Research Article Open Access
Necrotizing Enterocolitis in Rat Offspring Exposed to Placental Insufficiency: Role of Aldosterone, Oxidative Stress and Leptin
| Norma B Ojeda* | |
| Department of Pediatrics-Neonatology Division, University of Mississippi Medical Center, Jackson-Mississippi, USA | |
| Corresponding Author : | Norma B Ojeda Department of Pediatrics-Neonatology Division University of Mississippi Medical Center Jackson-Mississippi, USA Tel: +1 601-984-1855 E-mail: nojeda@umc.edu |
| Received: Septemebr 16, 2015; Accepted: October 17, 2015; Published: October 24, 2015 | |
| Citation:Ojeda NB (2015) Necrotizing Enterocolitis in Rat Offspring Exposed to Placental Insufficiency: Role of Aldosterone, Oxidative Stress and Leptin. J Preg Child Health 2:196. doi:10.4172/2376-127X.1000196 | |
| Copyright: © 2015 Ojeda NB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Necrotizing enterocolitis (NEC) is a leading cause of morbidity and mortality in the neonatal intensive care unit among premature and low birth weight infants. Formula feeding, cold exposure, and altered intestinal bacterial colonization are frequently associated with this condition. However, there are few studies linking factors associated with placental function and necrotizing enterocolitis.
Aim: To investigated the role of aldosterone, oxidative stress and leptin in the development of NEC. The hypothesis proposes that Aldosterone, Oxidative Stress and Leptin are associated with development of NEC in rat offspring exposed to placental insufficiency.
Method: Premature low birth weight rat’s offspring were exposed to induced-NEC immediately after birth during 24hs. Leptin, aldosterone, and oxidative stress markers (superoxide dismutase activity, total antioxidant capacity and lipid peroxidation) were assessed at birth. Intestinal injury was evaluated after 24 hr of induced-NEC. Male and female offspring were divided in 4 groups with “n”=8, per group.
Results: Plasma levels of Aldosterone and Oxidative Stress markers were significantly greater, and leptin levels were significantly lower in premature low birth weight offspring compared to normal offspring (P<0.05). Intestinal injury was greater, and survival rate was lower in premature low birth weight offspring compared to normal offspring (P<0.05).
Conclusion: These results suggest that plasma levels of aldosterone, oxidative stress and leptin at birth are associated with the susceptibility to develop necrotizing enterocolitis in premature low birth weight offspring in a rat model of placental insufficiency.
| Keywords |
| Placental insufficiency; Necrotizing enterocolitis; Aldosterone; Oxidative stress; Leptin |
| Introduction |
| NEC is an acute inflammatory disease of the intestine of neonates and can result in intestinal necrosis, systemic sepsis and multi-system organ failure [1,2]. The incidence of NEC is inversely related to birth weight and gestational age [3,4], and when intestinal necrosis is already stablished the process is rarely reversible and mortality rates can reach up to 50-80% in severe cases [1,2]. Despite, the effort of physicians and researchers the morbidity and mortality of NEC have increased in the last decade, with more severe complications and poor response to treatment [5,6]. This is partially explained by the improvement in health care practices to maintain alive premature infants, but it does not explain individual differences in outcomes within the same institutional setting [7]. One of the factors involved in infant’s susceptibility to develop NEC is perinatal morbidity, which is strongly associated with fetal-placental unit function [8]. Currently, there are no identified factors linking placental function and the risk to develop NEC; therefore, identification of these factors could help to develop perinatal preventive strategies to decrease the morbidity and mortality associated with NEC. |
| Experimental and epidemiological studies suggest a multifactorial etiology for NEC including predisposing factors such as enteral feeding, hypoxia and or hypothermia, but with unclear pathogenesis [9,10]. We induced NEC in premature low birth weight (LBW) rat’s offspring from a rat model of placental insufficiency [11]. Premature birth is the major determinant of NEC [4,12]. Increased oxidative stress is among the factors associated with NEC in premature infants [13,14]. Oxidative stress has been observed in several maternal conditions associated with placental insufficiency [15]. Experimental studies report a direct correlation between increased oxidative stress and Aldosterone plasma levels in newborns [16]. Moreover, epidemiological studies reported increases in plasma aldosterone levels associated with LBW and preterm delivery [17-19]. Aldosterone can regulate oxidative stress [20], hence it can be suggested that increased levels of aldosterone and oxidative stress may be associated with NEC in premature and low birth weight infants exposed to placental insufficiency. Aldosterone is involved in the developmental changes of Na+ electrogenic transport in immature intestines [21], resulting in alteration in the homeostasis of gastrointestinal mucus barrier [21,22], and abnormal microbial colonization of the gastro intestinal tract with exacerbated inflammatory response and necrosis [23]. The digestive and absorptive capacity of the gastrointestinal tract is also compromised in premature infants [23,24]. Experimental studies report that postnatal leptin treatment enhances digestive function in intrauterine growth restricted offspring [25,26], by increasing cell mitosis and promoting growth of intestinal mucosa [27]. Leptin levels were reduced in animal models of placental insufficiency induced by reduced uterine perfusion. [28,29]. Therefore, leptin levels at birth could be associated with growth capacity and maturation of gastrointestinal tract in newborns. |
| The identification of factors associated with the risk to develop NEC in infants exposed to placental insufficiency will help to develop preventive strategies and modify current paradigms in postnatal care to prevent development of NEC. |
| Materials and Methods |
| Animals |
| All experimental procedures were conducted in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals with approval by the Animal Care and Use Committee at the University of Mississippi Medical Center. Timed pregnant Sprague Dawley rats were purchased from Charles River Laboratories International, Inc. (Raleigh, NC) and housed in a temperature-controlled room (23°C) with a 12:12-hour light/dark cycle with food and water available ad libitum. At day 14 of gestation, rats underwent reduced uterine perfusion surgical procedure as described below or sham procedure. Dams were allowed to either deliver at term for a normal gestational period for the Sprague Dawley rat (day 21-23 of gestation) [30], or Dams underwent cesarean section to surgically deliver the offspring at day 20 of gestation. C-section at day 20 of gestation ensures that offspring will not have access to dams’ milk or been nurtured by dams. Offspring from dams that underwent C-section were placed in an incubator for small animals with controlled temperature and humidity under the care of investigators and exposed to Formula feeding, Asphyxia and Cold (FAC); offspring from dam’s left for normal delivery remained under dams nurture (DN). |
| Offspring’s weight was recorded at birth and at 12 hours postdelivery. Litters were culled to 8 pups per group to ensure equal nutrient access for all offspring. Blood samples were collected using heparinized capillaries (2 X 100 ul capillary) from the umbilical cord of each offspring at the time of delivery. Dams exposed to reduced uterine perfusion delivered low birth weight (LBW) offspring and dams exposed to sham surgery delivered normal birth weight offspring (NBW) as reported earlier [11,31]. |
| Male and female offspring from 8 control dams and 8 reduced uterine perfusion dams were randomly assigned into four groups (n=8 per group): Normal Birth Weight-Dams Nurtured (NBW-DN); Normal Birth Weight-Formula Asphyxia and Cold (NBW-FAC); |
| Low Birth Weight-Dams Nurtured (LBW-DM); Low Birth Weight- Formula Asphyxia and Cold (LBW-FAC). All animals undergoing surgical procedures were anesthetized using 2-5% Isoflurane gas by inhalation. The experiment was stopped at 24 hours after birth due to the high mortality rate in LBW offspring exposed to FAC (Table 1). |
| Reduction in uterine perfusion |
| Dams were exposed to reduced uterine perfusion by placing silver clips around abdominal aorta and both branches of ovarian arteries at 14 days of gestation as previously described [32]. This procedure leads to fetal intrauterine growth restriction and results in low birth weight offspring as previously reported [11]. Low birth weight was defined as the birth weight equal or below the tenth percentile of birth weights of offspring delivered from control litters from dams exposed to sham surgical procedure. |
| Formula feeding, asphyxia and cold (FAC) |
| The protocol previously described by Caplan et al. [33] with modification was utilized to induce NEC. Briefly, the offspring selected for FAC were delivered via C-section at 20 days of gestation. After delivery normal birth weight and low birth weight rat offspring selected for FAC were placed in an incubator with controlled temperature and humidity and without contact with the dam. Formula feeding was initiated between 5 to 10 minutes after delivery using commercially available puppy milk (ESBILAC Pet-Ag) prepared according to manufacturers at a dose of 100 every 3 hours by gavage. Asphyxia exposure was accomplished by placing the offspring in a special chamber with air composition controlled to deliver 100% CO2 for 1 minute every 12 hours initiated at 2 hours post-delivery. Cold exposure was done by placing offspring in a refrigerated chamber at 4°Celsius for 5 minutes every 12 hours initiated at 1 hour post-delivery. |
| Circulating aldosterone and leptin levels |
| Plasma levels of aldosterone, and leptin were measured using commercially available kits (Siemens and R&D Systems,) from umbilical cord blood obtained at delivery. To increase the volume of our samples we pooled samples from 2 offspring of the same sex from the same litter per each animal group. |
| Oxidative stress markers |
| We utilized commercially available ELISA kits (Cayman Chemicals) following manufacturer assays techniques to measure Extracellular Superoxide Dismutase (E-SOD), Total Antioxidant Capacity (TAC) and Lipid Peroxidation (T-BARS) in plasma from umbilical cord blood. To increase the volume of our samples we pooled samples from 2 offspring of same sex from the same litter per each animal group. |
| Tissue morphology |
| Intestines were harvested for histological assessments after euthanasia at the end of experiment at 24 hours post-delivery and after exposure to FAC in the group of induced NEC. A section of 2 cm of the distal ileum was selected for evaluation in each offspring. Each intestinal section was placed in 10% phosphate buffered formalin, embedded in paraffin, sectioned (4 um thickness) then stained with hematoxylin and eosin (Suripath Medical Industries, Leica, IL). Evaluation of the slides was performed in a blinded manner to samples identity using a modified semiquantitive evaluation previously published by Dvorak et al. [34]. For scoring, all slides were examined under light microscope at 400X magnification. The magnitude of necrosis, and structural damages were scored as follows: 0 (normal), no damage; 1 (mild), slight submucosa and/or lamina propria separation; 2 (moderate), moderate separation of submucosa and/or lamina propria, and/or edema in submucosa and muscular layers; 3 (severe), severe separation of submucosa and/ or lamina propria, and/or severe edema in submucosa and muscular layers region, villous sloughing; 4 (necrosis), loss of villi and necrosis. Scores were reported as the average of 10 different fields per slide from each sample. |
| Most representative microphotographs were taken at 200X magnification to show details of the structure of mucosae villous. |
| Statistical analysis |
| GraphPad 5 and SPSS 22 statistical software were utilized to perform data analysis of the results. ANOVA with multivariate analysis was used to calculate differences between groups. Survival rate was calculated using Prism survival curves by performing both the logrank (Mantel-Cox) test and the Gehan-Breslow-Wilcoxon test. P values were calculated using both test, and Log-rank (Mantel-Cox) Chi square test were reported. |
| Results |
| Birth weight and weight gain |
| As previously reported offspring from dams exposed to reduced uterine perfusion were born with low birth weight (LBW) (Figure 1A). Weight gain within 12 hours postnatal was significantly lower (P<0.05) in LBW offspring exposed to experimental FAC compared to other groups; however, no difference in weight gain was observed between LBW and NBW in the groups nurtured by dams. (Figure1B). |
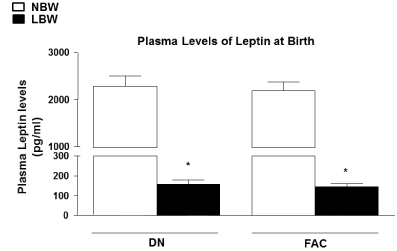
| Plasma levels of leptin at birth |
| Premature LBW offspring showed significantly lower plasma levels of leptin at birth compared to NBW offspring in both DN and FAC groups (P<0.05) (Figure 2). |
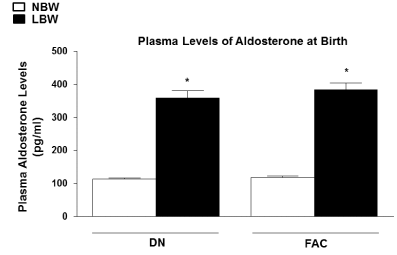
| Plasma levels of aldosterone at birth |
| Premature LBW offspring showed significantly higher plasma levels of aldosterone at birth compared to NBW offspring in both DN and FAC groups (P<0.05) (Figure 3). |
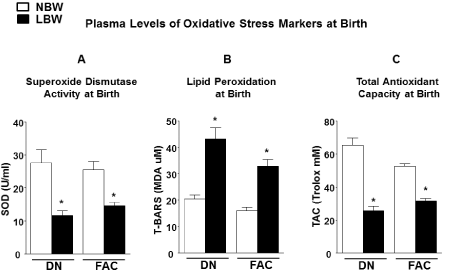
| Plasma levels of oxidative stress markers |
| Premature LBW offspring showed significant (P<0.05) lower levels of extracellular superoxide dismutase activity (Figure 4A), higher levels of lipid peroxidation (Figure 4B), and lower total antioxidant capacity (Figure 4C) at birth compared to NBW offspring. |
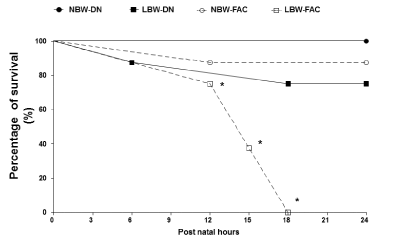
| Survival rate after FAC |
| LBW offspring had a significantly lower survival rate after exposure to FAC in comparison to NBW offspring (X2=20.53, df=3, P=0.0001). The survival percentage for LBW exposed to FAC was 75% at 12 hours, 38% at 15 hours and 0% at 18 hours (Figure 5). |
| Tissue morphology |
| The intestinal sections from offspring nurtured by dams showed normal structural parameters in both NBW and LBW offspring. Offspring exposed to FAC showed intestinal structural damage, which was significantly greater in LBW offspring compared to NBW offspring. LBW offspring exposed to FAC exhibited tissue damage score with an average of 3.6 ± 0.2 (P<0.05) vs. all other groups (Figure 6A). Most representative microphotographs show intestine section of LBW offspring exposed to FAC with intestinal villi disintegration, loss of cell nuclei, edema in the muscular wall and lamina propria and necrosis with perforation (Figure 6B). |
| Discussion |
| The main findings in this study are that offspring exposed to placental insufficiency have 1) Low birth weight 2) Increased plasma levels of aldosterone at birth, 3) Increased levels of oxidative stress markers at birth, 4) Decreased plasma levels of leptin at birth, 5) Decreased survival rate after exposure to formula feeding, asphyxia and cold temperature. This is the first time that an experimental model of NEC induced by formula feeding, asphyxia and cold exposure is utilized in a rat model of premature LBW induced by placental insufficiency. This model is mimicking the human condition of NEC in premature and LBW infants. |
| NEC is one of the leading causes of morbidity and mortality in the neonatal intensive care unit (NICU) [35]; it is almost an exclusive disease of pre-term and LBW infants, with unclear mechanistic pathways involved in its etiology. Moreover, preventive strategies are inadequate and the prognosis of NEC in the NICU is often associated with long-term consequence [36]. Therefore, diagnostic tools to identify infants “at risk” to develop NEC may help prevent this condition and improve survival outcomes. Other investigators have examined other factors implicated in the etiology of NEC utilizing animal models [2]. However, few reports have examined potential biomarkers for early identification of infants at risk to develop NEC [37,38]. We designed our study to investigate potential early biomarkers that may predict an increased risk for NEC in premature LBW offspring. |
| In this study, LBW offspring were more susceptible to develop NEC in comparison to NBW offspring from dams exposed to sham operation. The experimental model to induce NEC utilized in this study is a modification of the protocol developed by Caplan et al. [39]. The protocol used by Caplan et al. induces NEC in newborn rat offspring via exposure to formula feeding, asphyxia and cold for a period of 48 to 96 hours until NEC is developed. However, in the current study the survival rate was lower in LBW offspring compared to NBW offspring. Premature LBW offspring showed clear evidences of development of NEC with abdominal distention and lethargy as early as 12 hours after initiation of formula feeding, asphyxia and cold exposure. The group of LBW exposed to FAC resulted in no survivors after 18 hours of exposure. This finding suggests that premature low birth weight offspring are more susceptible to the development of NEC with a lower survival rate in comparison with NBW offspring. |
| Oxidative stress is strongly involved in the etiology of morbidity in premature and LBW infants. A reduction in antioxidant capacity leading to the formation of free radicals may contribute to increased morbidity in preterm and LBW infants including NEC [14]. Increased oxidative stress induce a state of imbalance in the normal cell redox homeostasis resulting in shifting cell metabolism from anabolic to catabolic and increased apoptosis [20]. The fast cell turnaround observed in the intestinal mucosa is affected by oxidative stress resulting in disruptions of intercellular tight junctions and more susceptibility to shear stress [14]. There are strong evidences supporting that oxidative stress biomarkers in cord blood could help in the early identification of infants at risk to develop NEC [14]. |
| There are several epidemiological studies reporting an increases in plasma aldosterone levels associated with LBW and preterm delivery [17-19]. Experimental studies report that aldosterone can mediate the regulation of oxidative stress [16] by decreasing the activity of glucose-6-phosphate dehydrogenase [20] the rate-limiting enzyme in the pentose phosphate pathway for production of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) [20]. NADPH limits the production of reactive oxygen species (ROS) and reduces oxidative stress [20]. |
| Additionally, aldosterone is the most important factor responsible for electrogenic Na+ absorption in the immature gut, and this action has a genomic effect [40]. Therefore, an elevation in aldosterone levels could result in deregulation of Na+ absorption and alterations in water and electrolyte homeostasis in the intestinal epithelium. A fully developed intestinal barrier provides selective permeability and controlled bidirectional fluid flow to flush away pathogens and toxins from the intestinal lumen [41]. Plasma aldosterone levels are elevated in infants delivered prematurely and in infants with perinatal asphyxia [17,19], and these two conditions are also associated with NEC [42 ]. Therefore, we investigated the association between aldosterone levels and NEC in our experimental model. |
| We found that LBW offspring have increased levels of aldosterone in plasma samples obtained from the umbilical cord at the time of delivery. The significant elevation of plasma aldosterone observed in LBW offspring could be a contributing factor for the development of NEC Further studies will be necessary to investigate this paradigm and to test a causality effect. |
| Immaturity of the gastrointestinal tract is another factor involved in the etiology of NEC. The injury in NEC begins with a breach in the intestinal mucosal barrier leading to microbial colonization and exacerbated inflammatory response [23]. Intestinal digestive and absorptive capacity are also impaired in premature infants creating a favorable environment for bacterial colonization [23,24]. Leptin is a peptide with neuroendocrine actions and regulatory effects on several systems related to growth and maturation [43,44]. Leptin is known for promoting growth and maturation during fetal and early postnatal life [25,26], and it is important in proper infant development [45,46]. Experimental studies report that postnatal leptin treatment enhances digestive function in intrauterine growth restricted offspring [25,26]. Leptin is also associated with the cell mitosis to apoptosis ratio and enhanced growth of the mucosa in newborns [27]. Epidemiological studies report that prematurity and LBW are associated with low leptin levels at birth [47,48]. Consequently, we examined the correlation of leptin levels at birth and NEC in our model of low birth weight offspring. |
| In this study plasma leptin levels were lower in umbilical cord samples obtained from LBW rat offspring at the time of delivery. Leptin actions and regulatory effects are extended to several systems related to fetal growth [43,44]; and it seems to have a protective effect against cell apoptosis and autophagy in the neonatal gut epithelium [27]. Moreover, other investigators reported that leptin treatment promotes small intestine growth and significantly increases the total surface area of Peyer’s patches in growth restricted piglets [26]. Low leptin levels found in LBW offspring in our study could be associated with immaturity of gut epithelium, impairment of digestive and absorbance functions, and increased apoptosis [1,36] often associated with NEC. |
| In conclusion the findings from this study demonstrate that the rat model of LBW induced by placental insufficiency is a suitable model to investigate NEC and the factors associated in the link between placental insufficiency and NEC. We found parallelisms between our data and prior humans reports that low birth weight fetuses have higher aldosterone and lower leptin levels at birth. In addition, findings from this study indicate that LBW induces a significant susceptibility to a secondary insult that contributes to the development of NEC. We speculate that alterations in aldosterone and leptin levels at birth may be critical factors in predisposing premature and low birth weight infants to an increased risk to develop NEC. It is important to point out that the measurement of aldosterone, oxidative stress and leptin were performed at birth in offspring not exposed yet to formula feeding, asphyxia and cold temperature. The limitation of this study is related to the investigation of causality effects between the biomarkers measured at birth and the development of NEC. Further studies are warranted to examine causality effects and potential mechanistic pathways linking these biomarkers at birth with NEC. |
References
- Lin PW, Stoll BJ (2006) Necrotisingenterocolitis. Lancet 368: 1271-1283.
- Sodhi C, Richardson W, Gribar S, Hackam DJ (2008) The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech 1: 94-98.
- Llanos AR, Moss ME, Pinzòn MC, Dye T, Sinkin RA, et al. (2002) Epidemiology of neonatal necrotisingenterocolitis: a population-based study. PaediatrPerinatEpidemiol 16: 342-349.
- Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, et al. (2003) Necrotizing enterocolitis among neonates in the United States. J Perinatol 23: 278-285.
- Gibbs K, Lin J, Holzman IR (2007) Necrotisingenterocolitis: the state of the science. Indian J Pediatr 74: 67-72.
- Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, et al. (2007) New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res 62: 510-514.
- Ahle M,Drott P, Andersson RE (2013) Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987-2009. Pediatrics 132: e443-451.
- Moore SW, Arnold M, Wright C (2013) Necrotizing enterocolitis and the placenta - a key etiological link. J PediatrSurg 48: 359-362.
- Hunter CJ,Upperman JS, Ford HR, Camerini V (2008) Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 63: 117-123.
- Kanto WP Jr, Hunter JE, Stoll BJ (1994) Recognition and medical management of necrotizing enterocolitis. ClinPerinatol 21: 335-346.
- Ojeda NB (2011) Low birth weight increases susceptibility to renal injury in a rat model of mild ischemia-reperfusion. Am J Physiol Renal Physiol 301: F420-426.
- Patel RM, Denning PW (2015) Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res 78: 232-238.
- Perrone S,Tataranno ML, Negro S, Longini M, Marzocchi B, et al. (2010) Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum Dev 86: 241-244.
- Perrone S,Tataranno ML, Negro S, Cornacchione S, Longini M, et al. (2012) May oxidative stress biomarkers in cord blood predict the occurrence of necrotizing enterocolitis in preterm infants? J Matern Fetal Neonatal Med 25 Suppl 1: 128-131.
- Pereira RD, De Long NE, Wang RC,Yazdi FT, Holloway AC, et al. (2015) Angiogenesis in the placenta: the role of reactive oxygen species signaling. Biomed Res Int 2015: 814543.
- Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T (2007) Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49: 355-364.
- Bourchier D (2005) Plasma aldosterone levels in the 1st week of life in infants of less than 30 weeks gestation. Eur J Pediatr 164: 141-145.
- Doerr HG,Versmold HT, Bidlingmaier F, Sippell WG (1989) Adrenocortical steroids in small-for-gestational-age term infants during the early neonatal period. Pediatr Res 25: 115-118.
- Pereira DN,Procianoy RS (1997) Transient elevation of aldosterone levels in perinatal asphyxia. ActaPaediatr 86: 851-853.
- Leopold JA, Dam BA, Maron, AW Scribner, R Liao, et al. (2007) Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 13: 189-97.
- Pácha J1 (2000) Development of intestinal transport function in mammals. Physiol Rev 80: 1633-1667.
- Treszl A,Tulassay T, Vasarhelyi B (2006) Genetic basis for necrotizing enterocolitis--risk factors and their relations to genetic polymorphisms. Front Biosci 11: 570-580.
- Claud EC (2009) Neonatal Necrotizing Enterocolitis -Inflammation and Intestinal Immaturity. AntiinflammAntiallergy Agents Med Chem 8: 248-259.
- Claud EC, Walker WA (2001) Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. Faseb J. 15: 1398-403.
- Attig L,Larcher T, Gertler A, Abdennebi-Najar L, Djiane J (2011) Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis 7: 88-94.
- Attig L,Brisard D, Larcher T, Mickiewicz M, Guilloteau P, et al. (2013) Postnatal leptin promotes organ maturation and development in IUGR piglets. PLoS One 8: e64616.
- Godlewski MM,Słupecka M, Woliński J, Skrzypek T, Skrzypek H, et al. (2005) Into the unknown--the death pathways in the neonatal gut epithelium. J PhysiolPharmacol 56 Suppl 3: 7-24.
- Anderson CM, Lopez F, Zhang HY, Pavlish K, Benoit JN (2005) Characterization of changes in leptin and leptin receptors in a rat model of preeclampsia. Am J ObstetGynecol 193: 267-272.
- Anderson CM, Lopez F, Sandeen A, Johnson L (2009) Placental insufficiency: programming of leptin secretion, blood pressure, and postnatal growth in two generations of Sprague-Dawley rats. Biol Res Nurs 10: 284-291.
- Pritchettt-Corning KR GA, Avellanded G, Fritz PE, Chou S, Brown MJ (2010) Handbook of Clinical Signs in Rodents and Rabbits. Charles River Laboratories 1: 1-136.
- Alexander BT1 (2003) Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457-462.
- Ojeda NB, Johnson WR, Dwyer TM, Alexander BT (2007) Early renal denervation prevents development of hypertension in growth-restricted offspring. ClinExpPharmacolPhysiol 34: 1212-1216.
- Caplan MS, Russell T, Xiao Y, Amer M, Kaup S, et al. (2001) Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr Res 49: 647-652.
- Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, et al. (2002) Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J PhysiolGastrointest Liver Physiol 282: G156-164.
- Hsueh W,Caplan MS, Qu XW, Tan XD, De Plaen IG, et al. (2003) Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. PediatrDevPathol 6: 6-23.
- Lin PW, Nasr TR, Stoll BJ (2008) Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. SeminPerinatol 32: 70-82.
- Young C, Sharma R, Handfield M, Mai V, Neu J (2009) Biomarkers for infants at risk for necrotizing enterocolitis: clues to prevention? Pediatr Res 65: 91R-97R.
- Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, et al. (2010) Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr 157: 757-761.
- Caplan MS,Jilling T (2001) The role of polyunsaturated fatty acid supplementation in intestinal inflammation and neonatal necrotizing enterocolitis. Lipids 36: 1053-1057.
- Pácha J,Pohlová I, Karen P (1995) Regulation of amiloride-sensitive Na+ transport in immature rat distal colon by aldosterone. Pediatr Res 38: 356-360.
- Hecht G1 (1999) Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol 277: C351-358.
- Blakely ML,Lally KP, McDonald S, Brown RL, Barnhart DC, et al. (2005) Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg 241: 984-989.
- Soliman AT, MM ElZalabany, M Salama, BM Ansari (2000) Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism 49: 819-825.
- Ahima RS,Saper CB, Flier JS, Elmquist JK (2000) Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21: 263-307.
- Mehta R,Petrova A (2011) Biologically active breast milk proteins in association with very preterm delivery and stage of lactation. J Perinatol 31: 58-62.
- Martin LJ, Woo JG, Geraghty SR, Altaye M, Davidson BS, et al. (2006) Adiponectin is present in human milk and is associated with maternal factors. Am J ClinNutr 83: 1106-1111.
- Mellati AA,Mazloomzadeh S, Anjomshoaa A, Alipour M, Karimi F, et al. (2010) Multiple correlations between cord blood leptin concentration and indices of neonatal growth. Arch Med Res 41: 26-32.
- Ho SP, Wang LJ, Cheng I, Chen YL, Sung TC, et al. (2010) Association of plasma leptin levels with maternal body weight and body mass index in premature and term newborns. PediatrNeonatol 51: 19-25.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
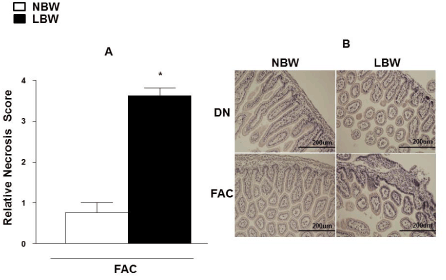 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 10304
- [From(publication date):
December-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 9380
- PDF downloads : 924
