Morbid Obesity in End Stage Heart Failure: How Safe is Bariatric Surgery in Ventricular Assist Device Recipients?
Received: 30-Apr-2019 / Accepted Date: 30-Jul-2019 / Published Date: 06-Aug-2019 DOI: 10.4172/2165-7904.1000388
Abstract
Introduction: About 25% of patients suffering end stage heart failure (ESHF) are obese. BMI>40 prevents patients from receiving an organ graft because morbid obesity (MO) dramatically increases mortality after heart transplantation (HTx). Moreover, MO (BMI>40) increases the risk of thromboembolic events by 20%. The treatment of ESHF in obese patients must include the treatment of their obesity.
Bariatric surgery (BS) is the most effective treatment for MO, but has prohibitive surgical mortality in ESHF patients. One strategy is to first implant a Left Ventricular Assist Device (LVAD) to provide hemodynamic stability during BS and eventually reduce patient’s BMI to values compatible with HTx (bridge-to-candidacy approach). However, stopping the anticoagulation for BS increases the risk of LVAD thrombosis and/or thromboembolic events, particularly in presence of MO. We report the therapeutic pathway we applied to solve this challenging situation.
Method: A 54 years old man, former smoker, with a BMI of 43.8 kg/m2 and sleep apnea syndrome, suffered from ESHF due to ischemic (LAD occlusion) and rhythmic cardiomyopathy (non valvular atrial fibrillation) with CHA2DS2- VASC score 5. Left ventricle ejection fraction was 20%. Mean pulmonary pressure was 35 mm Hg and cardiac index was 2.0 l/min/m2. His BMI was the only contraindication to HTx. Our institutional Heart Failure Team decided to implant a continuous flow LVAD to improve patient’s hemodynamic condition and then perform a sleeve gastrectomy (SG).
Results: LVAD implant (Abbott HeartMate 3) was performed under CPB (75 min) using a minimal invasive approach through an upper ministernotomy and a left anterior hemi thoracotomy. The operation and post-operative phase were uneventful. The patient was extubated less than 12 hours after the surgery and left ICU on postoperative day (POD) 3. Clopidogrel and anti-vitamin K treatment were introduced on POD 3. Despite nutritional management and rehabilitation, the patient’s weight increased by 7 kg during the first postoperative months. Thus, it was decided to perform SG using a laparoscopic approach 10 months after LVAD implant. Sintrom was suspended 3 days before the procedure and replaced by prophylactic IV Heparin (anti-Xa<0.1). Sintrom was reintroduced on POD 3. The operation was uneventful despite three previous abdominal open surgeries, with no need of laparotomy conversion. The patient was discharged at POD 10. Patient lost 22 Kg in the first 3 months after SG (BMI<35), he is now listed to HxT.
Conclusion: LVAD followed by BS represents an effective therapeutic strategy to make ESHF obese patients eligible for HTx. New generation LVADs make the management of anticoagulation treatment safer than ever even in patients at high risk for thromboembolic complications.
Keywords: Left ventricular assist device bariatric surgery; Laparoscopic sleeve gastrectomy; End stage heart failure; Heart transplant
Introduction
Heart failure (HF) prevalence keeps constantly going up reaching 60000 patients worldwide. End stage Heart Failure (ESHF) is often associated with metabolic comorbidities, such as HTA, diabetes or obesity. So that, 80% of HF patients are either diabetic or obese. According to the International Society of Lung and heart transplant of 2016 recommendations [1], heart transplantation is contraindicated in patients with a BMI higher than 35. Morbid obesity (MO) increases mortality and thromboembolic events by 20% after heart transplantation (HTx). Yang et Al demonstrate that BMI up to 30 increases the risk of coronary heart disease requiring Primary Coronary Angioplasty after HTx. Only 2200 hearts are available each year for more than 100000 registered receptors with ESHF. Thus, medical societies have established eligibility restrictions for being on the transplant list, according to the chances of survival after HTx. The treatment of ESHF in obese patients must include the treatment of their obesity. Bariatric surgery (BS) is the most effective treatment for MO, it will eventually reduce the patient’s BMI to values compatible with HTx, but has prohibitive surgical mortality in ESHF patients. One strategy is to first implant a Left Ventricular Assist Device (LVAD) support in a “Bridge to candidacy approach”.
LVAD support provides hemodynamic stability during BS. However, stopping the anticoagulation in obese patients for BS increases the risk of LVAD thrombosis and/or thromboembolic events. We report the therapeutic pathway we applied to solve this challenging situation.
Case Description
The patient was treated between 02.2018 and 01.2019 at Vaud University Hospital (CHUV, Lausanne, Switzerland).
Mr. R. C, a fifty four years old man, former smoker at 45 PA, suffering from high blood pressure, stage III obesity with BMI 43.8 kg/m2 (134 kg for 175 cm) and sleep apnea syndrome treated by CPAP. The patient had furthermore a history of numerous abdominal surgeries (9 by open surgeries and one by laparoscopic approach).
In the cardiac background, he has an ischemic heart disease after an occlusion of the LAD, with a chronic restenosis of a stent in that position. He has a persistent non-valvular atrial fibrillation (CHA2DS2-VASC score 5 pts) as rhythmic disturbances, anticoagulated by apixaban. The patient also carries an Implantable Cardioverter Defibrillator in primary prevention of sudden cardiac death. A cardiac MRI done three years earlier showed a LV dilatation with reduced ejection fraction
(LVEF 28%), dyskinesia of the anterior wall and apex on seven myocardial segments together with transmural fibrosis.
He was admitted with an acute heart failure with dyspnea NYHA stage IV. The diagnosis of advanced HF on ischemic heart disease (IHD) was retained. He was diagnosed as being in advanced heart failure due to IHD.
A transthoracic echocardiogram 4 months before LVAD surgery confirmed a severe dilatation of the left ventricle, with severely depressed ejection fraction (LVEF 20%), anterior and anteroseptal akinesia with apical dyskinesia, diastolic dysfunction and left atrial dilatation with a volume of 39 cc/m2. No significant cardiac valvulopathy was identified. Pulmonary artery systolic pressure was measured at 30 mm Hg.
Pharmacological treatment of the HF was limited because of severe hypotension. After discussion by the heart team, a LVAD implantation in “Bridge to candidacy approach” was retained.
Right heart catheterization showed a severely reduced cardiac index at 2.0 l/min/m2, pulmonary capillary pressure at 24 mm Hg and a mean pulmonary artery pressure of 35 mmHg. No response to Dobutamin on frequency, flow or filling pressures. We completed the preoperative evaluation by ergometer cycle with submaximal stress test at the metabolic level, which was interrupted because of severe dyspnea (9/10). We concluded on highly altered aerobic capacity with a MVO2 max at 11.0 ml/kg/min and a slope VE/VCO2 to 38. We added at least a Computerized Tomography (CT) scan as well as a sinus CT, revealed no abnormalities contraindicating the surgery.
LVAD implant (Abbott HeartMate 3) was performed under cardiopulmonary bypass (CPB; 75 min) using a minimal invasive approach through an upper mini-sternotomy and a left anterior hemithoracotomy. The operation and post-operative phase were uneventful. The patient ’ s postoperative course was uneventful (mechanical ventilation duration<12 hours; ICU stay, 3 days). Oral anticoagulation (Acenocoumarol, INR 2 to 3) and antiplatelet (Clopidogrel, 75 mg/ day) treatment were introduced on postoperative day (POD) 3.
Five months after the procedure, he consults for chest pain and dyspnea with pain in the path of the LVAD supply cable without erythema or suppuration. An important biological inflammatory syndrome was found and empirical antibiotic treatment was granted with co-amoxicillin. We first performed a US revealing no collection around the cable on its subcutaneous path, blood cultures at 48 hours were negative. Clinical and biological outcomes showed the progression of inflammatory syndrome with persistence of fever despite antibiotic therapy. We performed a whole body Positron Emission Tomography with FDG injection, showing an uptake at the Interventricular septum level, associated with minimal pleural effusions and pericardial faintly hyper metabolic, reason why We decided to stop antibiotic therapy. Taking into account the PET CT result and the clinical and biological improvement without specific treatment, we hold the diagnosis of acute pleuropericarditis.
Metabolic follow-up and dietary counseling was conducted for weight loss, with a complete evaluation of body composition by Dualenergy X-ray absorptiometry, using indices of body composition makes it possible to standardize the value of Lean mass index: 10.16 kg/m2, absolute fatty mass according to the stature of the patient. Total fat mass was 47.8% and fat mass Index (IMF)=19.05 kg/m2.
Despite nutritional management and rehabilitation, the patient’s weight increased by 7 kg during the postoperative period. Thus, it was decided to perform Sleeve Gastrectomy using a laparoscopic approach 10 months after LVAD implantation. In preparation for bariatric surgery (BS), oral anticoagulation was suspended 3 days before the procedure and replaced by prophylactic IV Heparin (anti-Xa<0.1).
Clopidogrel was substituted by Aspirin 100 mg. Laparoscopic Sleeve Gastrectomy (LSG) was performed 10 months after LVAD implant using a conventional technique. There was no intraoperative or postoperative adverse event. The patient was discharged from hospital on POD 10.
At follow-up, no pump thrombosis or thrombo-embolic events were observed despite six days therapeutic anticoagulation window. Two weeks after SG, the patient presented syncope on orthostatic hypotension. Iatrogenic injury (diuretic and vasodilators) was retained. The HM III interrogation reveals several "PI events" without low flow episodes.
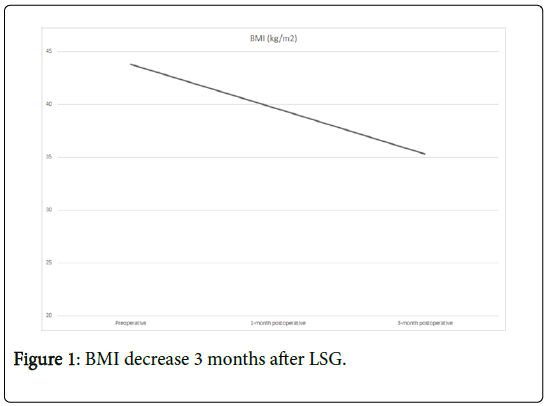
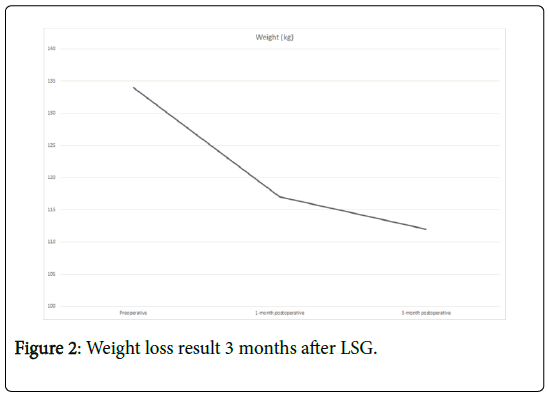
Only a month after LSG, there was a significant improvement in physical activity with 30 min of walking on a daily basis (2.5 km) and a tolerance of 4-5 flights of stairs. During the first month post LSG he had a 10 Kg weight loss and a decrease of his BMI to 39.63 kg/m2. After the firsts 3 post-operative months the patient achieves a total reduction of BMI of 9 points and a weight loss of 22 kg. The patient is now listed for heart transplantation with a BMI of 35.3 kg/m2.
Discussion
ESHF (NYHA IV) is correlated with high mortality, up to 40 to 50% to a year without HxT. About 25% of patients suffering end stage heart failure (ESHF) are obese. BMI>40 prevents patients from receiving an organ graft because morbid obesity (MO) dramatically increases mortality after heart transplantation (HTx). In a Bridge to Htx by weight, the only way is to implant LVAD support which is not contraindicated by their obesity. Indeed, obesity does not impact midterm survival after LVAD implantation but it ’ s associated with moderately increased risk of driveline infection [2,3] and thrombosis event.
In our patient, despite nutritional management and rehabilitation whose clearly improves patient’s physical abilities, weight increased by 7 kg during the six months following implantation of a continuousflow left ventricular assist device (CF-LVAD). In fact, exercise capacity in heart failure patients remains reduced under LVAD support [4] which make weight loss more difficult. Our institutional Heart Failure Team decided to perform a sleeve gastrectomy (SG) to eventually reduce patient ’ s BMI to values compatible with HTx (bridge-tocandidacy approach).
Before us, few teams worked on a combined approach LVAD/SG in ESHF obese patients as a «bridge to candidacy», however no largescale studies have been conducted. Indeed, the widest include no more than eleven patients [5]. In all the cases described, no patient was under HM III and thrombotic events were reported [6].
Chronic heart failure was a powerful predictor of mortality in patients undergoing surgery. Reason why, it seemed reasonable to implant a continuous flow LVAD (Abbott Heartmate III™) first to improve patient’s hemodynamic condition before considering a sleeve gastrectomy (SG).
We have used previous studies to choose SG technique. Several works having shown the superiority of the SG compared to the gastrectomy in Y, which seems Induce immunological disorder compromising the immunosuppressive therapy after HTx. Structural modification of the mucosa was suspected. The laparoscopic approach was the safest surgical technique [7]. Thus, an incision that would be too close to the cable of the pump or the transmission cable can be avoided. Other teams try BS with a different approach using (Roux en– Y- gastric bypass) [8].
Ten months establishment between the LVAD implantation and the LSG. Although several studies have been conducted to demonstrate the safety of a non-cardiac surgical procedure in patients equipped with LVAD, bariatric surgery was rarely described making us face several challenges.
During the surgery, on one hand, there was pump complications. The first difficult step of the surgery was to manage hemodynamic disturbances due to Pneumoperitoneum. Indeed, gas insufflation and anti-trend on venous return exponent to a risk of pump defusing, forcing us to keep an insufflation pressure constantly<12 mmHg. We were also concerned about Pump thrombosis and/or thromboembolic event requiring an emergency reoperation and pump change. MO (BMI>40), increases the risk of thromboembolic events by 20% more increasing by Stopping the anticoagulation for BS increases the risk of LVAD thrombosis and/or thromboembolic events, particularly in presence of MO.
The use of HM III, a continuous flow-through rotary pump (nonpulsatile), large blood flow gaps suspension with electromagnetic suspension, blood contacting textured surfaces, is revolutionary Ventricular Assistance device offering a lower risk of thrombosis. Elevate study Compare the HM II to HM III, and concluded that HM III device was more efficacy comparing to HM II but also reduce thrombotic complication [9]. Therefore, Mehra et al. in momuntum trial show 9.4 percent of difference in survival at 6 mouth without brain stroke or reoperation for pomp thrombosis between HM II and HM III [9]. New generation LVADs make the management of anticoagulation treatment safer than ever even in patients at high risk for thromboembolic complications, making possible to consider LSG in a serene manner.
The possibility of Converting to open surgery was not negligible in our patient because of the history of numerous abdominal surgeries, 9 by open surgeries and one by laparoscopic approach. Hemorrhagic complication due to adhesiolysis, angiodysplasia and arteriovenous malformations were increased by aspirin treatment.
We were also concerned about the risk of poor gastric scarring. Indeed, non-pulsatile perfusions due to LVAD causing hypo perfusion to the digestive system.
Three months after LSG the patient lost 22 kg (Figure 1) reaching BMI of 35.3 kg/m2 (Figure 2). Despite an excessive weight loss, no cable injury, fracture or move was observed as a collateral effect.
The patient is now listed to heart transplant. Still further, Many teams [10] have shown such an improvement in EF that they have been able to explant the LVAD, therefore, “the bridge to candidacy” was rather a “ bridge to recovery ” . Indeed, LVAD increase the myocardial recovery, the pathophysiological implication of The obesity in the HF mechanism suggests that in treating obesity, myocardial recovery under LVAD would be optimized, even if the ex-plantation is still relatively rare, the positive impact of the treatment of obesity in the Treatment of heart failure. Therefore, ”the bridge to candidacy” can be imagine like a “bridge to recovery” Unfortunately the short follow up does not allow us to conclude as to this benefit on our patient.
Option is to first implant a Left Ventricular Assist Device (LVAD) to provide hemodynamic stability during BS.
Conclusion
LVAD followed by BS represents an effective safe therapeutic strategy to make ESHF obese patients eligible for HTx. Indeed new generation LVADs make the management of anticoagulation treatment safer than ever even in patients at high risk for thromboembolic complications. LSG can be safely considered as an option for efficient weight loss in heart failure patients with new generation LVAD.
References
- Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, et al. (2017) The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth adult heart transplantation report-2017; Focus theme: Allograft ischemic time. J Heart Lung Transplant 36: 1037-1046.
- Akay MH, Nathan SS, Radovancevic R, Poglajen G, Jezovnik MK, et al. (2018) Obesity is associated with driveline infection of left ventricular assist devices. ASAIO J.
- Raymond AL, Kfoury AG, Bishop CJ, Davis ES, Goebel KM, et al. (2010) Obesity and left ventricular assist device driveline exit site infection. ASAIO J 56: 57-60.
- Jung MH, Gustafsson F (2015) Exercise in heart failure patients supported with a left ventricular assist device. J Heart Lung Transplant 34: 489-496.
- Hawkins RB, Go K, Raymond SL, Ayzengart A, Friedman J (2018) Laparoscopic sleeve gastrectomy in patients with heart failure and left ventricular assist devices as a bridge to transplant. Surg Obes Relat Dis 14: 1269-1273.
- Chaudhry UI, Kanji A, Sai-Sudhakar CB, Higgins RS, Needleman BJ (2015) Laparoscopic sleeve gastrectomy in morbidly obese patients with end-stage heart failure and left ventricular assist device: Medium-term results. Surg Obes Relat Dis 11: 88-93.
- Lin MYC, Tavakol MM, Sarin A, Amirkiai SM, Rogers SJ, et al. (2013) Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis 9: 653-658.
- Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, et al. (2008) Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: A pilot study. Clin Transplant 22: 281-291.
- Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC, et al. (2017) A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 376: 440-450.
- Leviner DB, Keidar A, Ben-Gal T, Medalion B (2014) Cardiac function recovery following LVAD implantation and bariatric surgery in a morbidly obese patient. J Card Surg 29: 740-742.
Citation: Ait-Tigrine S, Tozzi P, Hullin R, Yerly P, Regamey J, et al. (2019) Morbid Obesity in End Stage Heart Failure: How Safe is Bariatric Surgery in Ventricular Assist Device Recipients? J Obes Weight Loss Ther 9: 388. DOI: 10.4172/2165-7904.1000388
Copyright: © 2019 Ait-tigrine S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3227
- [From(publication date): 0-2019 - Dec 04, 2025]
- Breakdown by view type
- HTML page views: 2351
- PDF downloads: 876