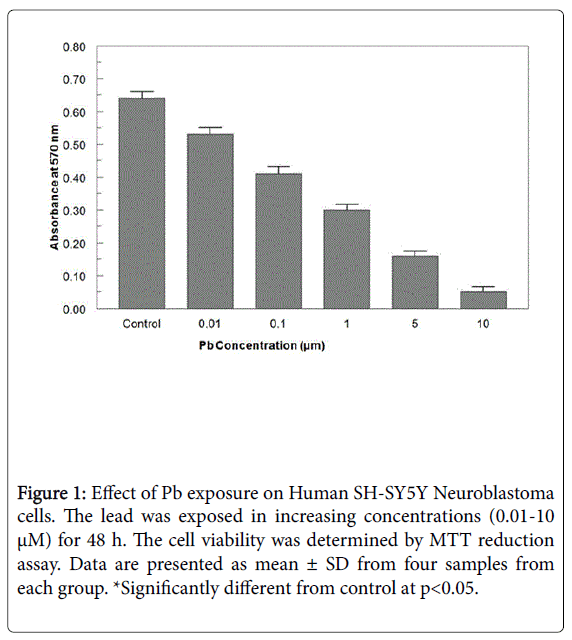
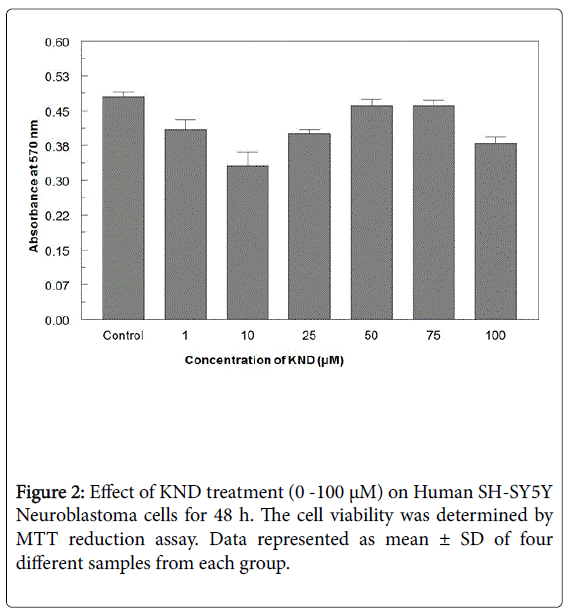
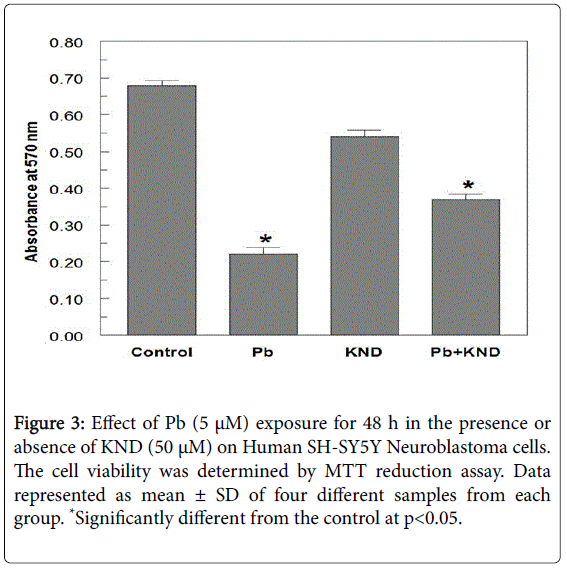
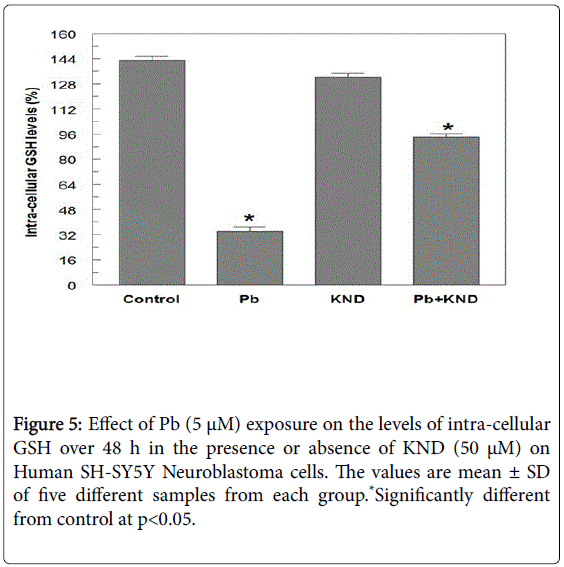
When exposed to different concentrations of Pb ranging from 0.01 μM to 10 μM for 48 h, the cell viability was significantly decreased from 96.4-23.2% (Figure 1). A 50% decrease in cell viability (IC50) was observed at 5 μM Pb. When cells exposed to KND with a range of concentration from 0-100 μM for 48 h, the cell viability has not shown any significant alterations (Figure 2). KND was treated (50 μM) to the cells to verify its protective effects against the pre-treatment of Pb at a concentration of 5 μM 48 h, resulted in the significant increase in the cell viability at least by 29.4% from 12.1% when compared with Pb alone treated group (Figure 3). This indicates that, KND can effectively function as a protective compound in enhancing the cell viability affected by Pb exposure. A significant decrease in the levels of intracellular GSH was observed in the cells exposed to Pb for about 48 h in a concentration dependent (Figure 3). 53.2% of decrease in GSH levels were observed in the cells at 5 μM of Pb exposure, whereas cells treated with KND alone (1-100 μM) for 48 h the GSH levels were unaltered (Figure 4). When pre-treated with KND (50 μM) along with Pb (5 μM) has shown a significant increase (48.2%) in the levels of GSH when compared with the group with Pb alone indicating the fact that, KND has an antioxidant effect (Figure 5).
Figure 1: Effect of Pb exposure on Human SH-SY5Y Neuroblastoma cells. The lead was exposed in increasing concentrations (0.01-10 μM) for 48 h. The cell viability was determined by MTT reduction assay. Data are presented as mean ± SD from four samples from each group. *Significantly different from control at p<0.05.
Figure 3: Effect of Pb (5 μM) exposure for 48 h in the presence or absence of KND (50 μM) on Human SH-SY5Y Neuroblastoma cells. The cell viability was determined by MTT reduction assay. Data represented as mean ± SD of four different samples from each group. *Significantly different from the control at p<0.05.
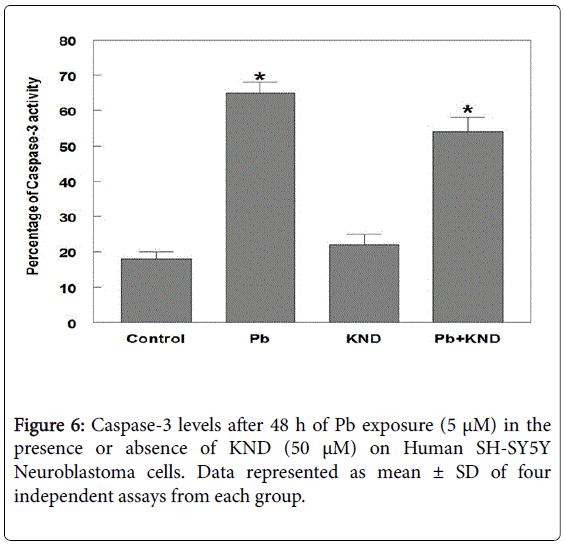
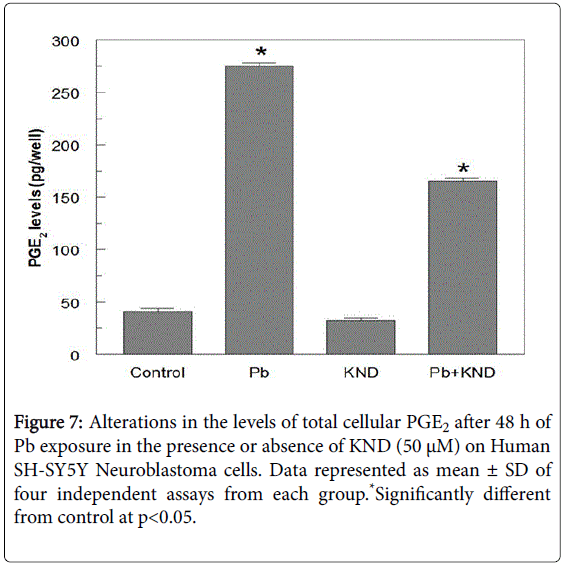
Levels of caspase-3, an executioner enzyme in the apoptosis pathway were significantly increased at least by 52.5% when compared with the untreated control group. Rather, the cells treated with KND did not show any increase in the enzyme levels when compared with the Pb alone group, but, the combination of Pb and KND has a significant decrease in the levels of caspase-3 by 21.4% when compared with the Pb alone group (Figure 6). Seven fold increase in the PGE2 levels were observed in the Pb but not KND group, whereas, the combination of KND (50 μM) and Pb (5 μM) exposure resulted in a significant decrease in PGE2 by 112.5 pg per well when compared the Pb alone group (Figure 7).
Discussion
Literature indicates that, Pb has induced alterations in both cellular and animal models [35-38] but reports on the effects of low levels of Pb exposure and subsequent damage to Central Nervous System (CNS) is very limited. Pb-induced toxicity to the cells mediated by the oxidative stress is one of the very important events that are been associated with the cell death [36]. Present study deals with the investigations involving the exposure of low levels of Pb on human neuroblastoma cells in culture and role of koenidine in protective the cells from the toxic assaults.
Our results were encouraging and suggested that cell viability has gone down drastically with the exposure of Pb in a dose dependent manner. Murraya koenigii leaves to be a potent source of proteasome inhibitors that lead to cancer cell death. The active component(s) such as koenidine from the leaf extract might be playing role in the reducing the proliferation rate of the cells [39]. When we tested the KND on human brain cells, the similar kind of effects such as a significant reduction on cell proliferation was observed. Koenidine alone has not shown any effect on the proliferation of neuroblastoma cells but its pre-treatment has significantly mitigated the influence of Pb on cell proliferation. Abnormal behavioural, motor and cognitive impairments resulted because of the Pb induced brain damage could be through the process of apoptosis, a process of programmed cell death is triggered by variety of stimuli.
Caspases a family of cysteine proteases, transducer apoptotic cell death signals in a hierarchical fashion, where the initiator caspases cleave and activate the effector caspases leading to cell death [40]. One of the important executioner enzymes in the group of caspases is caspase-3 which promotes the cell dis-assembly via the cleavage of several structural proteins and repairs the enzymes essential for maintaining cellular homeostasis. The proteolytic activity of caspase-3 is a critical determinant of whether or not cell commits suicide [41]. It was observed that alkaloids from Murraya has induced the loss of mitochondrial membrane potential and subsequently activated caspase-9/caspase-3 cascade resulting in induction of apoptosis [31].
In the present study, Pb exposure stimulated the specific activity of caspase-3 and it was evident with the significant increase in its levels. However, the cells pre-treated with KND followed by Pb exhibited a decrease in caspase-3 activity suggesting that KND could elicit effective anti-apoptotic effect. In fact, caspase-3, a member of the caspase family has been shown to be the key modulator of apoptotic changes [42].
Our results shows a significant decrease in the levels of GSH, a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and the amine group of cysteine, and the carboxyl group of cysteine is attached by normal peptide linkage to a glycine, suggesting the fact that oxidative stress has been generated. Oxidative stress is one of the molecular events that are closely associated with Pb induced toxicity. Studies have shown that environmental Pb can stimulate oxidative haemolysis in erythrocytes by inhibiting the superoxide dismutase [12,16]. From our results it is evident that the cells pre-treated with KND were resistant to the effects of Pb, suggesting that KND acts as neuroprotective agent in countering Pb toxicity. KND might have strongly bonded to Pb thus leading to increase in intracellular GSH levels could be via up-regulation of machinery of GSH-synthesizing enzymes [43-45]. It is possible that the metal-chelating effect of KND has reduced cell damage and increased the production of intracellular GSH.
A very low concentration of Pb has resulted in the significant release of PGE2. Activation of cyclooxygenase and production of PGE2 is an essential key mechanism in the process neuronal loss [46]. It gives an impression that Pb could have raised the release of PGE2 by activating of the cyclooxygenase pathway. Cells that are treated with KND resulted in the significant alterations in the PGE2 levels indicating the fact that KND has an important role in the inflammatory pathway.
Conclusion
Exposure of Pb to the cells has induced oxidative stress, apoptosis and inflammation; these pathways might have played an important role in developing neurotoxicity. Further, pre-treatment of the cells with natural bio-active compounds such as koenidine has significantly mitigated the toxicity that is been generated by Pb.
Acknowledgement
This research work was supported by ICMR Grant No. 58/57/2012- BMS.
Conflict of Interest
The authors declared that they have no conflict of interest.
References
- Verity MA (1990) Comparative observations in inorganic and organic Pbneurotoxcity.Environ Health Persp 89: 43-48.
- Silbergeld EK (1992) Mechanisms of Pb neurotoxicity or looking beyond the lamp post. FASEB J 6: 3201-3206.
- Tong S, Von Schirnding YE, Prapamontol T (2000) Environmental lead exposure: a public health problem of global dimensions. Bull. World Health Organ 78: 1068-1077.
- Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, et al. (1979) Deficits in psychologic and classroom performance of children with elevated dentine lead levels. New Eng J Med 300: 689-695.
- Wilson MA, Johnson MV, Goldstein GW, Blue ME (2000) Neonatal lead exposure impairs development of rodent barrel filed cortex. ProcAcadSci USA 97: 5540-5545.
- Shih TM, Hamin N (1978) Chronic lead exposure in mature animals: neuro chemical correlates. Life Sci 21: 877-888.
- Silbergeld EK, Fales JT, Goldberg AM (1974) The effects of inorganic lead on the neuromuscular junction. Neuropharmacology 13: 795-801.
- Silbergeld EK, Goldberg AM (1974) Lead-induced behavioral dysfunction: an animal model of hyperactivity. ExpNeurol 42: 146-157
- Zawia NH, Harry GJ (1995) Exposure to lead acetate modulates the developmental expression of myelin genes in the rat frontal lobe. Int J Dev Neurosci 13: 639-644.
- Sattar A, Xie S, Hafeez MA, Wang X, Hussain HI, et al. (2016) Metabolism and toxicity of arsenicals in mammals. Environ ToxicolPharmacol 48: 214-224
- Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ ToxicolPharmacol 48: 203-213.
- Gurer H, Ozygunes H, Saygin E, Ercal N (2001) Antioxidant effect of taurine against lead-induced oxidative stress. Arch Environ ContamToxicol 41: 397-402.
- Scortegagna M, Chikhale E, Hanbauer I (1998) Lead exposures increases oxidative stress in serum deprived E14 mesencephalic cultures: Role of metallothionein and glutathione. RestorNeurol Neurosci 3: 95-101.
- Flora SJ, Pande M, Kannan GM, Mehta A (2004) Lead induced oxidative stress and its recovery following co-administration of melatonin or N-acetylcysteine during chelation with succimer in male rats. Cell Mol Biol 50: 543-551.
- Flora SJ, Pande M, Mehta A (2003) Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiolchelators in the treatment of chronic lead intoxication. Chem Bio Interact 145: 267-280.
- Gurer H, Ercal N (2000) Can oxidants be beneficial in the treatment of lead posisoning. Free Rad Biol Med 29: 927-945.
- Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126: 5-19.
- Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev 49: 529-554.
- Biswas A, Bhattacharya S, Dasgupta S, Kundu R, Roy SS, et al. (2010) Insulin resistance due to lipid-induced signaling defects could be prevented by mahanine. Mol Cell Biochem 336: 97-107.
- Ahmad K, Tan S, Sukari M, Ali A, Nafiah M (2014) Cytotoxic and Anti-Tumour Promoting Activities of Carbazole Alkaloids from Malayan Murraya koenigii (L.) Sp reng. Am J Plant Sci 5: 2869-2877.
- Rao LJM, Ramalakshmi K, Borse BB, Raghavan B (2007) Antioxidant and radical-scavenging carbazole alkaloids from the oleoresin of curry leaf (Murraya koenigiiSpreng). Food Chem 100: 742-747.
- Gupta RS, Singh D (2007) Protective nature of Murrayakoenigii leaves against hepato-suppression through antioxidant status in experimental rats. Pharmacologyonline 1: 232-242.
- Birari R, Roy SK, Singh A, Bhutani KK (2009) Pancreatic lipase inhibitory alkaloids of Murraya koenigii leaves.Prod Commun 4: 1089-1092.
- Nayak A, Banerji J, Banerji A, Mandal S (2010) Review on chemistry and pharmacology of Murraya koenigiiSpreng (Rutaceae). J Chem Pharm Res 2: 286-299.
- Gupta S, Prakash J (2009) Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum Nutr 64: 39-45.
- Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N (2001) Antioxidative activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem49: 5589-5594.
- Khan BA, Abraham A, Leelamma S (1997) Anti-oxidant effects of curry leaf, Murraya koenigii and mustard seeds, Brassica juncea in rats fed with high fat diet. Indian J ExpBiol 35: 148-150.
- Gupta S, George M, Singhal M, Sharma GN, Garg V (2010) Leaves extract of Murrayakoenigii for anti-inflammatory and analgesic activity in animal models. J Adv Pharm Technol Res 1: 68-77.
- Shah AS, Wakade AS, Juvekar AR (2008) Immunomodulatory activity of methanolic extract of Murraya koenigii (L) Spreng. Leaves. Indian J ExpBiol 46: 505-509.
- Ito C, Itoigawa M, Nakao K, Murata T, Tsuboi M, Kaneda N, Furukawa H (2006) Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine 13: 359-365.
- Roy MK, Thalang VN, Trakoontivakorn G, Nakahara K (2004) Mechanism of mahanine-induced apoptosis in human leukemia cells (HL-60). BiochemPharmacol 67: 41-51.
- Bhattacharya K, Samanta SK, Tripathi R, Mallick A, Chandra S, et al.(2010) Apoptotic effects of mahanine on human leukemic cells are mediated through crosstalk between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways. BiochemPharmacol 79: 361-372.
- Black L, Berenbaum MC (1964) Factors affecting the dye exclusion test for cell viability. Exp Cell Res 35: 9-13.
- Sandhir R, Gill KD (1995) Effect of lead on lipid peroxidation in liver of rats. Biol Trace Elem Res 48: 91-97.
- Adonaylo BN, Oteiza PI (1999) Lead intoxication, antioxidant defense and oxidative damage in brain. Toxicology 135: 77-85.
- Huang F, Schneider JS (2004) Effects of lead exposure on proliferation and differentiation of neural stem cells derived from different regions of embryonic rat brain. Neurotoxicology 6: 1001-1012.
- Jahromi MF, Liang JB, Ebrahimi R, Soleimani AF, Rezaeizadeh A, et al. (2016) Protective potential of Lactobacillus species in lead toxicity model in broiler chickens. Animal 2: 1-7.
- Noolu B,Ajumeera R, Chauhan A, Nagalla B, Manchala R, et al. (2013) Murraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cells. BMC Complement Altern Med 13: 7.
- Wolf BB, Green DX (2001) Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem 274: 20049-20052.
- Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281(5381): 1312-1316.
- Mandlekar S, Yu R, Tan TH, Kong AN (2000) Activation of caspase-3 and c-Jun NH2-terminal kinase-1 signaling pathways in tamoxifen-induced apoptosis of human breast cancer cells. Cancer Res 60: 5995-6000.
- Limson J, Nyokong T, Daya S (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J Pineal Res 24: 15-21.
- Martin GM, Austad SN, Johnson TE (1996) Genetic analysis of aging: Role of oxidative and environmental stresses. Nature Genet 13:25-34.
- Martin V, Sainz RM, Antolin I, Mayo JC, Herrera F, et al (2002) Several antioxidant pathways are involved in astrocyte protection by melatonin. J Pineal Res 33: 204-212.
- Bate C, Rutherford S, Gravenor M, Reid S, Williams A (2002) Cycloxygenase inhibitors protect against prion-induced neurotoxicity in vitro. Neuroreport 13: 1933-1938.
Citation: Suresh C, Dixit PK (2016) Mitigative Effects of Koenidine in Reducing the Toxicity Induced by Heavy Metal Lead in Human Neuroblastoma Cells. J Chem Biol Ther 1: 113. DOI: 10.4172/2572-0406.1000113
Copyright: © 2016 Suresh C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12812
- [From(publication date): 11-2016 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 11877
- PDF downloads: 935