Review Article Open Access
Nonalcoholic Fatty Liver Disease and the Gut Microbiota: Exploring the Connection
Edward C. Oldfield1, Ray Z. Dong1 and David A. Johnson2*
1Eastern Virginia Medical School, Norfolk VA, USA
2Division of Gastroenterology, Eastern Virginia Medical School, Norfolk VA, USA
- *Corresponding Author:
- David A Johnson
Department of Internal Medicine Chief
Eastern Virginia Medical School, Norfolk VA, USA
Tel: 7576416685
Fax: 7574405713
E-mail: dajevms@aol.com
Received date: November 17 2014; Accepted date: December 19 2014; Published date: December 23 2014
Citation: Oldfield EC, Dong RZ, Johnson DA (2014) Nonalcoholic Fatty Liver Disease and the Gut Microbiota: Exploring the Connection. J Gastrointest Dig Syst 4:245. doi:10.4172/2161-069X.1000245
Copyright: © 2014 Oldfield EC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use distribution and reproduction in any medium provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
As the gut microbiota continues to be implicated in an increasing number of disease processes, a plethora of new literature surrounding its complexity and role in the maintenance of intestinal homeostasis has become available. Nonalcoholic fatty liver disease (NAFLD) has become the most common nonviral liver disease worldwide and a number of predisposing risk factors for NAFLD have been identified, including obesity and insulin resistance. Recent evidence supports a role for the gut microbiota in the pathogenesis of these risk factors and NAFLD itself. Additionally changes in the gut microbiota can lead to activation of immune responses that have the potential to promote progression of NAFLD to the more severe nonalcoholic steatohepatitis (NASH). Furthermore, the gut microbiota may serve as a potential target for therapeutic options to treat NAFLD. This review seeks to explain the role of the gut microbiota in the pathogenesis of NAFLD and its risk factors, while also discussing potential future treatment options directed at correcting imbalances with in the gut microbiota.
Keywords
Non-alcoholic fatty liver disease; Microbiota; Insulin resistance; Metabolic syndrome; Steatohepatitis; Inflammosomes
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver disease defined as the presence of lipids in >5% of hepatocytes or a lipid content >5% of liver weight in the absence of significant alcohol intake (>20g of alcohol/day), hepatic viral infections or the use of potentially hepatotoxic medications [1,2]. Worldwide NAFLD has become the most common nonviral liver disease affecting over one billion individuals with an estimated prevalence of 6-30% in the general population in part due to the increasing incidence of obesity and as well due to related other metabolic risk factors [1-4]. Currently NAFLD related chronic liver disease is the 3rd leading indication for liver transplantation in the U.S. and is expected to be the leading cause in 2020 [2]. Steatosis in NAFLD can progress to non-alcoholic steatohepatitis (NASH) with fibrosis. This may further be subject to progressive changes in inflammation and fibrosis that can lead to liver cirrhosis,end stage liver disease and also an increased risk for hepatocellular carcinoma (HCC) [2,4]. The initial diagnosis of NAFLD is often suggested incidentally during abdominal ultrasonography as most patients with NAFLD are asymptomatic [3]. Predisposing factors for the development of NAFLD include those of the metabolic syndrome: abdominal obesity, hypertriglyceridemia, low HDL, hypertension and insulin resistance.
With >1014 different microorganisms the gut microbiota is considered as a major metabolic internal organ intimately involved in molecular “cross-talk with the intestinal epithelium and affecting the intestinal barrier function [5,6]. Recent attention has focused around the gut microbiota not only as part of the disease process but also as a potential target for treatment. The focus of this article is to explore the link between the human gut microbiota and NAFLD as disruption of the gut microbiota may predispose patients to developing NAFLD.
Beginning with a review of the relevant pathophysiology this article will address the role of the liver and gut microbiota in both metabolic and immune regulation. Further discussion of specific alterations in the gut microbiota in direct relation to each of the major risk factors for NAFLD will follow. Lastly a review of the therapeutic options functioning to modify the gut microbiota will be addressed.
Pathophysiology
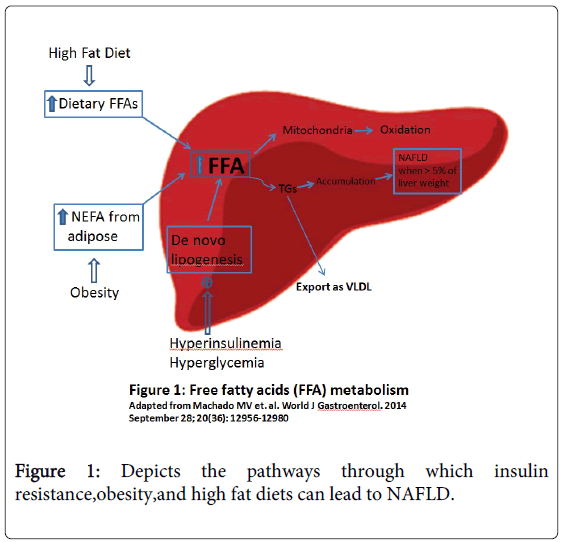
In order to understand the pathogenesis of NAFLD it is essential to have a basic understanding of hepatic function and its relationship to the predisposing risk factors for NAFLD. The liver is the main warehouse for various lipids including triglycerides free fatty acids (FFA), diacylglycerol, free cholesterol, cholesterol esters, ceramides and phospholipids. The hallmark pathogenesis of NAFLD is the presence of ectopic fat within hepatocytes which results from an imbalance in the levels of lipogenesis and lipolysis [2]. Triglycerides are synthesized from FFAs that accumulate that within the liver; therefore the concentration of FFAs functions as a regulator of lipogenesis. Importantly the hepatic uptake of FFAs is unregulated and is directly proportional to the level of nonesterified fatty acids (NEFAs) which accounts for 60% of FFAs accumulation within the liver primarily from lipolysis in adipose tissue [2]. Other sources of FFAs include de novo lipogenesis (25%) and dietary fatty acids (15%) in the form of chylomicrons lipoproteins [2]. After FFAs are taken up by the liver they have three potential fates: oxidation within mitochondria VLDL (very low-density lipoprotein) assembly and export or triglyceride synthesis and storage as lipid droplets (Figure 1). Over time an abundance of triglycerides accumulates and leads to increased hepatic storage of lipid droplets promoting the progression towards NAFLD [2].
Many of the risk factors for NAFLD alter the balance of these three pathways. For instance in patients with insulin resistance there is a decreased ability of insulin to suppress adipose tissue lipolysis which leads to increased hepatic uptake of FFA [2]. Also in patients with NAFLD de novo lipogenesis during fasting state increases by 3 fold compared to those with a lean liver [2]. Furthermore excess insulin induces sterol response element-binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor-γ (PPAR-γ) to promote the expression of several lipogenic genes generating more lipids and conferring a greater burden to the liver [7]. Lastly dietary FAs are positively correlated with a high fat diet in which >30% of total energy requirement is provided as fat [8].
Another critical factor in the pathogenesis of NAFLD is the interactions between the specific risk factors for NAFLD. The result is a complex pathway that leads to a cyclic pattern of inflammation and injury. To start high fat diet and obesity lead to increased peripheral adipose tissue which initiates insulin resistance (IR). The excessive accumulation of fat in adipocytes promotes an increase in oxidative stress and low grade inflammatory state through the release of inflammatory markers including interleukin-6 (IL-6) and monocyte chemotactic protein 1(MCP-1) [9]. Subsequently the activation of macrophages and lymphocytes promotes further release of proinflammatory cytokines associated with insulin resistance namely tumor necrosis factor-α (TNF-α) and interferon-γ (INF-γ), promoting a continuation of the cycle [10].
Progression from NAFLD to NASH occurs in roughly 20% of cases and is characterized by the hallmark lobular chronic inflammatory infiltrate without any secondary causes of hepatic fat accumulation e.g. significant alcohol consumption use of steatogenic medication or hereditary disorders [2,4,11]. Injury and inflammation are thought to be the major factors that lead NAFLD progression to NASH and fibrogenesis [2]. One potential explanation for the progression to NASH is lipotoxicity a process in which increased oxidative stress secondary to accumulation of lipids overwhelms the hepatic function of metabolism. Lipotoxicity also leads to impaired autophagy causes cell damage and cell death and induces an inflammatory and wound healing response that can lead to fibrogenesis [2]. Additionally a variety of bacterial products can activate various immune responses further promoting inflammation through the expression of proinflammatory cytokines [12]. These immune responses will be analyzed and discussed more thoroughly in a later section.
icrobiota in NAFLD risk factors
Although a number of genetic and environmental factors have been linked in the pathogenesis of NAFLD, obesity, insulin resistance and immune responses are the more dominant risk [2,12]. First obesity in particular central obesity is highly predictive of hepatic steatosis and disease progression. In overweight (BMI >25) patients the prevalence of steatosis is at least two times more frequent than in lean subjects directly proportional to elevated body mass index (BMI) [2]. In extreme obesity (BMI >40) most patients have NAFLD steatosis and more than one third have NASH [13]. Secondly insulin resistance plays a huge role in developing NAFLD evidenced by a 5-9 fold increased risk for NAFLD in patients with type 2 diabetes mellitus (T2DM) as compared to the general population; further two thirds of these patients with T2DM develop NAFLD [14,15]. Third the immune system regulates inflammatory responses to a variety of bacterial products that can be altered in NAFLD. This section seeks to more closely explore the relationship between each of these risk factors and their association with changes in the gut microbiota.
Obesity
The gut microbiota has been recently linked to the pathogenesis of obesity through a number of pathways [16]. In particular modification of appetite and alteration of de novo lipogenesis appear to be essential mechanisms by which the gut microbiota maximizes hepatic triglyceride content [5,11]. Evidence for these mechanisms comes from animal studies where germ-free (GF) animals born and raised in a sterile environment lacking gut flora were resistant to the development of obesity when fed a high-fat high-sugar diet; however after introducing gut flora to these GF mice there was an increase in energy harvested from the diet with increased intestinal monosaccharide uptake. Additionally these mice had increased weight and body fat content with increased hepatic lipogenesis and fat deposition which eventually led to the development of insulin resistance [11,16,17].
Within the gut microbiota two predominate species of bacteria Firmicutes and Bacteroidetes have been influential in the development of metabolic syndrome [11]. The balance of these two bacteria is dysregulated in patients with metabolic syndrome and obesity evidenced by multiple studies showing an excess of Firmicutes and reduction of Bacteroidetes compared to lean counterparts [11,16,18]. In these studies more Firmicutes resulted in increased fermentation end products such as short-chain fatty acids (SCFAs). These SCFAs in turn play a major role in appetite regulation by not only diffusing passively into circulation but also by acting as signaling molecules [11,19]. Certain SCFAs such as propionate and acetate can bind to G protein-coupled receptors (GPCRs) to induce release of peptide YY (PYY) [20]. PYY is an enteroendocrine cell-derived hormone that normally inhibits gut motility and increases nutrient absorption so abundant SCFAs increase calorie absorption by stimulating PYY leading to obesity. Furthermore excess SCFAs will also be converted into triglycerides in the liver which can cause hepatic steatosis [19]. These studies give us insight that further therapeutic approaches to obesity could target this specific gut flora [21].
These “typical” changes in the obese human gut microbiota however have not been found by all investigators. Schwiertz et al. reported lower ratios of Firmicutes to Bacteroidetes in obese human adults compared to lean controls [22]; howeversignificant diet-dependent reductions in a group of butyrate-producing Firmicutes were found [23]. In 2011, Arumugam et al studied the phylogenetic composition of 39 fecal samples from individuals representing 6 nationalities and found that there was no correlation between body mass index and the Firmicutes/Bacteroidetes ratio [24]. On the other hand the identification of three metagenomic-derived functional biomarkers that strongly correlate with body mass index (BMI),suggests that differences at the phylum level are probably less important than metagenomic-based functional aspects [20,24].
Besides the gut flora changes and metagenomic biomarkers there are also a few studies targeting how the gut microbiota puts patients at risk for obesity on a molecular level. Bäckhead et al showed that fasting-induced adipocyte factor (Fiaf) a member of the angiopoietin-like family of proteins is suppressed in the intestinal epithelium by the microbiota [25]. This suppression leads to increased lipoprotein lipase (LPL), a key regulator of fatty acids which results in increased cellular uptake of fatty acids and adipocyte triglyceride accumulation. Further investigation revealed that when the gut was colonized with Bacteroides thetaiotaomicron and Methanobrevibacter smithii there was a significant increase in suppression of Fiaf which leads to obesity [26].
More than just the bacteria living in the gut microbiota may influence energy homeostasis. Zhang et al reported an association between methanogenic Archaea (microorganisms which produce methane as a byproduct during anoxic conditions) and obesity [27]. Increased levels of Archaea-derived gene fragments were detected in obese mice compared to their lean relatives suggesting that methanogens in the gut may play a pivotal role in fermentation and ultimately lead to production of SCFAs with the net result being energy harvest and weight gain [28,29]. A proposed explanation is that methanogens remove fermentation intermediate such as H2 (hydrogen gas) or formate relieving thermodynamic limitations and allowing greater production of SCFAs that are available to be absorbed across the intestinal epithelium while at the same time extracting more energy from indigestible polysaccharides [27]. The study concluded that interspecies H2 transfer between bacterial and archaeal species affects energy uptake in humans and puts patients at risk for obesity [27]. SCFAs also regulate gut hormones via free fatty acid receptors 2 (FFAR2) and 3 (FFAR3) which promote energy storage by stimulating adipogenesis and inhibiting lipolysis. This decrease in energy expenditure ultimately leads to obesity and other metabolic diseases [28–30].
Bottom line
Obesity is clearly a strong risk factor in the pathogenesis of NAFLD with a prevalence twice that of lean comparators. High fat diets increase the accumulation of FFAs within the liver ultimately leading to NAFLD. The gut microbiota has been shown to be intimately involved in this pathway as a characteristic increase in Firmicutes and reduction in Bacteroidetes have been widely documented. This alteration in the normal ratio affects the regulation of gut hormones such as PYY and also number of regulatory factors for lipolysis and lipogenesis including Fiaf, LPL, FFAR2 and FFAR3. Continued investigation into the alterations in the gut microbiota in obesity may help to further our understanding NAFLD and explain key differences in environmental versus genetic factors.
Insulin Resistance
Environmental factors and host genetics play major roles in establishing and maintaining gut microbiota while in turn interacting to sustain the homeostasis of gut weight control and insulin sensitivity [31,32]. Previously discussed inflammatory mediators such as TNF-alpha, IL-6, inducible nitric oxide and nuclear factor (NF- κB) have already been shown to be increased when the gut microbiota is altered or disrupted. Here we will discuss the mechanisms behind which changes in gut microbiota may promote insulin resistance.
Certain inflammatory mediators involved in the development of insulin resistance are controlled by Toll-like receptor 4 (TLR4) activated by lipopolysaccharide (LPS) from gram negative bacteria highlighting a link between insulin resistance and liver inflammation through several pathways responsible for the regulation of hepatocyte apoptosis and insulin signaling [16,33]. Important functions of TLR4 in relation to insulin resistance are the upregulation of both c-Jun NH2-terminal kinase (JNK) and IκB kinase complex (IKKβ) and also decreased phosphorylation of insulin receptor substrate (IRS)-1. The IRS-1 is needed for glucose transport in muscle and adipose tissue, glycogen synthesis in muscle and liver and lipogenesis in adipose tissue while JNK and IKKβ disrupt appropriate insulin signaling leading to insulin resistance [34,35]. The LPS also induces insulin resistance by promoting the expression of NF-κB and activation of the MAPK pathway in adipocytes [34,36]. New evidence also suggests LPS can promote the expression of iNOS (inducible nitric oxide synthase) by hampering LPL activity and increasing lipolysis ultimately worsening insulin resistance by increasing levels of circulating fatty acids [34,37].
Other bacterial factors that play a role in the development of insulin resistance could be nucleotide oligomerization domain (NOD)-1 and -2 proteins. These NOD proteins are intracellular pattern recognition receptors that can sense bacterial cell wall peptidoglycan (PGN) moieties which then induce stress and inflammation pathways [34,38]. NOD-1 detects PGN found in gram-negative bacteria whereas NOD-2 detects gram-positive bacteria [38]. Activation of NOD-1 in adipocytes leads to impaired insulin signaling and decreased insulin-stimulated glucose uptake [39]. While activated NOD-2 leads to muscle cell-autonomous insulin resistance [40].
Adenosine monophosphate-activated protein kinase (AMPK) is an enzyme which plays an active role in energy homeostasis. It is activated to offset the energy deprived state by stimulating fatty acid oxidation, ketogenesis and glucose uptake insulin secretion while inhibiting cholesterol synthesis lipogenesis and triglyceride synthesis [28,41]. Bäckhead et al. demonstrated that the expression of AMPK is suppressed by microbiota thereby predisposing the host to obesity and insulin resistance [26].
A few animal studies have also investigated the link between insulin resistance and the gut microbiota in particular how the translocation of gut microorganisms and their byproducts into portal and systemic circulation may cause hepatic inflammation and insulin resistance. It has been shown that mice on a HFD have greater accumulation of bacteria close to the mucosa of the intestinal lumen, which facilitates their translocation through the epithelium [42]. This high level of bacteria at the mesenteric adipose tissue (MAT) triggers inflammatory markers through LPS released by bacteria, eventually leading to systemic inflammation and insulin resistance [42]. Interestingly mice given one month of probiotics showed complete normalization of insulin sensitivity, inflammation and fasting hyperinsulinemia further supporting the gut microbiota as a potential target in insulin resistant diabetic patients [42]. Another study done by Caricilli et al looked at gut microbiota on a molecular level in association with insulin resistance [31]. Their results showed that in TLR2 knockout mice conventionalization (as opposed to “germ-free” condition) results in a phenotype reminiscent of metabolic syndrome, characterized by different gut flora, with a 3-fold increase in Firmicutes and a slight increase in Bacteroidetes compared with control; further, antibiotics were able to reverse these adverse outcomes [31]. Once again, LPS absorption, subclinical inflammation, insulin resistance and glucose intolerance are all sequelae of these changes in microbiota.
Bottom-line
As compared to obesity, which primarily predisposes to NAFLD on the basis of increased FFA within the liver, insulin resistance appears to affect a wider variety of biochemical pathways involved in the pathogenesis of NAFLD. Insulin resistance is closely linked to inflammatory mediators and regulation of signaling cascades that affect glucose transport in muscle and adipose tissue, glycogen synthesis and lipogenesis. In these respect alterations in the gut microbiota that affect activation of immune response can potentially modify insulin resistance.
Cellular Immunity and Inflammation
While obesity and metabolic syndrome are undoubtedly the most important risk factors for the development of NAFLD, the relationship between the immune system and the gut microbiota appears have a more essential role in the inflammatory processes that drive the change from NAFLD to NASH. The pathogenesis of NASH was originally described as a “two-hit” hypothesis in which the “first hit,” hepatic steatosis, acts to sensitize the hepatocytes for the “second hit,” either genetic factors, oxidative stress, gut-derived endotoxins, or inflammatory cytokines [43]. More recently, new evidence has emerged suggesting that inflammation may be able to proceed steatosis in some cases, suggesting that multiple parallel hits may occur to initiate the progression to NASH [44]. While there a number of factors involved in this complex pathway leading to NASH, this review will focus on the role of the innate immune system and its relationship to endotoxin and gut derived signals.
During the progression from NAFLD to NASH, injured cells and necrotic tissues release molecules such as damage-associated molecular patterns (DAMPs), which trigger inflammation through the binding of several receptors. These receptors can be specific or shared with pathogen-associated molecular patterns (PAMPs) that recognize molecular patterns associated with microbial pathogens or cellular stress. The essential foundation for the relationship between the immune system and the gut microbiota is the recognition of these PAMPs and DAMPs via Toll-like receptors (TLRs) or Nod-like receptors (NLRs). Both TLRs (located on the cell surface or within endosomes) and NLRs (located within the host cytosol) function to recognize microbial products and activate signaling pathways of both innate and adaptive immune responses [45]. In order to understand the impact that gut microbiota alterations can have on the immune system, it is important to more closely analyze the major receptors in each of the families.
Toll-like Receptors
The TLRs often represent a first line of defense based on their cell surface location and recognition of a variety of microbial signals. In the liver, TLRs are an essential piece of immunity as the portal system has the potential to be a significant source of microbial products and any disruption in the balance can lead to excess inflammation within the liver. The four main TLRs involved in NAFLD and NASH is: TLR2 recognizing peptidoglycan and lipoteichoic acid both components of gram-positive bacterial cell walls; TLR4 recognizing lipopolysaccharide (LPS) from gram-negative bacteria; TLR5 a receptor for bacterial flagellin; and TLR9 recognizing unmethlyated CpG motifs in bacterial DNA [12].
To date a number of studies performed in animal models have helped to explain the significance of these receptors in the development of NAFLD. Evidence for the relationship between the gut microbiota and TLRs is multifocal although key factors are alterations in the gut microbiota along with a related increased intestinal permeability. These factors have been demonstrated in rodent models through a variety of diets including high-fat diet (HFD), methionine-choline deficient diet (MCD), and choline–deficient amino acid-defined diet (CDAA) [12]. For example it has been shown that rodents placed on a high-fat diet (HFD) have increased inflammation through the induction of TLR4 which leads to increased intestinal permeability and increased endotoxin levels further accelerating obesity; importantly, this effect was not reproducible with the HFD in TLR4 deficient mice [46]. Additionally, a number of other studies have shown that TLR4 mutant mice are resistant to the development of NAFLD [47-49]. Similar models using a methionine choline-deficient (MCD) diet were able to induce NASH evidenced by increased liver triglyceride accumulation, lipid peroxidation, serum ALT, TNF-α, NADPH and markers of liver fibrosis [48]. When knockout mice deficient for TLR4 and its co-receptor MD-2 (myeloid differentiation factor) were also placed on the MCD diet however, these increases were attenuated. The authors of this study suggest that these results demonstrate a role for LPS recognition via TLR4 and MD-2 for inducing liver steatosis and fibrosis in a NASH model in mice [48]. This conclusion is supported by several mouse models in which LPS injections in NAFLD mice were able to further promote liver injury through increased levels of proinflammatory cytokines [50,51]. This represents an important finding, as levels of LPS in humans are also elevated in those with metabolic syndrome and NAFLD [12].
Among patients with biopsy-proved NAFLD, increased small intestine bacterial overgrowth has been associated with disrupted intercellular tight junctions, leading to increased intestinal permeability and delivery of LPS to the portal system [52]. In patients with type 2 diabetes mellitus, circulating levels of LPS were shown to be 76% higher than in matched controls and further associated with significant increases in TNF-α and IL-6 [53]. Another mechanism by which TLR increases inflammation is through the potent activation of Kupffer cells within the liver [47]. This activation of Kupffer cells can induce a pathological effect by inducing reactive oxygen species (ROS)-dependent activation of X-box binding protein-1 (XBP-1), which is a key transcription factor mediating unfolded protein response in ER (endoplasmic reticulum) stress [54]. Additionally, in this rodent model of NASH, Kupffer cell depletion led to an abrogation of the high-fat, high-cholesterol diet induced TLR4 expression; this suggests that Kupffer cells are a major source of pro inflammatory mediators through an increased expression of TLR4 [54].
Toll like Receptor 9, which recognizes unmethylated CpG motifs in bacterial DNA has also been shown to play an important role in the progression to NASH. Using a CDAA diet induced NASH model, researchers were able to show that TLR9 signaling induced IL-1β production leading to steatosis, inflammation and fibrosis which was also associated with insulin resistance and weight gain; in this same model TLR9 deficient mice showed less steatosis, inflammation, liver fibrosis, insulin resistance and weight gain compared to controls [55].
One of the major changes in the gut microbiota associated with obesity and high fat diets is a significant decrease in the gram-negative Bacteroidetes and a proportional increase in the gram-positive Firmicutes [18]. This change in the gut microbiota represents a major shift in the balance of the gram-negative to gram-positive bacteria that has the potential for alteration of the inflammatory activity secondary to TLR activation. In this environment TLR2 which recognizes components of gram-positive cell walls likely acts in concert with TLR4 to mediate changes in the proinflammaotry cytokines and alterations in intestinal permeability. Interestingly while TLR2 deficient mice on a HFD show decreased insulin resistance [56,57] when placed on an MCD diet these mice have significantly enhanced histological and molecular evidence of steatohepatitis compared to controls [58,59]. The proposed mechanism for this phenomenon is increased sensitivity of TLR4 to LPS in the absence of TLR2 [47]. These results would also suggest a protective role of TLR2 against the development of liver injury, a potential mechanism of which would be maintenance of the mucosal integrity as evidenced by disruption of tight junctions in TLR2 deficient mice that was preserved in wild type mice with a TLR2 agonist [60].
Toll like Receptor 5, which recognizes bacterial flagellin may also play a protective role, as a study with TLR5 deficient mice showed the development of obesity and steatosis which was further exacerbated by a high-fat diet [61]. Subsequent decimation of the gut microbiota in these TLR5 deficient mice corrected the metabolic syndrome relative to the wild type mice. Looking more closely at the gut microbiota in these TLR5 deficient mice both Firmicutes and Bacteroidetes were similar with wild type mice; however more specific analysis showed that species concentrations within these two phyla were significantly different [61]. When the gut microbiota from TLR5 deficient mice was transplanted into wild type germ-free mice phenotypic aspects of the TLR5 deficient mice were transferred to wild type mice including hyperphagia, obesity, hyperglycemia, insulin resistance, colomegaly and elevated proinflammatory cytokines [61].
In summary, a wide range of rodent models have shown the significance of interactions between immune regulation through TLRs and the gut microbiota in the development of NAFLD. The primary mechanisms behind these changes are increased proinflammatory cytokines and altered intestinal permeability that create a predisposition to the major risk factors for NAFLD namely obesity, insulin resistance and metabolic syndrome.
Nod-like Receptors
In contrast to the TLRs which function primarily to recognize extracellular ligands the NLRs are located intra-cellularly and have a more complex mechanism of action including activation of inflammasomes. NLRs are complicated receptor proteins that have a variable N-terminal domain and a centrally located nucleotide-binding oligomerization domain (NOD) and a C-terminal leucine rich repeat region that recognizes PAMPs [45]. Within the host cytosol these NODs recognize specific microbial molecules; NOD1 recognizes iE-DAP (γ-D-glutamyl-meso-diaminopimelic acid) which contains fragments from most gram-negative and some gram-positive bacteria while NOD2 recognizes muramyl dipeptide (MDP) found in the majority of both gram-positive and gram-negative bacteria [45]. Within the N-terminal domain there is further protein modules involved in downstream signaling pathways including a caspase recruitment domain (CARD). These CARDs are particularly important as multiple NLRs can join together through an adaptor protein such as ASC (apoptosis-associated speck-like protein) to form an inflammasome which controls caspase activation and subsequent production of pro-inflammatory cytokines [45,62].
These inflammasomes and caspases play critical roles in the immune response through regulation of inflammation and also cell death. Caspase-1 activation by inflammasomes leads to the cleavage of pro-IL-1β and pro-IL-18 into their biologically active forms, causing recruitment of inflammatory cells, production of INF-γ, and enhancement of natural killer cell activity [45]. One inflammasome in particular NLRP6 appears to have a critical role in controlling intestinal homeostasis; NLRP6 deficiency has been associated with: decreased levels of IL-18, increased concentrations of Bacteroidetes and the bacterial phylum TM7 enhanced activation of MAP kinase and NF-Kβ upon TLR ligation defective autophagy of goblet cells, impaired mucin secretion into the gut lumen and improved resistance to infection with Listeria, Salmonella, and E. coli [62–66]. As such NLRP6 may serve to dampen certain inflammatory signals by promoting bacterial dissemination and colonization of systemic organs while at the same time clearing enteric pathogens from the mucosal surface to maintain intestinal homeostasis.
In this manner inflammasome function is intrinsically related to the gut microbiota and regulation of TLR activation, which also has an important role in controlling the progression of liver injury. This has been evidenced in animal studies showing that NLRP6 and NLRP3 along with IL-18 negatively regulate progression of injury in NAFLD and NASH [64]. Further, inflammasome deficiency may lead to increased TLR4 and TLR9 agonists into the portal circulation, thereby triggering increased inflammation and driving progression of the injury mainly through hepatic TNF-α production. A key regulator of this increased TLR4 and TLR9 agonist production may be microbiota-induced subclinical colonic inflammation through chemokine CCL5 secretion [64]. Additionally, some of the metabolic alterations in these inflammasome-deficient mice can be horizontally spread with the resulting altered gut microbiota negatively impacting NAFLD progression [64].
Proinflammatory Cytokines
Both the TLRs and the NLRs ultimately affect downstream pathways that lead to alterations in the levels of proinflammatory cytokines. Among these cytokines TNF-α and IL-1β are the major cytokines driving liver injury and progression of NAFLD. The primary role of TNF-α is in the regulation of immune cells. Dysregulation of TNF production has been implicated in a variety of human diseases including a spectrum of rheumatologic diseases and inflammatory bowel disease. Animal models have also shown that TNF-α and IL-1β deficiencies confer resistance to NAFLD and NASH respectively while on a HFD [67,68].
The cytokine TNF-α is involved in a number of pathways that can ultimately affect the predisposing factors for NAFLD. Most importantly TNF-α cause increased insulin resistance through alteration of insulin receptor function and also increasing cholesterol accumulation in hepatocytes through the inhibition of LDL receptors and efflux transporters. [12]. Increased lipid levels in these hepatocytes alter normal signaling and lead to an increase in reactive oxygen species which drives cell death signaling. In addition increased cholesterol accumulation with in hepatocytes can result in increased TLR4 through suppression of the endosomal-lysosomal degradation pathway of TLR4 [52]. In recent years researchers have been able to identify that Kupffer cells resident macrophages in the liver seem to play a crucial role in detecting DAMPs and activating inflammasome responses. Studies with human biopsies have shown an increase in CD68 a pan-macrophage marker in patients with NASH as compared with simple steatosis and that Kupffer cell depleted animals develop less features of NASH [2]. Additionally, Kupffer cell phagocytosis of excess cholesterol also leads to increased expression of proinflammatory cytokines and TLR4 activation [52]. Similarly, IL-1β is involved in lipid accumulation within hepatocytes however IL-1β suppresses PPARα causing accumulation of triglycerides within hepatocytes, thereby leading to increased expression of pro-apoptotic pathways [12].
Bottom-line
The immune response to changes in the gut microbiota is complex and multifocal; however, it remains clear that increased knowledge of these pathways leads to a more comprehensive understanding of the potential interactions between the risk factors for NAFLD and cellular immunity. This immune response is an important factor in driving the progression of NAFLD to NASH through induction of inflammation. Additionally these immune pathways may serve as potential therapeutic targets, as restoring the normal microflora has been shown to attenuate NAFLD in a number of animal studies.
Treatment Options for Altering the Gut Microbiota
There is a number of treatment options available for NAFLD aimed at a variety of pathways involved in the development of NAFLD. Among these the use of diabetes medications clearly functions to combat the increased insulin resistance, while pentoxyfylline aims to decrease levels of inflammation. In this manner available treatment options focus on different aspects of disease pathogenesis including risk factors and progression. In our review we will focus on those treatments options that alter the gut microbiota as a predominant mechanism of action. These include antibiotics, prebiotics, and probiotics.
Antibiotics
At present there is no concise evidence supporting the use of antibiotics in the treatment of NAFLD. There are, however a number of potential mechanisms by which antibiotics can alter the gut microbiota in favor of attenuating the severity of NAFLD. As discussed earlier in the paper levels of endotoxemia and inflammation secondary to activation of TLRs by microbial products represents major factors in the progression of NAFLD liver injury. Antibiotic administration leading to a reduction in these bacterial products in particular LPS would then theoretically attenuate the inflammation. This in turn would allow for decreased intestinal permeability through increased expression of tight junction proteins. Both of these mechanisms are supported by evidence from animals models where antibiotics decreased circulating LPS and TLR4 activation in addition to increasing expression and function of tight junction proteins [69,70].
Rifaximin a non-absorbable antibiotic is one potential candidate for the treatment of NAFLD. Rifaximin has been shown in a number of studies to improve liver injury in patients with cirrhosis, most notably for its effects in treating hepatic encephalopathy [71-73]. Currently there is an ongoing randomized trail assessing efficacy of rifaximin in NAFLD/NASH through measurements of proinflammatory cytokine and endotoxin levels including TNF-α and TLR4 activation [74]. Given the high cost and adverse effects associated with chronic antibiotic use, however results of this study and others will be needed before rifaximin or other antibiotics can be recommended as a therapeutic option for NAFLD.
Prebiotics
Prebiotcs were originally defined as “nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon”; however they are now more loosely defined as “selectively fermented ingredients that allow specific changes both in the composition and/or activity in the gastrointestinal microflora that confer benefits.” [75-77]. In order for a food to be classified as a prebiotic it must resist gastric acidity, hydrolysis by mammalian enzymes and absorption in the upper gastrointestinal tract, such that it is able to be fermented by the gut microbiota into short-chain fatty acids (SCFAs),including acetate, propionate and butyrate, that can be used for energy [12,77]. The primary prebiotics used the two inulin-type fructans oligofructose (OFS) and fructo-oligosaccharides (FOS) and the galactan, galacto-oligosacchardies (GOS) [78,79]. The fructans are the most extensively studied prebiotics for use in metabolic syndrome with the differences between the fructans being only the number of repeating units of D-fructose in the polymer chain [78].
The role of prebiotics in the treatment of NAFLD centers largely on the functional roles of improved glucoregulation and modified lipid metabolism [78]. Specific alterations of the gut microbiota by these prebiotics include favored growth of indigenous bifidobacteria and/or lactobacilli and decreased luminal pH which impedes the growth of pathogens [78,79].
Modification of lipid metabolism by prebiotics is centered on regulation of de novo fatty acid synthesis. While healthy individuals usually have minimal hepatic de novo lipogenesis NAFLD patients with hyperinsulinemia can have up to 26% of the hepatic triglyceride content as a result of de novo lipogenesis [8]. Importantly, this increased de novo lipogenesis is also an important phenotypic factor in genetically obese mice, another clinical feature that has strong implications in the development of NAFLD in humans [80]. Prebiotics have been shown to attenuate de novo lipogenesis, likely through a mechanism of action that includes alterations in gene expression of regulatory enzymes for lipogenesis [78]. Additionally, prebiotics may decrease lipogenesis by altering the by-products of microbiota fermentation. Of the SCFA by-products, acetate and propionate are the major constituents delivered to the liver, whereas most butyrate is metabolized in the colon; in the liver, acetate promotes lipogenesis while propionate inhibits lipogenesis [81–83]. One suggested mechanism of prebiotics in NAFLD is an increased ratio of proprionate to acetate, which may promote a decrease in hepatic lipogenesis.
Alteration of the gut microbiota by prebiotics may also affect the levels of proinflammatory cytokines secondary to changes in intestinal permeability and levels of LPS. Using the prebiotic (oligofructose) in mice fed a HFD gut microbiota showed an increase in the levels of Bifidobacterium, which was positively associated with decreased endotoxemia and proinflammatory cytokines as a result of decreased levels of LPS [84]. The complexity of this relationship between the gut microbiota and intestinal permeability is further highlighted as researchers have also shown decreased intestinal permeability and LPS absorption in prebiotic treated mice who have increased production of glucagon-like-peptide 2 [85].
There is currently little data from human studies concerning the use of prebiotics as it pertains to alterations in inflammation with only one randomized placebo controlled pilot study of 7 patients with NASH showing decreased levels of aminotransferases after 8 weeks; however, there is some evidence for prebiotics in lowering lipid levels improving both weight loss and insulin resistance. In eight studies using prebiotics in human subjects with diabetes or hyperlipidemia levels of cholesterol and triglycerides were shown to decrease between 6-20% and 14-27%, respectively [86]. One randomized control trail assigned patients to receive either the prebiotic oligofructose or placebo for 12 weeks and found a significant reduction in weight of 1.03 ± 0.43 kg in the prebiotic group versus a weight gain of 0.45±0.31 kg in the placebo group (P = 0.01) [87]. Additionally patients in the prebiotic group reported a decreased caloric intake that was associated with decreased ghrelin and increased peptide YY levels.
In summary prebiotcs may serve a role in the modification of lipid metabolism by attenuating de novo lipogenesis and alerting byproducts of microbial fermentation. Other potential benefits of prebiotics include decreased intestinal permeability and alteration of gut hormones that may lead to decreased caloric intake. While there is insufficient clinical evidence to support routine use of prebiotics in NAFLD patients the evidence from animal studies supports consideration for the use of prebiotics in select patients who may not have responded to other therapeutic options.
Probiotics
Probiotics are live microoganisms that, when administered in adequate quantities, confer a health benefit to the host [88]. Probiotics have been used in a number of disease processes, including NAFLD, in an attempt to produce a health benefit through the correction of gut dysbiosis. The use of probiotics in NAFLD is focused on the basis that many patients with NAFLD have increased intestinal permeability secondary to small intestinal bacterial overgrowth (SIBO) [11]. As discussed earlier, the increased intestinal permeability results from disruption of intercellular tight junctions and leads to increased translocation of bacterial products into the bloodstream, causing increased endotoxemia and delivery of these products to the liver activating inflammatory cytokines. There are several different mechanisms to justify a potential role for the use of probiotics in the treatment of NAFLD. First, probiotics have been shown to produce a number of antimicrobial factors which lead to a decreased pH and inhibition in the growth of pathogenic gram negative bacteria [89]. In addition, some probiotic strains can compete with and displace pathogenic bacteria from epithelial surface receptors in the gut [89]. Intestinal permeability is also improved as lactobacillus and bifidobacteria mixtures have been shown to increase mucin secretion through upregulation of the mucin producing genes MUC2 and MUC3 [89]. Overall, the activity of probiotics should lead to improvements in NAFLD by partially correcting the dysbiosis of the gut microbiota and by limiting SIBO and its resultant increased intestinal permeability and endotoxemia.
The efficacy of probiotics in NAFLD animals models has been well established in a variety of Lactobacillus species, with a number of studies showing reductions in LDL, cholesterol and triglycerides along with histological improvement and amelioration of the inflammation and steatosis [89]. Despite this, there have been a limited number of human trials investigating the efficacy of probiotics in NAFLD largely related to the complex pathology of the disease and the ethical considerations required with invasive diagnostic procedures and histological sampling. To date the best clinical evidence in humans comes from a recent meta-analysis covering 134 patients from four randomized control trials receiving probiotics (including Lactobacillus, Bifidobacterium, and Streptococcus species) for the treatment of NAFLD or NASH. Results showed that compared to placebo probiotics significantly decreased ALT, AST, total cholesterol, HDL and TNF-α1; however, no significant changes in BMI, glucose or LDL1 were associated with probiotic use [90]. Some limitations exist when interpreting this data namely the difficulties in ascertaining changes in liver fatty infiltration as it requires a histologic specimen. Of the three studies using histologic analysis only one had post-treatment histology results. The remaining study used ultrasonography which cannot identify fatty infiltration of the liver below a threshold of 30% [90]. Lastly there remains a potential for confounding as dietary restrictions exercise and physical activity were not reported.
In summary probiotics appear to be a potential treatment option for NAFLD. Numerous studies have shown improvements in the intestinal dysbiosis leading to decreasing intestinal permeability endotoxemia and subsequent inflammation. While the majority of evidence supporting the use of probiotics is from animal studies with only a few clinical trials given the technical difficulties of performing this research in humans the positive findings from the clinical trials should be encouraging for efficacy of probiotics in NAFLD.
Bottom-line
There are number of potential therapeutics roles for antibiotics, prebiotics and probiotics in the treatment of NAFLD based on alterations of the gut microbiota. While currently there is limited evidence to support the use of antibiotics both prebiotcs and probiotcs have encouraging results in animal studies for improving the gut dysbiosis and potentially inducing a clinical benefit in NAFLD patients. As such clinicians should be aware of these options and consider them for patients either not responding to other treatment approaches or who desire an adjunctive treatment option.
Conclusion
The global epidemic of obesity and the increasing prevalence of type 2 diabetes has propelled NAFLD as the most common chronic non-viral liver disease. The complication of NASH in this population is formidable given the numbers of patients affected. Additionally, the burden of NAFLD on the healthcare system is expected to increase, as by 2020 this is projected to be the number one indication for liver transplantation in the US [2]. Accordingly, it is essential that clinicians understand the modifiable risk factors for NAFLD. A summary of the currently available data and our core tips are provided in Table 1.
| Risk Factor | Pathogenesis | Current Evidence | Core Tip |
|---|---|---|---|
| Obesity | Increased Firmicutes/Decreased Bacteroidetes in comparison to lean counterparts Suppression of Fiafleads to increased lipoprotein lipase (LPL), thereby increasing cellular uptake of fatty acids and adipocyte triglyceride accumulation Increased SCFAs induce the release of peptide YY (PYY) | Germ-free mice fed a high-fat, high-sugar diet were resistant to development of obesity; introduction of gut flora led to increased body weight, body fat, increased hepatic lipogenesis, and fat deposition [11,16,17] Colonization of gut with Bacteroidetesthetaiotaomicron and Methanobrevibactersmithii leads to increased suppression of Fiaf and subsequent obesity [26] Excess Firmicutes can result in increased SCFA production and increased calorie absorption via PYY, ultimately leading to obesity; further, excess SCFAs can be converted into triglycerides within the liver increasing hepatic steatosis [11,19,20] | Obesity is clearly a strong risk factor in the pathogenesis of NAFLD with a prevalence twice that of lean comparators. Obese patients may have a characteristic increased Firmicutes/decreased Bacteroidetes within their gut microbiota. Further investigation into the regulation of gut hormones and the regulatory factors for lipolysis and lipogenesis will help expand our understanding of the relationship between the gut microbiota and obesity in NAFLD. |
| Insulin Resistance | Primarily driven by inflammatory mediators and immune responses Link between insulin resistance and liver inflammation through these inflammatory pathways | Gut microbiota can suppress the expression of AMP kinase and thereby predispose the host to insulin resistance [26] Lipopolysaccharide (LPS) from gram negative bacteria can lead to decreased activation of insulin receptor substrate 1 (IRS-1) leading to insulin resistance in muscle and adipose tissue [34,35] LPS can also hamper the activity of LPL, worsening insulin resistance by increasinglevels of circulating fatty acids [34,37] | Insulin resistance affects a wide variety of biochemical pathways involved in the pathogenesis of NAFLD. In particular, it appears to incorporate both the immune responses and inflammatory changes that occur in NAFLD. Currently, specific alterations in the gut microbiota relating to insulin resistance have not been found in NAFLD due to the vast role of insulin signaling in metabolism; however, continued investigation may prove to isolate more specific gut alterations related to insulin resistance. |
| Immune Responses | TLR4 is activated by LPS from gram negative bacteria and leads to inflammatory responses TLR9 can lead to activation of IL-1β and a subsequent increase in liver steatosis, inflammation, and fibrosis TLR2 and TLR5 may play protective roles NLRP6 inflammasome may be protective in the maintenance of intestinal homeostasis by clearing enteric pathogens and dampening bacterial dissemination TNF-α and IL-1β are the major proinflammatory cytokines driving liver injury and progression of NAFLD à NASH | Rodents on a high-fat diet (HFD) have increased inflammation through induction of TLR4, resulting in increased intestinal permeability and endotoxin levels; these changes are not reproducible in TLR deficient mice. Additionally, TLR4 deficient mice are resistant to NAFLD[46,49] TLR9 deficient mice have decreased steatosis, inflammation, fibrosis, insulin resistance and weight gain in comparison to controls.[55] TLR2 deficient mice on a HFD have disrupted tight junctions that was preserved in wild type mice given a TLR2 agonist.[47,60] TLR5 deficient mice showed obesity and steatohepatitis that was exacerbated by a HFD. [61] NLRP6 deficiency has been associated with decreased IL-8, increased Bacteroidetes, defective autophagy of goblet cells, impaired mucin secretin into gut lumen, and enhanced activation of MAP kinase and NF-Kβ upon TLR binding [62-66]. TNF-α can increase insulin resistance by altering insulin receptor function; additionally, it can increase cholesterol accumulation in hepatocytes through inhibition of LDL receptors and efflux transporters. [12] IL-1β suppresses PPARα causing accumulation of triglycerides within hepatocytes and increasing expression of pro-apoptotic pathways. [12] | Disruption of the normal gut microbiota can result in altered immune system activation. These immune responses affect a number of pathways related to the risk factors for NAFLD. Additionally, they can promote the progression of NAFLD to NASH through increased inflammation. Particularly important to the maintenance of gut homeostasis are the control of intestinal permeability and the levels of bacterial products, which can activate TLRs, triggering additional immune responses. |
Table 1: Summary of NAFLD risks, pathogenesis, current evidence and core tips
The gut microbiota has long been understood to play a role in the pathogenesis of various diseases; however recent advances in technology have greatly increased our ability to analyze to composition of the gut microbiota and its alterations relative to specific diseases. Current evidence strongly supports the existence of certain characteristic changes in the gut microbiota affecting signaling pathways and immune responses which play a role in the development and progression of NAFLD. Additionally the gut microbiota may be a potential effective therapeutic target for improving outcomes associated with NAFLD.
References
- Arab JP, Candia R, Zapata R, Muñoz C, Arancibia JP, et al. (2014) Management of nonalcoholic fatty liver disease: an evidence-based clinical practice review. World J Gastroenterol 20: 12182-12201.
- Machado MV, Cortez-Pinto H1 (2014) Non-alcoholic fatty liver disease: what the clinician needs to know. World J Gastroenterol 20: 12956-12980.
- Sattar N, Forrest E2, Preiss D3 (2014) Non-alcoholic fatty liver disease. BMJ 349: g4596.
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, et al. (2012). The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55:2005–2023.
- Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K (2013) Gut microbiota and non-alcoholic fatty liver disease: new insights. ClinMicrobiol Infect 19: 338-348.
- Giorgio V, Alisi A, Kazem HM, Monti S, Nobili V (2014) NASH and the Cross-Talk Between the Gut and Liver. CurrPediatr Rep 2:211–217.
- Lambert JE, Ramos-Roman MA2, Browning JD3, Parks EJ4 (2014) Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146: 726-735.
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, et al. (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343-1351.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752-1761.
- Tateya S, Kim F, Tamori Y (2013) Recent advances in obesity-induced inflammation and insulin resistance. Front Endocrinol (Lausanne) 4: 93.
- Gangarapu V, Yıldız K, Ince AT, Baysal B (2014) Role of gut microbiota: obesity and NAFLD. Turk J Gastroenterol 25: 133-140.
- Miura K, Ohnishi H1 (2014) Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol 20: 7381-7391.
- Machado M, Marques-Vidal P, Cortez-Pinto H (2006) Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol 45: 600-606.
- Anstee QM, Targher G, Day CP (2013) Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev GastroenterolHepatol 10: 330-344.
- Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, et al. (2007) Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30: 1212-1218.
- Goel A, Gupta M, Aggarwal R (2014) Gut microbiota and liver disease. J GastroenterolHepatol 29: 1139-1148.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, et al. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027-1031.
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, et al. (2005) Obesity alters gut microbial ecology. ProcNatlAcadSci U S A 102: 11070-11075.
- Musso G, Gambino R, Cassader M (2010) Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: mechanisms and implications for metabolic disorders. CurrOpinLipidol 21: 76-83.
- Tilg H, Kaser A (2011) Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 121: 2126-2132.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022-1023.
- Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, et al. (2010) Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18: 190-195.
- Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, et al. (2008) Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 32: 1720-1724.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, et al. (2011) Enterotypes of the human gut microbiome. Nature 473: 174-180.
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, et al. (2004) The gut microbiota as an environmental factor that regulates fat storage. ProcNatlAcadSci U S A 101: 15718-15723.
- Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. ProcNatlAcadSci U S A 104: 979-984.
- Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, et al. (2009) Human gut microbiota in obesity and after gastric bypass. ProcNatlAcadSci U S A 106: 2365-2370.
- Parekh PJ, Arusi E, Vinik AI2, Johnson DA3 (2014) The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. Front Endocrinol (Lausanne) 5: 47.
- Shen J, Obin MS, Zhao L (2013) The gut microbiota, obesity and insulin resistance. Mol Aspects Med 34: 39-58.
- Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, et al. (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7: e35240.
- Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, et al. (2011) Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoSBiol 9: e1001212.
- Spor A, Koren O, Ley R (2011) Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9: 279-290.
- Raso GM, Simeoli R, Iacono A, Santoro A, Amero P, et al. (2014) Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J NutrBiochem 25:81–90.
- Caricilli AM, Saad MJ (2013) The role of gut microbiota on insulin resistance. Nutrients 5: 829-851.
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, et al. (2007) Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56: 1986-1998.
- Chung S, LaPoint K, Martinez K, Kennedy A, Sandberg MB, et al. (2006)Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 147:5340–5351.
- Carvalho-filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira BC, et al. (2005) S-Nitrosation of the insulin receptor , insulin receptor substrate , and protein kinase B/Akt: A novel mechainism of insulin resistance. Diabeteses 54:959–967.
- Schertzer JD, Klip A (2011) Give a NOD to insulin resistance. Am J PhysiolEndocrinolMetab 301: E585-586.
- Zhao L, Hu P, Zhou Y, Purohit J, Hwang D (2011) NOD1 activation induces proinflammatory gene expression and insulin resistance in 3T3-L1 adipocytes. Am J PhysiolEndocrinolMetab 301: E587-598.
- Tamrakar AK, Schertzer JD, Chiu TT, Foley KP, Bilan PJ, et al. (2010) NOD2 activation induces muscle cell-autonomous innate immune responses and insulin resistance. Endocrinology 151: 5624-5637.
- Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277: E1-10.
- Amar J, Chabo C, Waget A, Klopp P, Vachoux C, et al. (2001) Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3:559–572.
- Day CP, James OF (1998) Steatohepatitis: a tale of two "hits"? Gastroenterology 114: 842-845.
- Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52: 1836-1846.
- Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10: 241-247.
- Kim KA, Gu W, Lee IA, Joh EH, Kim DH (2012) High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7: e47713.
- Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, et al. (2007) Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol 47: 571-579.
- Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, et al. (2001) Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J PhysiolGastrointest Liver Physiol300:G433–441.
- Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, et al. (2007) C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50:1267–1276.
- Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, et al. (2012) Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab 16:44–54.
- Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, et al. (2009) Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol 51:168–175.
- Takaki A, Kawai D, Yamamoto K (2013) Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J MolSci 14: 20704-20728.
- Creely SJ, McTernan PG, Kusminski CM, Fisher ff M, Da Silva NF, et al. (2007) Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J PhysiolEndocrinolMetab 292:E740–7.
- Ye D, Li FY, Lam KS, Li H, Jia W, et al. (2012) Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut 61: 1058-1067.
- Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, et al. (2010) Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139: 323-334.
- Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, et al. (2010) Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 53: 1795-1806.
- Himes RW, Smith CW (2010) Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J 24: 731-739.
- Rivera CA, Gaskin L, Allman M, Pang J, Brady K, et al. (2010) Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol 10: 52.
- Szabo G, Velayudham A, Romics L Jr, Mandrekar P (2005) Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol ClinExp Res 29: 140S-145S.
- Cario E, Gerken G, Podolsky DK (2007) Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359-1374.
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, et al. (2010) Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228-231.
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, et al. (2011) NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145: 745-757.
- Chen GY, Liu M, Wang F, Bertin J, Núñez G (2011) A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol 186: 7187-7194.
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, et al. (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482: 179-185.
- Wlodarska M, Thaiss CA2, Nowarski R3, Henao-Mejia J3, Zhang JP4, et al. (2014) NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156: 1045-1059.
- Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, et al. (2012) NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488: 389-393.
- Salles J, Tardif N, Landrier JF, Mothe-Satney I, Guillet C, et al. (2012) TNFa gene knockout differentially affects lipid deposition in liver and skeletal muscle of high-fat-diet mice. J NutrBiochem 23:1685–1693.
- Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, et al. (2011) Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol 55: 1086-1094.
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, et al. (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470-1481.
- Bergheim I, Weber S, Vos M, Krämer S, Volynets V, et al. (2008) Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 48: 983-992.
- Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, et al. (2013) Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 8: e60042.
- Bajaj JS, Hylemon PB, Younossi Z (2012). The Intestinal Microbiota and Liver Disease. Am J GastroenterolSuppl 1: 9–14.
- Kalambokis GN, Tsianos EV (2012) Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology 55: 655-656.
- NCT02009592. Efficacy of Rifaximin on Hepatosteatosis and Steatohepatitis Patients. .
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125: 1401-1412.
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17: 259-275.
- Slavin J1 (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5: 1417-1435.
- Parnell JA, Raman M, Rioux KP, Reimer RA (2012) The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int 32: 701-711.
- Macfarlane S, Macfarlane GT, Cummings JH (2006) Review article: prebiotics in the gastrointestinal tract. Aliment PharmacolTher 24: 701-714.
- Parnell JA, Reimer RA (2010) Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose-response study in JCR:LA-cp rats. Br J Nutr 103: 1577-1584.
- Daubioul C, Rousseau N, Demeure R, Gallez B, Taper H, et al. (2002) Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zuckerfa/fa rats. J Nutr 132: 967-973.
- Levrat MA, Rémésy C, Demigné C (1991) High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J Nutr 121: 1730-1737.
- Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81: 1031-1064.
- Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, et al. (2007) Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50: 2374-2383.
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, et al. (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091-1103.
- Beylot M1 (2005) Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br J Nutr 93 Suppl 1: S163-168.
- Parnell JA, Reimer RA (2009) Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J ClinNutr 89: 1751-1759.
- Sanders ME1 (2008) Probiotics: definition, sources, selection, and uses. Clin Infect Dis 46 Suppl 2: S58-61.
- Iacono A, Raso GM, Canani RB, Calignano A, Meli R (2011) Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J NutrBiochem 22: 699-711.
- Ma YY, Li L, Yu CH, Shen Z, Chen LH, et al. (2013) Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 19: 6911-6918.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 16681
- [From(publication date):
December-2014 - Sep 01, 2025] - Breakdown by view type
- HTML page views : 11900
- PDF downloads : 4781