Research Article Open Access
Novel Microchip Technique for the Transfer of Spheroids as Floating Cultures to Micropatterned-Adherent Cultures
| Sakai Y1,2 and Nakazawa K1* | |
| 1Department of Life and Environment Engineering, The University of Kitakyushu, Hibikino, Wakamatsu-ku, Kitakyushu, Fukuoka 808-0135, Japan | |
| 2Research Fellow of the Japan Society for the Promotion of Science (JSPS), Hibikino, Wakamatsu-ku, Kitakyushu, Fukuoka 808-0135, Japan | |
| Corresponding Author : | Kohji Nakazawa Department of Life and Environment Engineering The University of Kitakyushu, Hibikino Wakamatsu-ku, Kitakyushu Fukuoka 808-0135, Japan Tel: +81-93-695-3292 Fax: +81-93-695-3359 E-mail: nakazawa@kitakyu-u.ac.jp |
| Received June 18, 2010; Accepted December 05, 2011; Published December 10, 2011 | |
| Citation: Sakai Y, Nakazawa K (2011) Novel Microchip Technique for the Transfer of Spheroids as Floating Cultures to Micropatterned-Adherent Cultures. J Biochip Tissue chip S4:001. doi:10.4172/2153-0777.S4-001 | |
| Copyright: © 2011 Sakai Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
Abstract
Spherical multicellular aggregate (spheroid) culture has attracted attention as useful technique for tissue engineering or regenerative medicine research and cell-based assay studies. Although spheroids are generated using floating culture method on non-adhesive surface, the transfer of floating spheroids to adherent culture holds importance for various applications. In this paper, we successfully established a novel microchip technique which allowed the formation of spheroids with uniform size and their transfer from a floating condition to a micropatterned adherent culture. A spheroid transfer chip (ST chip) contained 270 microwells (600 ?m in diameter, 600 ?m in depth, and 660 ?m in pitch) composed of a through-hole PMMA frame and a PDMS sheet, and the surface of microwells was modified with PEG to create non-adhesive surface. Mouse ES cells, NIH 3T3 cells, HepG2 cells, and rat primary hepatocytes spontaneously formed spheroids in each microwell of the chips. The micropatterned adherent culture of spheroids was realized by simple procedures, which the ST chip was flipped onto a collagencoated dish, and then the PDMS sheet was peeled off after the spheroids fell onto the dish. The transferred spheroids gradually spread on the collagen-coated dish, and the overlap of cells extending from each spheroid occurred in order of mouse ES cells, 3T3 cells, HepG2 cells, and primary rat hepatocytes. This difference may be due to cell proliferation ability, cell viability in the spheroid, and spheroid size. Because the chip characteristics such as the microwell size, the pitch between spheroids, spheroid sizes, and spheroid number can be designed according to the experimental needs, this chip technique is a promising tool for spheroid study.
| Keywords |
| Spheroid; Micropatterned culture; Transfer; Microchip; Mouse ES cells; 3T3 cells; HepG2 cells; Primary rat hepatocytes |
| Introduction |
| Spherical multicellular aggregates (spheroids) of various animal cells such as hepatocytes, ES cells, and neural cells are utilized for tissue engineering, regenerative medicine research, toxicological assays, and fundamental research. For example, hepatocyte spheroids display a tissue-like architecture and can maintain high specific functions for a long term [1–3]. Furthermore, spheroids of embryonic stem (ES) cells, named embryonic bodies (EBs), have been widely used as the first step of ES cell differentiation [4,5]. Although many researchers reported that the control of spheroid size is extremely important from the viewpoint of their central necrosis and/or the differences of cell differentiation [6–8], mass production of spheroids of uniform size by traditional spheroid methods such as hanging drop, rotational, and round-bottomed 96-well cultures is difficult. On the other hand, microchips containing multiple microwells are usually efficient in solving these problems. Indeed, it has been reported that microwell chips can produce a large number of homogeneous spheroids [9–12]. Spheroids are generated using floating culture method on non-adhesive surface such as microwell chips. The transfer of floating spheroids to adherent culture holds importance in biological research. For example, floating spheroids of ES cells must be moved onto an extracellular matrix (ECM)-coated substrate for a subsequent differentiation step [13,14]. However, adherence results in random organization and/or fusion of spheroid. Therefore, it is difficult to control the culture microenvironment of adhered spheroids and to evaluate individual spheroids cultured on ECM substrates. For transfer from floating culture to micropatterned adherent culture, Rosenthal et al. have established a cell micropatterned method by using a bio flip chip having microwell structure [15], while Kang et al. have developed a multi-layer microfludic array chip which spheroids can transfer to adherent culture without manual cell retrieval [16]. However, to our knowledge, there is no technique that floating spheroids are transferred to micropatternedadherent culture in stationary condition. In this paper, we established a novel microchip technique that allows the formation of spheroids with uniform size and their transfer from a floating condition to micropatterned adherence onto an ECM substrate. This system composed of a through-hole poly(methyl methacrylate) (PMMA) frame and a poly(dimethylsiloxane) (PDMS) sheet, the spheroid transfer was realized by simple concept that peel off the PDMS sheet of the flipped chip. |
| Materials and Methods |
| Preparation of spheroid transfer chip |
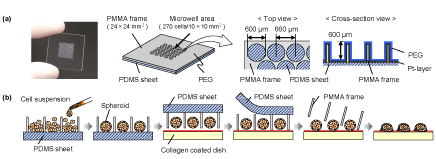
| Figure 1a shows a schematic diagram of the novel microchip termed “spheroid transfer chip (ST chip)”. This microchip consisted of a through-hole PMMA frame and a PDMS (Sylgard 184; Dow Corning, Midland, MI, USA) sheet. The PMMA frame comprised 270 throughholes bored in a triangular arrangement by using a programmable micro-milling system (PMT Corp., Fukuoka, Japan); each circular hole was 600 μm in diameter, 600 μm in depth, and 660 μm in pitch. In order to configure the culture microwells appropriately, the PDMS sheet (thickness, 400 μm) was adhered directly to the PMMA frame. The surface of microchip was coated with a 6-nm-thick layer of platinum using an ion-sputtering unit (Hitachi Science Systems Ltd., Ibaragi, Japan). It was immersed in 2.5 mM PEG-SH (molecular weight, 30,000) (NOF Co., Tokyo, Japan) in 50% ethanol solution to create non-adhesive cell surface and then thoroughly rinsed with 50% ethanol and distilled water for sterilization and removal of any unbound PEG-SH. Finally, the surface was covered with culture medium until use. |
| Preparation of various animal cells |
| Mouse ES cells (129SV; DS Pharma Biomedical Co. Ltd., Osaka, Japan) were subcultured on an inactivated mouse embryonic fibroblast (MEF; ReproCELL Inc., Tokyo, Japan) feeder layer in a 100-mm gelatin-coated dish (BD Biosciences, CA, USA) containing 10-mL Dulbecco’s modified Eagle’s medium (DMEM; DS Pharma) supplemented with 15% fetal bovine serum (FBS; DS Pharma), 1% nonessential amino acids (NEAA; DS Pharma), 1% nucleosides (DS Pharma), 110-μM 2-mercaptoethanol (DS Pharma), 1% glutamine, 1% penicillin, 1% streptomycin, and 1000 U/mL leukemia inhibitory factor (LIF; Wako Pure Chemical Industries Ltd., Osaka, Japan). The ES cell suspension was obtained by treating the confluent monolayer formed on the dish with 0.25% trypsin (DS Pharma). NIH 3T3 cells (JCRB0615; Health Science Research Resources Bank, Japan Health Science Foundation, Osaka, Japan) and HepG2 cells (RCB1648; RIKEN Cell Bank, Ibaraki, Japan) were cultured in DMEM (Invitrogen Corp., Carlsbad, Calif., USA) and Williams’ medium E (Sigma, St. Louis, MO, USA), respectively; each medium was supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Each cell suspension was obtained by treating the confluent monolayer formed on the tissue culture dish with 0.25% trypsin (Invitrogen). Hepatocytes were isolated from the whole liver of an adult Wistar rat (male, 7–8 weeks old, weighing approximately 200 g) by liver perfusion using 0.05% collagenase (Wako) [17]. The cell viability was determined by using the trypan blue exclusion method, and the cells with more than 85% viability were used for this study. The hepatocytes were cultured in the following medium: DMEM (Invitrogen) supplemented with 10 mg/L insulin (Sigma), 7.5 mg/L hydrocortisone (Wako), 50 μg/L epidermal growth factor (EGF; Biomedical Technologies Inc., Stoughton, MA, USA), 60 mg/L proline (Wako), 50 μg/L linoleic acid (Sigma), 0.1-μM CuSO4 5H2O, 3 μg/L H2SeO3, 50-pM ZnSO4 7H2O, 100 U/mL penicillin, and 100 μg/mL streptomycin. |
| Spheroid transfer process |
| Figure 1b shows the process of spheroid transfer onto collagen-coated dishes with arrangement. Cell suspensions of mouse ES cells, 3T3 cells, HepG2 cells, and rat primary hepatocytes were inoculated onto the ST chip at a density of 1.0 × 105 cells/chip, and a single spheroid in each microwell was formed in a floating condition (the detailed procedures have been already reported [12]). These ST chips that contained spheroids were contacted to pre-wetted collagen-coated dish (Asahi Techno Glass, Tokyo, Japan) held vertically and flipped in 3-day-old cultures. Two milliliters of culture medium were added to the dish after the ST chip was fixed with a stainless ring, and the PDMS sheet was peeled off after spheroids fell onto the collagen-coated dish (within 10 minutes after the ST chip was flipped). The stainless ring and the PMMA frame were removed after the spheroids adhered onto the dish. |
| Evaluation of spheroid adhesion |
| To evaluate the spheroid adhesion area at each time point, phase-contrast micrographs of the spheroids adhered onto the collagen-coated dish were captured. The spheroid extension area was measured by using the WinROOF software (Mitani Corp., Fukui, Japan). The data are expressed as the mean ± standard deviation (SD) of 15 spheroids. |
| Cell viability assay |
| Calcein-AM and propidium iodide (PI) were used to identify live and dead cells, respectively (Cellstain Double Staining Kit; DOJINDO, Kumamoto, Japan). The assay solution mixing calcein-AM and PI was prepared by adding 10 μL of 1mmol/mL calcein-AM stock solution and 15 μL of 1.5 mmol/mL PI solution to 5 mL of phosphate buffered saline (PBS). Cells were incubated with the assay solution for 15 min and then washed with PBS solution. The double-stained cells were assessed using a fluorescence microscope (Biorevo BZ-9000; Keyence, Osaka,Japan). |
| Results and Discussion |
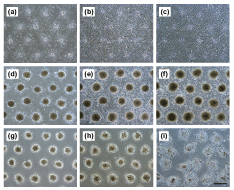
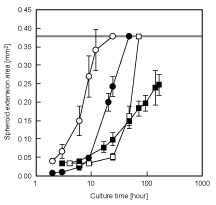
| Figure 2,3 show the phase-contrast micrographs of the formation process of spheroids on the ST chip and of the transferred spheroids. Mouse ES cells, 3T3 cells, HepG2 cells, and rat primary hepatocytes formed spheroids within 3 days of culture on the ST chip (Figure 2a,b). The contact between the ST chip and the collagen-coated dish and the peeling-off of the PDMS sheet were achieved without any air bubbles in the microwells or loss of spheroids (Figure 2c). When a dry collagen-coated dish was used, a large number of air bubbles remained in each microwell. As a consequence, most of the spheroids could not establish direct contact with the collagen-coated dish. Therefore, pre-wetting treatment of the material to which the cells can adhere (a collagen-coated dish in the case of this study) is important for efficient transfer of the spheroids. Spheroids of mouse ES cells, 3T3 cells HepG2 cells, and rat primary hepatocytes adhered onto the collagen-coated dish within 2, 2, 4, and 3 hours after the ST chip was flipped, respectively; the PMMA frame could be peeled off without any spheroid losses (Figure 2d). In the spheroid or cell transfer process, the supply of enough oxygen and nutrients is required in order to maintain cell viability. Although Rosenthal et al. solved this problem by installing a spacer gasket between cell flip chip and tissue culture dish [15], we solved by the combination of through-hole PMMA frame and PDMS sheet. The lack of oxygen during transfer processes of spheroids does not happen, since the PDMS sheet has high oxygen diffusion ability and can be peeled off in a short time (approximately 10 minutes) after the ST chip is flipped. Spheroids on the ST chip are supplied enough nutrients and not crushed by the pressure that is formed when the chip contacts the dish, because each microwell of the ST chip is large enough for these spheroids. In addition, the regular adhesion of spheroids is possible because this chip can directly contact to ECM-coated substrate. The spheroid diameters before flipping the ST chip in mouse ES cells, 3T3 cells, HepG2 cells, and rat hepatocytes were 207 ± 14, 100 ± 5, 195 ± 13, and 206 ± 9 μm, respectively. The transferred spheroids gradually spread on the collagen-coated dish (Figure 2e,f,g,h and 3). The spheroids of ES cells adhered and spread immediately and covered over the dish within approximately 12 hours, and the morphology of spheroids was barely distinguishable within 24 hours after the ST chip was flipped (Figure 2e,f,g,h). The spheroid behaviors of 3T3 cells closely resemble ES cells. The 3T3 spheroids also adhered immediately and covered over within approximately 48 hours, and their morphology was barely distinguishable within 72 hours (Figure 3a,b,c). The HepG2 spheroids spread slowly compared to the other cell types and covered over the surface of the dish within 72 hours (Figure 3d and e). Interestingly, HepG2 spheroid morphology was clearly distinguished during the culture period (Figure 3f). On the other hand, rat primary hepatocyte spheroids immediately adhered but slowly spread; their boundaries were almost unrecognizable within 7 days after the ST chip was flipped, but the cells did not entirely cover the dish surface (Figure 3g,h,i). Change in the spheroid extension areas are shown at (Figure 4). The linear threshold was set at 0.377 mm2/spheroid—value at which the cells extending from the spheroids overlapped (maximum area). The overlap of cells extending from each spheroid occurred in order of mouse ES cells, 3T3 cells, and HepG2 cells. This difference may be due to cell proliferation ability, cell viability in the spheroid, and spheroid size. To evaluate the cell viability, the adhered HepG2 cells on the collagen-coated dish after 15 days of culture from spheroid transfer were stained by Calcein-AM and PI (Figure 5). Many cells extending with the disintegration of spheroid were viable cells (green). Although some dead cells (red) were observed, there were many of dead cells in the spheroid area. Many researchers including us have reported that large spheroid of more than 100 – 150 μm occurs to the central necrosis, and the cell proliferation activity is active in the periphery region of spheroid [6,7,18,19]. In this study, we used HepG2 spheroid of approximately 200 μm in diameter. Therefore, the observed dead cells may occur in the spheroid generating process on the ST chip, and after spheroid transfer, the dead cells may gradually move to the outside area of spheroid from its center area with the extension of viable cells. The problem of central necrosis in the spheroid can solve by the use of small spheroids. microstencil methods, which the spheroids are formed by proliferation from cell monolayer on micropatterned spots [19–21]. In these methods, however, it is difficult that the formed spheroids under floating condition are directly micropatterned onto ECM-coated substrate. In contrast, our technique can easily micropattern the floating spheroids. Although we designed an ST chip with certain characteristics in this study, the chip characteristics such as the microwell size, the pitch between spheroids, spheroid sizes, and spheroid number can be designed according to the experimental needs [11,12]. In addition, culture times of spheroid transfer should be considered in this assay because the cell compaction levels of spheroids may relate to spheroid disintegration. Furthermore, the kinds of ECMs would be freely chosen in this technique. Thus, this microchip technique will be utilized for the evaluation of spheroid-spheroid and spheroid-ECM interactions. |
| Conclusions |
| We thus developed a novel microchip composed of a through-hole PMMA frame and a PDMS sheet. This microchip technique allowed the formation of spheroids with uniform size and their transfer from a floating condition to a micropatterned adherent culture. In this study, we designed a microwell chip 600 μm in diameter for spheroid formation and a collagen-coated dish for culturing adherent spheroids. However, we found that spheroid behavior greatly differed from cell to cell. Thus, this chip technique is a promising tool for studying spheroid-spheroid and spheroid-ECM interactions under controlled microenvironments. |
| Acknowledgements |
| This work was partly supported by a grant from the Regional Innovation Cluster Program (Global Type – 2nd Stage) and a Grant-in-Aid for Scientific Research (C) (23560949) from 18 the Ministry of Education, Culture, Sports, Science and Technology (MEXT). |
| References |
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13742
- [From(publication date):
specialissue-2011 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 9163
- PDF downloads : 4579
