Neurodevelopment, Intestinal Function, and Autism
Received: 20-Aug-2018 / Accepted Date: 14-Sep-2018 / Published Date: 22-Sep-2018 DOI: 10.4172/2572-4983.1000166
Keywords: Autism spectrum disorders; The enteric system; Enteric microbial assembly; Hippocampus memory; Aberrant development trajectory
Sensory Development before Functional and Cognitive Development
Studies of brain development noted sensory and motor functions maturing earlier as compared to higher-order integrative functions maturing later [1-4]. This can be correlated with respective cortical gray matter maturation in young children compared to older children and adolescents [5].
Rapid cortical gray matter growth in overall volume with regional differences is seen in the first 2 years of life. The sensory and sensorimotor regions are the first to grow and mature starting before birth. These enable motor, somatosensory, and auditory systems to function prior to birth, and vision for the newborn [6-8].
Brain regions develop around information processing sensory modalities. Regions expanding faster in the first year include the inferior frontal gyrus and angular gyrus, cortical regions involved with language [9], the fusiform gyrus, involved with face recognition and color processing [10,11] and the inferior temporal gyrus, involved with higher-order visual processing, including shape and faces [12,13]. The insula is also one of the most rapidly growing regions.
These are followed by frontal and parietal cortex expansion in the second year of life, probably associated with development of functional networks, since the default network which involves parietal and prefrontal cortex does not fully develop until 2 years of age [14]. Functional network hubs are noted earlier in sensorimotor regions [15,16] and in motor, sensory, auditory, and visual primary cortex in infants [17].
Maturational through Stimulation
The maturational trajectories are subjected to a variety of genetic and environmental factors. While heredity has a part to play, activity and experience would shape formation and elimination of synapses in the developing brain [18]. This probably accounts for the heterogeneity of developmental trajectories of subcortical structures in older children [19,20]. Nevertheless, the relative contribution of genetic and environmental factors to human gray matter development in this period of rapid growth and development is not clear.
Memory plays a significant part. There is a rapid increase in hippocampal volume in the first two years of life [5] while it continues to grow to 14 years of age [21,22]. It is less in the first year compared with the other subcortical structures, but becomes one of the faster growing structures in the second year of life. It supports, with more mobility of the child, the acquisition of episodic memory [23] as well as spatial working memory and path integration abilities [24,25].
Gut Influence on Brain Development
Visceral sensations develop as the enteric nervous system (ENS), some called the ‘little brain’ [26], develops during interactions of the neural crest-derived precursors (mostly vagal neural crest cells [27]) with the enteric microenvironment. Luminal stimuli activate mucosal enteroendocrine cells, which secrete signaling molecules, such as serotonin, that stimulate intrinsic primary afferent neurons (IPANs) to initiate peristaltic and secretory reflexes [28]. The insula, the most rapidly expanding regions in the first year, is involved with a variety of functions: awareness of interoceptive or visceral sensations, pain, body movement, emotions, vocalizations, and perhaps even consciousness [29-31].
Assembly of the developing nervous system for a final functional neural circuitry is dependent of a series of temporally regulated developmental processes. Development is not just following senses. Studies recently have provided much evidence that, besides the mainframe brain, microorganisms in the gut play a role in neurodevelopment [32]. Germ free (GF) mice displayed an exaggerated stress response, which could be restored by oral replacement of a single strain of bacterium, Bifidobacterium infantis [33].
Replacement for GF mice, using the microbiome from specific pathogen free mice raised with a normal gut flora, restored the stress response as well as reduced anxiety-like behavior and increased locomotor activity of GF mice to basal levels when given at an early developmental stage but not at a later age [33]. Serotonin levels in the hippocampus of GF mice were raised and correlated with reduced anxiety, but re-colonization of the mice after weaning did not restore the neurochemical differences (though it could revert the altered anxiety-like behavior) [34]. Time windows of microbial community might be critical in shaping the brain function and have long-lasting effects on behaviors. It needs be further revealed about the action of the gut microbiome on specific neuronal populations which could affect neurodevelopment of brain circuits. But perturbations in the delicate synergetic host-microbiota relationship may have serious consequences and lead to brain, digestive, and metabolic disorders [35-37].
Normal gut microbiota plays a critical role in shaping brain functions [38-41]. Microbe colonization after birth and gut microbiota assembly in the first 3 years of life, can shape development of the gut [42,43], endocrine system [36], and brain [38,44,45]. Gut microbiota and cognition in human infants are associated, as fecal microbial community diversity in infants affects later Mullen score (scale of early learning), visual reception scale, and expressive language scale at two years of age [46].
Developmental Outcome
Neurodevelopmental events including neurogenesis, axonal and dendritic growth, synaptogenesis, and refinement of these synaptic connections need be orchestrated to shape the functional neural circuitry matching needs that are critical for normal cognitive, motor, and emotional development. While plasticity allows a wide spectrum of outcome derivatives, systems matching [47,48] requires that the generated numbers of neurons and the appropriate synaptic density be matched exactly to the requirements of the pertinent neural circuit.
In the mainframe brain, development begins with an intricate process of neural cell generation, differentiation and migration, which is regulated intrinsically by expression of transcription factors as well as extrinsically by extracellular signals or morphogens [49,50]. This is followed by neuronal axonal growth as being guided by various attractive and repulsive cues and regulated by local mRNA translation [51,52]. Synapses are formed when the target synaptic partners are linked by the axons and then finally pruned to organize the final neural circuits [49,53]. The functions of these connections are further refined by synaptic plasticity.
Developmental outcome is certainly multifactorial. The brain is a plastic organ where both the intrinsic CNS milieu and extrinsic cues play important roles in shaping and wiring neural connections. Early life experiences can have profound and persistent effects on behaviors and traits with consequences for later life behavior and disease risk [54-56]. From psychosocial adjustment and self‐regulation and from wiring of neural networks, the balance of plasticity and stability, being critical for information processing and storage, precedes the associative learning.
Early life stress during this critical period can induces alterations in many body systems. As simple as maternal separation can produce alterations of the intestinal barrier function, altered balance in enteric microflora, exaggerated stress response and visceral hypersensitivity [57]. There is bidirectional communication pathway between gut bacteria and the central nervous system [45]. The microbiota–gut– brain axis exerts a profound influence on key brain processes, such as neuroinflammation, activation of the stress axes, neurotransmission, and neurogenesis, in addition to modulating complex behaviors, such as sociability and anxiety [35-37,58-61].
The gut microbial community is dynamic during the first 3 years of life before stabilizing to an adult-like state [62]. Microbiome composition and specificity differ across host species and lifestyles [63]. The complex and diverse alterations in gene expression in multiple neurotransmission pathways, including glutamatergic, GABAergic, serotonin and dopamine pathways as well as neurotransmitter transporters and ion channels can have diverse impacts on the brain [64]. Gut bacteria influence these central processes through their ability to synthesize neurotransmitters including gamma-aminobutyric acid (GABA), noradrenaline, and dopamine, modulate activation of the immune system, and produce metabolites, such as short-chain fatty acids (SCFAs), that possess neuroactive properties [65]. Moreover, additional pathways link the gut microbiota and the brain, through the vagus nerve and through the modulation of key dietary amino acids such as tryptophan [66-69].
Gastrointestinal Improvement Precede Recovery in Treated Autistic children
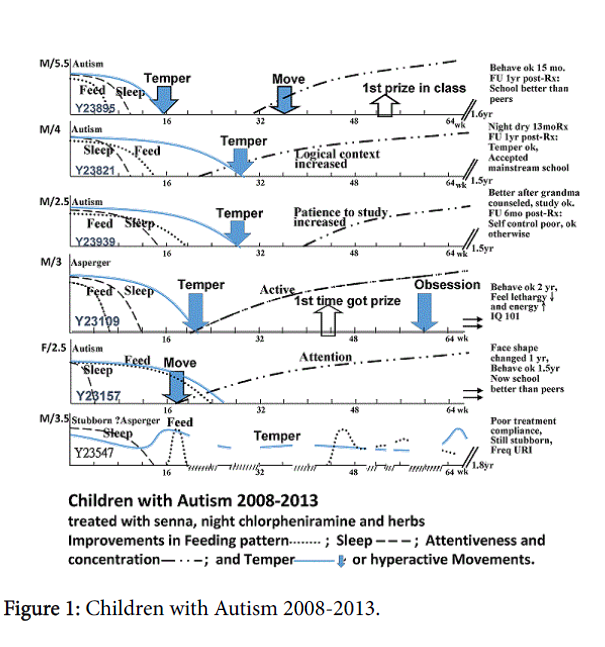
A series of autistic children (age 2.5-6.5 yrs) demonstrated the significance of gastrointestinal (GI) function. From 2008-13, 8 autistic children diagnosed by psychometric assessment by psychologists came to InteMed Specialist Centre. 6 were treated with a host of medicine and herbs for 1.5-2 years while 2 did not receive treatment. They were evaluated once every two weeks for GI health in terms of bowel motion, appetite, smelly stools and GI discomfort, sleep quality in terms of entry and restlessness, temper, concentration, and school acceptance and results. All other educational and social support were carried on unchanged as given outside the clinic. 5 patients improved greatly in final outcome, temper got significantly improved (5/6), and scoring high in schools (2/6). During recovery, appetite, speed of finishing feeds, sleep restlessness, concentration, temper, and school acceptance improved mostly in that sequence (Figure 1). 5 had treatment stopped within 1.5-2 years, while one with Asperger syndrome continued with stubbornness recovered after 2.5 years though negative and grudging thoughts changed positive only after 3 years. One child mainly stubborn was not compliant, feeding improved but worsened with frequent running nose and cough, and overall recovery variable with pattern not distinct. Follow-up in 2018 could not reach this child, being in foreign lands. For the remainder 5 patients, follow-up showed, for school: results good or upper grade in 3 and middle in 2, none need for special classes; for emotion: generally acceptable with peers in 5/5 and temper good in 1, easy to cry in 2, wrangling over issues in 2, none stubborn, all taken off special classes, nervous for work in 2, and none having mannerism; thus on the whole exceptionally effective.
The batch of medicine included chlorpheniramine 2mg and senna 3 mg daily. This is special low dose for longer-term use, even though they may not be obviously constipated, had no dependency problem displayed in all patients even afterwards. During treatment, it was particularly impressive that GI function in terms of appetite, speed of finishing feeds and smelly stools recovered within 6-24 weeks and tended to precede the whole sequence of recovery. Though cases were too few to be confident, there is some suggestion that the earlier GI function recovered, the earlier the rest of other symptoms recovered.
Gut motility in general depends on the gut luminal environment (including the gastrointestinal microbiota and fermentation), as well as factors related to the immune system, the ENS, and the central nervous system. Transit time is a key determinant and stool consistency strongly associated with gut microbiota richness and composition [70]. The increase of transit time is positively correlated with the increase of methanogens, of breath methane concentrations and pH, while inversely correlated to the proportion of sulfate reducing bacteria and SCFA concentration [71]. SCFAs, generated by enteric bacterial fermentation, may induce neurodegenerative diseases [72]. Besides, gut microbial cell wall components continually interact with the innate immune system to induce the secretion of cytokines. Neuroinflammation-related brain injuries are associated with [73] and cytokine imbalance is involved in autism spectrum disorders and schizophrenia [74].
There can be a lot more processes about the brain-gut. The ENS is a division of the autonomic system put in close apposition to effector systems that it controls; enterohormones also co-working. Brain development depends on nutrition and immune development from the gut. Long-chain polyunsaturated fatty acids, monounsaturated fatty acids, insulin-like growth factor 1 are among a long list of constituents and regulators of brain cell proliferation, apoptosis, myelination, neurogenesis, maturation and differentiation [75].
Aberrant Development Trajectory in Autistic Children
sAutism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by the presence of stereotypical behavior, communication, and social interaction deficits. ASD etiology remains unknown and pathogenesis is sought for genetic and environmental factors [76]. Cognitive models include domain-specific models elaborating primary deficit in social cognition, and domain general models elaborating primary deficit in nonsocial or domain-general processing. The disrupted cerebral connectivity hypothesis postulates that its clinical symptoms originate from deficiencies in the way the brain coordinates and synchronizes activity amongst different brains regions [77]. Physiological and behavioral indicators can be found in high-risk groups less than a year old that predict the disease [78,79]. In a recent paper, atypicalities of structural and functional development of the brain related to prenatal and perinatal causes (hypoplasia of the pons just after neural tube closure; and a deficient GABA developmental switch in the perinatal period) have been postulated to explain the diverse phenotypes of ASD [80]. Nevertheless, these specific prenatal and perinatal causes are expected to be infrequent, yet ASD is common, affecting approximately 2.25% of children [81,82].
Considering aberrant developmental processes, the Social Motivation Hypothesis views that an early neurobiological difference in response to rewarding social input could in turn lead to diminished social motivation, further increasing motivation for restricted interests [83]. Without appropriate input signals to develop the neural organization over a long period, decreased gray matter volume in the region, and synaptic strength is modified in activity- and experiencedependent ways, causing change in connectivity. Thus an array with high variability in patterns of widespread local overconnectivity, a mixed picture of local over- and underconnectivity as well as mixed patterns of long range under- and overconnectivity have been observed in ASD [77]. Meta-analysis of 13 functional magnetic resonance imaging studies found aberrant reward circuitry activation to both social and nonsocial rewards and increased activation to stimuli associated with their restricted interest [84]. Whether due to a circuit dysfunction [85] in reward and motivation, autistic brains might fail to register social experiences as rewarding, further reducing social interactions and social abilities, ultimately leading to heterogeneous social deficits with this ”aberrant development trajectory”.
Dopamine dysfunctions have been reported in ASD, and autisticlike behavior could arise from dopamine dysfunctions in midbrain dopaminergic modulatory systems affecting social motivation and goal-directed motor behavior [86]. Dopamine affects plasticity, synaptic transmission and the network activity in the hippocampal circuitry for memory [87]. Findings suggest that while memory representations are processed and activated by the hippocampus in both ASD and controls during successful retrieval, these are not searched for, transferred, or monitored in an efficient way during episodic memory retrieval as a result of widespread disrupted connectivity. In brief, memory deficits in the ASD could be driven by retrieval-related impairments that reduce the probability of recollection success [88]. Aberrant connectivity may lead to structural demonstrable differences in many brain areas, especially for developmental process involved in response to rewarding social input, which in turn may lead to the diminished social motivation.
Molding with Gut-related Brain Development
Neurogenesis with new neurons continues in the hippocampus to play an important role in learning and memory and responses to stress, even till adulthood [89,90]. Germ-free mice with absence of a gut microbiota have impaired abilities in spatial and object, but not olfactory memory related to less c-Fos-positive CA1 hippocampal cells [91].
ASD is associated with other GI abnormalities, particularly alterations in microbiota composition and function [39,92-96]. Studies demonstrated that autism-like behavioral and GI phenotypes are associated with altered microbiota in two separate mouse models of ASDs [39,97]. Gut microbiota dysbiosis [96] and altered fecal flora have been observed [97,98], Inflammation and neuro-immune system dysregulation can be a prominent clinical features of ASD [99-101]. Pro-inflammatory cytokines were found elevated [75] and dysfunctional immune responses could affect core behaviors in ASD [102]. Tumor necrosis factor alpha (TNF-α) were positively correlated with the ASD severity [103]. ASD blood monocytes show altered immune responses [104] while blood monocyte-derived macrophages show strong impairments in the endocannabinoid system [105]. These are endogenous agonists of cannabinoid receptors, involved in the suppression of synaptic transmission and mediate signaling in the brain and the ENS. Besides monocytes are precursors of macrophages and dendritic cells.
Neuro-inflammation can affect developmental trajectories. Inflammatory cells in the brain such as microglia and astrocytes regulate synaptic structure and function. Neuroglia responses are activated toward pro-inflammatory processes involving astroglia and microglia, and demonstratable by brain immunohistochemical analysis in ASD brain [106]. Microglia cells like the monocytes are more committed to molecular pro-inflammatory changes. Astrocytes are also involved in ASD development possibly related to the G-protein coupled receptors [107]. Activation of the immune system probably dynamically affects synaptic organization and function in the developing brain and microglia-mediate elimination of synapses [108].
Possibilities for Treatment
Our series open up a new treatment perspective for ASD through GI management and concurrent repatterning brain development. Synaptic remodeling and developmental reorganization could be important in development of cognitive abilities and the necessary behavioral transition when growing up [109]. The experience of our cases including others outside the series suggests that treatment before 6 years of age would have a high chance of achieving normal school and social outcomes, while late treatment only restore emotion stability and strengthen communication and learning ability. The effectiveness of therapy probably are made by normalization of GI function and concurrent actions to remedy the ‘aberrant developmental trajectory’.
There is a critical window in early life during which microbial colonization influences adult neurogenesis, including that in the hippocampus. Microbe colonization beginning around birth, produces a dynamic assembly during the first 3 years of life [62], and then gradually changes in composition onwards [110]. Increased intestinal permeability (leaky gut) has been associated with ASD, and is found in 37% of autistic patients and in 21% of their relatives [111]. With GI dysfunction present even in relatives and being subjectable to significant lowering with gluten-casein-free diet [112], a treatable postnatal element is present and may contribute to its associated neuro-inflammation in ASD. Gliogenesis essentially developed perinatally and postnatally [113,114]. Glial cells include astrocytes modulating the chemical environment by altering ion gradients and neurotransmitter transduction, oligodendrocytes producing myelin, and microglia to remove cellular and foreign debris within the central nervous system. Mal-assembly of gut microbiota during early childhood could enhance the individual’s susceptibility to environmental insults and poor diet whence GI dysfunction is resulted with a negative impact on mental health. Although the pathogenesis of ASD remains largely unknown [115], neuro-inflammation contributes to a significant subset of ASD [116]. The use of herbs including Potentilla chinenesis Ser. with inflammatory effects in our series may have contributed. Other herbs used in the early stage of treatment including Poria cocos (Schw.)Wolf, Triticum aestivum L., Ziziphus zizyphus , Taxillus chinensis , Lilii bulbus , Fructus oryzae and Morus alba L were also used to strength the GI system and the body. At a later stage for older chidren, Citrus aurantium L., Citrus reticulata Blanco, Bambusa tuldoides Munro and Pinellia ternata (Thunb.) Breit. were used to improve temperament. The use of probiotics has been proposed as a therapeutic intervention for ASD [117,118]. For ASD children with immune dysfunction, intravenous immunoglobulin infusion was demonstrated useful in behavioral issues, eye contact, and social interactions [119] and even with standardized cognitive and behavioral tests [120]. Along the same line, manipulation of the endocannabinoid system for the gut-brain axis, which can block and reduce the development of colitis [121], has yet to be explored in autism.
With the ongoing neuoinflammatory insults alleviated, concurrent restoration of the mental abilities from the deterring ‘aberrant developmental processes’ that block and lead to a diminished social motivation, would restore the necessary early rewarding social input. The hippocampus is activated by GI signals through the vagus nerve between the GI tract and the brain [122]. The hippocampus is linked with learning and memory control and with feeding behavior [123]. Vagus nerve stimulation (albeit non-physiological electrical stimulation) enhances memory [124,125], facilitates hippocampal neurogenesis, and increases hippocampal expression of brain-derived neurotrophic factor [126] and induce neuronal plasticity [127]. Certain herbs may restore brain synaptic remodeling and reorganization.
Normalization for ASD then depends on actions to re-direct and pattern the aberrant developmental trajectory. With developmental plasticity, early life experiences can have profound and persistent effects on traits expressed throughout the life course, with consequences for later life behavior. The effectiveness of treatment in the above cases could be also be related to sleep improvement, as enabled with antihistamine and restored by sleep-promoting herbs including spine date seed (Ziziphi Spinosae Semen). ASD tends to be associated with difficulty in falling asleep, wake up in the night frequently and a low frequency of saccadic eye movement during rapid eye movement (REM) sleep [128]. Slow wave sleep is also shortened in ASD, and sleeping time, particularly the proportion of REM sleep, is reduced [129]. As cerebral plasticity has a very important relationship with sleep, our series showed improvement of sleep precedes improvement of other symptoms.
There are other postnatal remedies demonstrated as effective. Oxytocin as a nasal spray can modulate human social behavior and cognition, while tics and anxiety related issues showed no improvement [130]. Postnatally, a lot can be done as shown by our series. Postnatal synaptic plasticity during the synaptic pruning begins at birth. Late steps of neurodevelopment including axon and dendrite growth and arborization, and experience-dependent synapse modification, are primarily involved in the development of neurocircuitry, and open to management to normalize developmental trajectory.
Conclusion
ASD as a neurodevelopmental disorder may have a lot of remedial postnatal elements, including the deleterious GI function and gut microbiota assembly, as well as aberrant developmental processes that deter useful interactions and lead to the diminished social motivation. Management of the GI system could target not only the microbial elements influencing neuro-inflammation and glial cells, but also the ENS sustaining signals to the hippocampus and brain for neurogenesis. Restoring the internal environment, management of the aberrant developmental processes may be facilitated by better sleep and stayed by behavioral modification or by herbs. The concurrent treatment of postnatal process has a high potential for ASD returning to normal trajectory for useful activities and life.
References
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, et al. (2003) Mapping cortical change across the human life span. Nat Neurosci 6: 309-315.
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174-8179.
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, et al. (2004) Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 24: 8223-8231.
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, et al. (2008) Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28: 3586-3594.
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, et al. (2012) Longitudinal Development of Cortical and Subcortical Gray Matter from Birth to 2 Years. Cerebral Cortex 22: 2478-2485.
- Kagan J, Herschkowitz N (2005) A Young Mind in a Growing Brain. Psychology Press, New York, US.
- Sanes DH, Bao S (2009) Tuning up the developing auditory CNS. Curr Opin Neurobiol 19: 188-199.
- Bourne JA (2010) Unravelling the development of the visual cortex: implications for plasticity and repair. Anat J 217: 449-468.
- Shalom DB, Poeppel D (2008) Functional anatomic models of language: assembling the pieces. Neuroscientist 14: 119-127.
- Bartels A, Zeki S (2000) The architecture of the colour centre in the human visual brain: new results and a review. Neurosci Eur J 12: 172-193.
- Kanwisher N, Yovel G (2006) The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci 361: 2109-2128.
- Denys K, Vanduffel W, Fize D, Nelissen K, Peuskens H, et al. (2004) The processing of visual shape in the cerebral cortex of human and nonhuman primates: a functional magnetic resonance imaging study. J Neurosci 24: 2551-2565.
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, et al. (2011) Emotional perception: meta-analyses of face and natural scene processing. Neuroimage 54: 2524-2533.
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, et al. (2009) Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci USA 106: 6790-6795.
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, et al. (2007) Resting-state networks in the infant brain. Proc Natl Acad Sci USA 104: 15531-15536.
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, et al. (2008) Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol 29: 1883-1889.
- Fransson P, Aden U, Blennow M, Lagercrantz H (2010) The functional architecture of the infant brain as revealed by resting-state FMRI. Cereb Cortex 21: 145-154.
- Hua JY, Smith SJ (2004) Neural activity and the dynamics of central nervous system development. Nat Neurosci 7: 327-332.
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, et al. (1996) Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 6: 551-560.
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, et al. (2009) Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 29: 11772-11782.
- Utsunomiya H, Takano K, Okazaki M, Mitsudome A (1999) Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am J Neuroradiol 20: 717-723.
- Knickmeyer RC, Gouttard S, Lin W, Evans DD, Wilber K, et al. (2008) A structural MRI study of human brain development from birth to age 2. J Neurosci 28: 12176-12182.
- Tustin K, Hayne H. (2010) Defining the boundary: age related changes in childhood amnesia. Dev Psychol 46: 1049-1061.
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB (2006) Path integration and the neural basis of the cognitive map. Nat Rev Neurosci 7: 663-678.
- Wolbers T, Wiener JM, Mallot HA, Buchel C (2007) Differential recruitment of the hippocampus, medial prefrontal cortex, and the human motion complex during path integration in humans. J Neurosci 27: 9408-9416.
- Cooke HJ (1989) Role of the "little brain" in the gut in water and electrolyte homeostasis. 3: 127-138.
- Uesaka T, Young HM, Pachnis V, Enomoto H (2016) Development of the intrinsic and extrinsic innervation of the gut. Dev Biol 417: 158-167.
- Gershon MD (2009) Enteric Nervous System Development. In: Binder MD, Hirokawa N, Windhorst U (Eds.) Encyclopedia of Neuroscience. Springer Verlag, Heidelberg, Germany.
- Augustine JR (1996) Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229-244.
- Nagai M, Kishi K, Kato S (2007) Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry 22: 387-394.
- Craig AD (2009) How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59-70.
- Tognini P (2017) Gut Microbiota: A Potential Regulator of Neurodevelopment. Front Cell Neurosci 11: 25.
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, et al. (2004) Postnatal microbial colonization programs the hypothalamic-pituitary adrenal system for stress response in mice. J Physiol 558: 263-275.
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, et al. (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiat 18: 666-673.
- Mayer EA (2011) Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci. 12: 453-466.
- Grenham S, Clarke G, Cryan JF, Dinan TG (2011) Brain-gut-microbe communication in health and disease. Front Physiol 2: 94.
- Cryan JF, O’Mahony SM (2011) The microbiome–gut–brain axis: from bowel to behavior. Neurogastroenterol Motil 23: 187-192.
- Diaz Heijtz R. Wang S, Anuar F, Qian Y, Björkholm B, et al. Normal (2011) gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108: 3047-3052.
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, et al. (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155: 1451-1463.
- De Palma G. Blennerhassett P, Lu J, Deng Y, Park AJ, et al. (2015) Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun 6: 7735.
- Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, et al. (2015) Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun 48: 165-173
- Hooper LV (2004) Bacterial contributions to mammalian gut development. Trends Microbiol 12: 129-134.
- Stappenbeck TS, Hooper LV, Gordon JI (2002) Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA 99: 15451-15455.
- Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF (2014) The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol 817: 373-403.
- Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, et al. (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20: 509-518.
- Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, et al. (2018) Infant Gut Microbiome Associated With Cognitive Development. Biol Psychiatry 83: 148-159.
- Rager G (1978) Systems-matching by degeneration. II. Interpretation of the generation and degeneration of retinal ganglion cells in the chicken by a mathematical model. Exp Brain Res 33: 79-90.
- Herrup K, Sunter K (1987) Numerical matching during cerebellar development: quantitative analysis of granule cell death in staggerer mouse chimeras. Neurosci J 7: 829-836.
- Colón-Ramos DA (2009) Synapse formation in developing neural circuits. Curr Top Dev Biol 87: 53-79.
- Lui JH, Hansen DV, Kriegstein AR (2011) Development and evolution of the human neocortex. Cell 146: 18-36.
- O’Donnell M, Chance RK, Bashaw GJ (2009) Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci 32: 383-412.
- Holt CE, Schuman EM (2013) The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 80: 648-657.
- Riccomagno MM, Kolodkin AL (2015) Sculpting neural circuits by axon and dendrite pruning. Annu Rev Cell Dev Biol 31: 779-805.
- Solı´s CB, Kelly-Irving M, Fantin R, Darnaudéry M, Torrisani J, et al. (2015) Adverse childhood experiences and physiological wear-and-tear in midlife: findings from the 1958 British birth cohort. Proc Natl Acad Sci USA 112: E738-E746.
- Felitti V, Anda R, Nordenberg D, Williamson DF, Spitz AM, et al. (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev 14: 245-258.
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, et al. (2004) Developmental plasticity and human health. Nature 430: 419-421.
- O’Mahony SM, Hyland NP, Dinan TG, Cryan JF (2011) Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology 214: 71-88.
- Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13: 701-712.
- Aziz Q, Doré J, Emmanuel A, Guarner F, Quigley EM (2013) Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil 25: 4-15.
- Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6: 306-314.
- Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10: 735-742.
- Yassour M, Vatanen T, Siljander H, Hämäläinen A-M, Härkönen T, et al. (2016) Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Science Translational Medicine 8: 343ra81.
- Bjork JR, O'Hara RB, Ribes M, Coma R, Montoya JM (2018) The dynamic core microbiome: Structure, dynamics and stability. bioRxiv 137885.
- Lu J, Lu L, Yu Y, Cluette-Brown J, Martin CR, et al. (2018) Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice. Scientific Reports 8: 5443.
- Sherwin E, Sandhu KV, Dinan TG, Cryan JF (2016) May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs 30: 1019-1041.
- Sherwin E, Rea K, Dinan TG, Cryan JF (2016) A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol 32: 96-102.
- Dinan TG, JF Cryan (2015) The impact of gut microbiota on brain and behavior: implications for psychiatry. Curr Opin Clin Nutr Metab Care 18: 552-558.
- Dinan TG, Stilling RM, Stanton C, Cryan JF (2015) Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res 63: 1-9.
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277: 32-48.
- Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, et al. (2016) Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65: 57-62.
- Oufir LEI, Flourié B, des Varannes SB, Barry JL, Cloarec D, et al. (1996) Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut 38: 870-877.
- Bourassa MW, Alim I, Bultman SJ, Ratan RR (2016) Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett 625: 56-63.
- Jin C, Londono I, Mallard C, Lodygensky GA (2015) New means to assess neonatal inflammatory brain injury. J Neuroinflammation 12: 180.
- Siniscalco D, Schultz S, Brigida AL, Antonucci N (2018) Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals 11: 56.
- Fernandez AM, Torres-Aleman I (2012) The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 13: 225-239.
- Grabrucker AM (2012) Environmental factors in autism. Front Psychiatry 3: 118.
- Vasa RA, Mostofsky SH, Ewen JB (2016) The Disrupted Connectivity Hypothesis of Autism Spectrum Disorders: Time for the Next Phase in Research. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 245-252.
- Jones W, Klin A (2013) Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature 504: 427-431.
- Ekberg TL, Falck-Ytter T, Bölte S, Gredebäck G, the EASE Team (2016) Reduced prospective motor control in 10-month-olds at risk for autism spectrum disorder. Clin Psychol Sci 4: 129-135.
- Inui T, Kumagaya S, Myowa-Yamakoshi M (2017) Neurodevelopmental Hypothesis about the Etiology of Autism Spectrum Disorders. Frontiers in Human Neuroscience 11: 354.
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. 6th Edn. American Psychiatric Press, Washington DC, US.
- Zablotsky B, Black LI, Maenner MJ, Schieve LA, Blumberg SJ (2014) Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 national health interview survey. Natl Health Stat Report 13: 1-20.
- Clements CC, Zoltowski AR, Yankowitz LD, Yerys BE, Schultz RT, et al. (2018) Evaluation of the Social Motivation Hypothesis of AutismA Systematic Review and Meta-analysis. JAMA Psychiatry 75: 797-808.
- Nover A (2018) fMRI Evidence for Social Motivation Hypothesis of Autism. Psychiatry Advisor.
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY (2010) Reward processing in autism. Autism Res 3: 53-67.
- Pavăl D (2017) A Dopamine Hypothesis of Autism Spectrum Disorder. Dev Neurosci 39: 355-360.
- McNamara CG, Dupret D (2017) Two sources of dopamine for the hippocampus. Trends in Neurosciences 40: 383-384.
- Cooper RA, Richter FR, Bays PM, Plaisted-Grant KC, Baron-Cohen S, et al. (2017) Reduced Hippocampal Functional Connectivity During Episodic Memory Retrieval in Autism. Cerebral Cortex 27: 888-902.
- Marin-Burgin A, Schinder AF (2012) Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res 227: 391-399.
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476: 458-461.
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF (2014) Microbiota is essential for social development in the mouse. Mol Psychiatry 19: 146-148.
- de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Knol J, et al. (2014) Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 37: 197-206.
- Douglas-Escobar M, Elliott E, Neu J (2013) Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr 167: 374-379.
- Mulle JG, Sharp WG, Cubells JF (2013) The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep 15: 337.
- MacFabe DF (2012) Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis 23: 19260.
- Doenyas C (2018) Gut Microbiota, Inflammation, and Probiotics on Neural Development in Autism Spectrum Disorder. Neuroscience 374: 271-286.
- Iovene MR, Bombace F, Maresca R, Sapone A, Iardino P, et al. (2017) Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 182: 349002D363.
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, et al. (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35: S6-S16.
- Onore C, Careaga M, Ashwood P (2012) The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun 26: 383-392.
- Xu N, Li X, Zhong Y (2015) Inflammatory cytokines: Potential biomarkers of immunologic dysfunction in autism spectrum disorders. Med Inflamm 2015: 531518.
- Siniscalco D, Bradstreet JJ, Antonucci N (2013) Therapeutic role of hematopoietic stem cells in autism spectrum disorder-related inflammation. Front Immunol 4: 140.
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, et al. (2011) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25: 40-45.
- Xie J, Huang L, Li X, Li H, Zhou Y, et al. (2017) Immunological cytokine profiling identifies TNF- α as a key molecule dysregulated in autistic children. Oncotarget 8: 82390-82398.
- Jyonouchi H, Geng L, Davidow AL (2014) Cytokine profiles by peripheral blood monocytes are associated with changes in behavioral symptoms following immune insults in a subset of ASD subjects: An inflammatory subtype? J Neuroinflamm 11: 187.
- Siniscalco D, Bradstreet JJ, Cirillo A, Antonucci N (2014) The in vitro GcMAF effects on endocannabinoid system transcriptionomics, receptor formation, and cell activity of autism-derived macrophages. J Neuroinflamm 11: 78.
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57: 67-81.
- Siniscalco D (2014) Adhesion G-protein Coupled Receptors in Autism. Autism Open Access 4: e126.
- Mottahedin A, Ardalan M, Chumak T, Riebe I, Ek J, et al. (2017) Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front Cell Neurosci 11: 190.
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, et al. (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 108: 13281-13286.
- Hopkins MJ, Sharp R, Macfarlane GT (2002) Variation in human intestinal microbiota with age. Dig Liver Dis 34 Suppl 2: S12-S18.
- De Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, et al. (2010) Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 51: 418-424.
- Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, et al. (2016) Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism 7: 49.
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, et al. (2006) Neocortical neurogenesis in humans is restricted to development. Proc. Natl Acad Sci USA 103: 12564-12568.
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, et al. (2014) Dynamics of oligodendrocyte generation and myelination in in the human brain. Cell 159: 766-774.
- de la Torre-Ubieta L, Won H, Stein JL, Geschwind DH (2016) Advancing the understanding of autism disease mechanisms through genetics. Nature 22: 345-361.
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK (2018) Beyond infection-Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol 299(Pt A): 241-251.
- Navarro F, Liu Y, Rhoads JM (2016) Can probiotics benefit children with autism spectrum disorders? World J Gastroenterol 22: 10093-10102.
- Santocchi E, Guiducci L, Fulceri F, Billeci L, Buzzigoli E, et al. (2016) Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 16: 183.
- Gupta S, Aggarwal S, Heads C (1996) Dysregulated immune system in children with autism: Beneficial effects of intravenous immune globulin on autistic characteristics. J Autism Dev Disord 26: 39-452.
- Melamed IR, Heffron M, Testori A, Lipe K (2018) A pilot study of high-dose intravenous immunoglobulin 5% for autism: Impact on autism spectrum and markers of neuroinflammation Autism Res 11: 421-433.
- Sharkey KA, Wiley JW (2016) The Role of the Endocannabinoid System in the Brain–Gut Axis. Gastroenterology 151: 252-266.
- Suarez AN, Hsu TM, Liu CM, Noble EE, Cortella AM, et al. (2018) Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nature Comm 9: 2181.
- Kanoski SE, Grill HJ (2017) Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry 81: 748-756.
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, et al. (1998) Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem 70: 364-373.
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA (1999) Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2: 94-98.
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, et al. (2007) Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 1179: 28-34.
- Biggio F, Biggio F, Gorini G, Caria S, Talani G, et al. (2009) Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Int J Neuropsychopharmacol 12: 1209-1221.
- Limoges E, Mottron L, Bolduc C, Berthiaume C, Godboutet R (2005) Atypical sleep architecture and the autism phenotype. Brain 128: 1049-1061.
- Buckley AW, Rodriguez AJ, Jennison K, Buckley J, Thurm A, et al. (2010) Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch Pediatr Adolesc Med 164: 1032-1037.
- Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, et al. (2017) Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci USA 114: 8119-8124.
Citation: Yu ECL (2018) Neurodevelopment, Intestinal Function, and Autism. Neonat Pediatr Med 4: 166. DOI: 10.4172/2572-4983.1000166
Copyright: © 2018 Yu ECL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5509
- [From(publication date): 0-2018 - Dec 17, 2025]
- Breakdown by view type
- HTML page views: 4550
- PDF downloads: 959