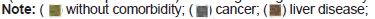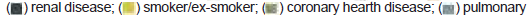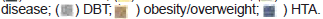Observational Study: Application of Therapy with Equine Serum for Patients Diagnosed with COVID-19 in Tucumán, Argentina
Received: 03-Mar-2022 / Manuscript No. JIDT-22-56065 / Editor assigned: 07-Mar-2022 / PreQC No. JIDT-22-56065(PQ) / Reviewed: 21-Mar-2022 / QC No. JIDT-22-56065 / Revised: 28-Mar-2022 / Manuscript No. JIDT-22-56065(Q) / Published Date: 31-Mar-2022
Abstract
Purpose: The objective of this work is to make an observational study of the usage in moderate or severe state COVID-19 patients of a new therapeutic commercial product obtained after immunization of horses: CoviFab® ELEA F(ab')2 fragmented equine immunoglobulins anti SARS-CoV2.
Methods: Participant centers depend on the Public Health System of Tucumán, Argentina were recruitment. Subjects were assigned to the Moderate Patient Group (MPG) and the Severe Patient Group (SPG), classified according to WHO criteria.
In total, n=84 were enrolled for this study. The subjects were divided into MPG and SPG. All participants were evaluated by physical examination and COVID-19 infection was diagnosed with positive RT-PCR. Each subject received two doses of 0.16 ml/kg, according to the subject's body weight. A generalized linear model with binomial distribution was adjusted for the number of symptoms. Data was analysed using proportion, bivariate and logistic regression. P-value was considered significant at the p<0.05 threshold..
Results: Both groups were similar in age, sex, and comorbidities. A higher proportion of patient with medical discharge was observed in MPG (91.4%) vs. SPG (55.3%) (p=0.004). MPG showed 9 times more chance of receiving medical discharge than SPG (9.33 CI=[1.65, 52.81]; p=0.012). Then, the chance to get medical discharge was independent of variables sex, age, and comorbidities.
Conclusion: Treatment with Equine Serum in patients with moderate and severe disease of COVID-19 managed to slightly reduce hospitalization time. This treatment improved the clinical state to obtain medical discharge. The bivariate analysis showed 8 times more chance in MGP versus SGP to receive of medical discharge and this chance was independent of the pre-existent comorbidities.
Keywords
Equine serum; COVID-19; Moderate; Severe patient
Introduction
The current Pandemic that has spread worldwide that originated the COVID-19 disease has generated more than 200,000,000 Coronavirus Cases in the world, along with approximately 5,000,000 deaths and more than 300,000,000 recovered patients [1]. In Argentina, despite the containment measures that the government has taken since its inception, the spread of COVID has grown and while the vaccines Thus, while plasma production is ongoing, vaccines are being distributed throughout the country; the population is being vaccinated in all age ranges and in the different possible groups. While these measures continue to be developed, new strategies have emerged to cope with the spread of COVID-19 disease, which are, in addition to social distance and hygienic measures There are medical substances produced from horses such as antibodies, immune serum and other. As these uses are to continue in the future, due to the lack of usage of non-animal alternatives or may be more expensive to produce or even unavailable. It is proven that the large volume of urine and blood that horses produce has been extracted to isolate antibodies proteins and hormones and it is also important to note that they are very low maintenance and easy to handle [2]. Because of this, horses have been useful to pharmaceuticals due to the fact that they have obtained products for human use and other known uses. As described in 1890 by von Behring and Kitasato, the development of equine neutralizing polyclonal antibodies ("antitoxins") to treat diphtheria and tetanus, equine antibodies were used to treat a child sick with diphtheria in 1891 [3]. As horse serum was used for equine-derived therapies, the treatment of humans has been successful for a wide range of diseases or conditions, such as Hemophilus influenzae [4]. Passive immunization of humans is being carried out with equine neutralizing antibodies until today, and this is the case of Ebola virus as well, and Junin virus infections, related to Argentine haemorrhagic fever [5,6]. Patients infected with SARSCoV2 are being treated with equine serum, as this is a new approach and use for this product. [7]. The immunotherapy of Equine polyclonal antibodies (EpAbs), is another solution viable because there is a difficulty in getting Convalescent Plasma donors. To be noted, it also has the benefits of reducing mortality, reducing admission to intensive therapy, reducing the requirement of mechanical respiratory assistance, and achieving clinical improvement (ordinal evaluation scale of the World Health Organization) at 7, 14 and 21 days. EpAbs recognize a vast array of epitopes (limiting the risk of viral escape mutations) and tend to develop greater avidity than monoclonal antibodies (mAbs) for their cognate antigens. Apart from this fact, their manufacture is not complicated and so its development is quick and scales up nowadays for human use. Although in past days the use of EpAbs was disfavoured because of the presence of Fc fragments, the new generation containing highly purified F(ab’)2 fragments is safe and well tolerated [7]. It has been pointed out that the receptor-binding domain (RBD) from the viral Spike glycoprotein elicits high titters of neutralizing antibodies against SARS-CoV-2 when it is used as an immunogen in horses. The binding of RBD to SARS-CoV-2 receptor, human ACE2, was verified and the efficacy of RBD in vivo was tested on horses. The result from this was that RBD triggered high-titer neutralizing antibodies in vivo, and immunoglobulin fragment F(ab’)2 was prepared from horse antisera through removing Fc. RBD-specific F(ab’)2 has shown via a Neutralization test that it inhibited SARS-CoV-2, and showed a potent inhibitory effect on SARS-CoV-2. This candidates RBD-specific F(ab’)2 as therapeutic for SARS-CoV-2 [8].
Objective
The objective of this work is to make an observational study of the usage in moderate or severe state COVID-19 patients of a new therapeutic product obtained after immunization of horses: CoviFab® ELEA F(ab')2 fragmented equine immunoglobulins anti SARS-CoV2 a new product for sale in pharmacy in Argentina.
Primary outcome: Hospital discharge at days 21. Proportion of patients discharged from hospital on days 21 since hospital admission date
Secondary outcomes:
• Relationship of medical discharge or death to sex, age and presence of comorbidities.
• Establish recommendations and guide according their clinical status in order to stop or prevent o more severe states of this illness.
Materials And Methods
Procedure
This is an observational cross-sectional study carried out 21 days after the therapeutic protocol was applied. The convenience sample limited.
Participants
The total group n=90 enrolled patients City San Miguel de Tucumán. The study was conducted between February 2021 and June 2021. The health coverage service was administered by the Health System of Tucumán Province (S.I.P.R.O.S.A, Tucumán, Argentina). The people who agreed to participate in the study gave their informed consent before starting the study (Research Ethics Committee/Health Research Directorate, File Number 49/2020). This study conforms to all CONSORT guidelines and reports the required information accordingly.
Inclusion criteria
Over 18 years of age of any sex.
Patients infected by SARS-CoV-2 confirmed by positive RT-PCR test.
Women of childbearing age with a negative pregnancy test.
Patients with COVID-19 classified according to the NIH scale as (REF NIH?):
Moderate: patients who have evidence of lower respiratory disease by clinical evaluation or imaging and SpO2 ≥ 94% in ambient air;
Severe: patients with any of the following criteria: respiratory rate (>30 breaths per minute); SpO2 at rest <93%; Arterial oxygen partial pressure (PaO2)/inspired oxygen fraction (FiO2) 50%.
Exclusion criteria
Pregnant or lactating.
Children or adolescents under 18 years of age.
Patients who have not completed/signed the informed consent.
Patients with COVID-19 classified according to the NIH scale as
Mild: Clinical symptoms were mild and there were no signs of pneumonia on imaging.
Critical: Patients who present any of the following criteria: Respiratory insufficiency requiring mechanical ventilation; shock; multi-organ failure.
Groups
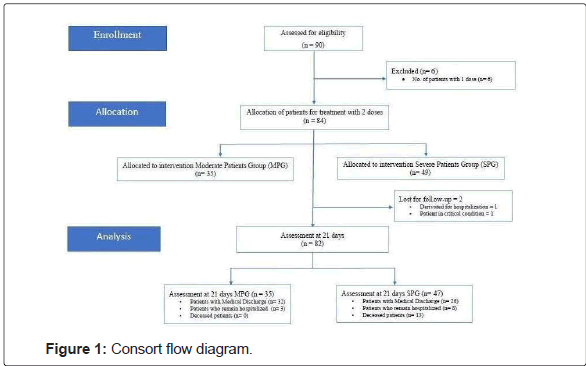
Participant Centres depend on the Public Health System of Tucumán, Argentina were recruitment. Subjects were assigned to the Moderate Patient Group (MPG) and the Severe Patient Group (SPG), classified according to WHO criteria [9]. The data processing group was blinded to analyse the database. Figure 1 shows the CONSORT flow diagram.
Note that 90 patients were initially assessed for eligibility, n=84 recruited, and allocated to this study n=82 distributed in MPG (n=35), and SPG (n=47). The clinical evaluation of symptoms considered to medical discharge was at 21th day. The total number of patients with Medical discharge (n=58); patients who remain hospitalized (n=11); and deceased patients (n=13).
Intervention protocol
Patients enrolled in the protocol received CoviFab® ELEA F(ab')2 fragmented equine immunoglobulins anti SARS-CoV2 mg/mL. Injectable Solution Package Leaflet Approved by ANMAT under special conditions: Hospital use with patient follow-up Informed consent signature [10]. The product is presented in a 10 ml glass vial, with a butyl rubber stopper and an aluminum seal, with a flip-top plastic lid, with a filling volume of 5 ml per vial. Each vial is packaged in an individual cardboard box. The dose of INM005 applied was 4 mg of protein/kg of subject body weight. Each vial contains 25 mg of protein/ml. Therefore, each subject received 0.16 ml/kg. According to the subject's body weight, the number of vials needed to prepare the indicated dose was calculated as:
V=0.16 [ml/kg].W (1)
Where:
[V]=[ml/subject];
W: body weight expressed in [kg]
The calculated INM005 volume was added to the 100 ml physiological saline infusion bag. Two doses of INM005 were administered as an infusion at a rate of 2.0 ml/min over a 50-minute period with a 24-hour interval between doses.
All participants were evaluated by physical examination and COVID-19 infection was diagnosed with positive RT-PCR. Clinical evaluation of symptoms was carried throughout the study period. Enrolled subjects completed symptom questionnaires (including reporting of any adverse effects of treatment), physical examinations and clinical assistance follow-up and received medical discharge 4 weeks after the start of the intervention.
Security definitions
An Adverse Event (AE) was defined as any medical event, sign, symptom, or disease temporarily associated with the use of the medication, which could occur in the subjects enrolled in the study. For the recording of adverse events such as injection-site reactions or hypersensitivity reactions, the same guidelines were followed as those proposed by the work RBD-specific polyclonal F(ab´)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial [11].
Statistics
Categorical variables were summaries with frequencies and percentages, and continuous variables with median and interquartiles. Differences between the categorical variables were estimated using the Chi-square test. A generalized linear model with binomial distribution was adjusted for the number of symptoms, out of a total of eight symptoms associated with COVID-19. The proportion test was applied to compare the proportion of participants with symptoms. Logistic regression was used to model the odds of medical release by sex, comorbidities, and age (Odd Ratio: OR). The level of statistical significance was reached when p<0.05. Calculations were performed using STATA 11.2.
Results
Demographic profile
In total, n=90 were enrolled for this study. The subjects were divided into Moderate Patients Group (MPG: n=35; median=52.0 years old, min=18.0, max=75.0, 10 (28.57%) female) and Severe Patients Group (SPG: n=49; median=51 years old, min=18.0, max=71.0, 7 (14.28%) female), p-Value>0.05. Table 1 shows the demographic profile and descriptions of comorbidity for booth group.
| Variables | COVID-19 moderate patients w/2 doses of ES N=35 | COVID-19 severe patients w/2 doses of ES N=47 | p-Value |
|---|---|---|---|
| Median age (in years) | 52 | 51 | NS |
| Interquartile Range (IQR) | [IQR25: 43; IQR75: 60] | [IQR25: 39; IQR75: 61] | |
| Gender (no. (%) | |||
| Male | 25 (71.43 %) | 41 (87.23 %) | NS |
| Female | 10 (28.57 %) | 6 (12.77 %) | NS |
| Weight (in Kg) | 83 | 85 | NS |
| Interquartile Range (IQR) | [IQR25: 75; IQR75: 98] | [IQR25: 77; IQR75: 98] | |
| Height (in m) | 1.7 | 1.7 | NS |
| Interquartile Range (IQR) | [IQR25: 1.60; IQR75: 1.75] | [IQR25: 1.68; IQR75: 1.75] | |
| BMI | 29.3 | 29.1 | NS |
| Interquartile Range (IQR) | [IQR25: 26; IQR75: 33.2] | [IQR25: 26.1; IQR75: 33.9] | |
| Comorbidity (no. (%)) | 27 (77.14 %) | 38 (80.85 %) | NS |
Note: RR: Respiratory rate; HR: Heart rate; SpO₂: Peripheral oxygen saturation.
Table 1: Demographic profile of patients with COVID-19 who received full Tx (two doses) with Equine Serum.
There were no significant differences in baseline characteristics between the MPG (77.14%) and SPG (80.85%). From the patients who received 2 doses of equine serum, the following patients had some comorbidities: 79.3% (65/82), (p<0.05).
Medical discharge: Patient evolution at 21 days after enrolment
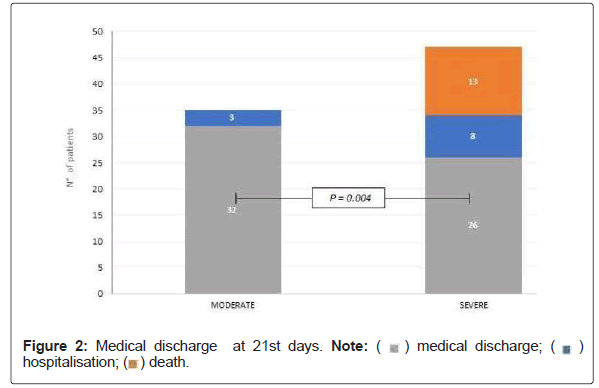
Figure 2 shows that there were significant differences in medical discharge in MPG 91.4% (32/35) compared to SPG 55.3% (26/47) (p=0.004). Being that the SPG 27.1% (13/47) died (p=0.004).
Of the 8 patients who were severely hospitalized, 5 went to ARM, of which 2 died.
The distribution of comorbidities associated with each group is shown in Figure 3.
MPG 77.1% (27/35) and SPG 81.25% (39/47) had some comorbidity (p=0.8). Figure 3 shows the following frequency of HTA, overweight and obesity, and DBT in the MPG relationship to SPG was observed; 66,7% (18/27): 55,3% (21/38); 29,6% (8/27): 44,7% (17/38); and 40,7% (11/27): 18,7% (7/35), respectively, however, this difference was not significant. The comorbidities associated with the 13 deceased patients who received two doses of Equine Serum, 5 of them had more than one underlying disease.
Logistic regression
Bivariate analysis showed 8 times more chance of receiving medical release in MPG than SPG (OR=8.69, 95%, 2.35-33.23, p-Value=0.001). The presence of comorbidity decreases by 11% (OR<1) the chance of being discharged in patients with COVID-19 who received Equine Serum.
The sex and age variables are independent in relation to the chance of receiving medical discharge (p>0.05).
When the logistic regression model was adjusted for multivariate analysis, with the variable medical discharge as the response variable in relation to the variables group (moderate or severe), comorbidity, UTI, sex, and age, the MPG patients treated with Equine Serum had 9.33 times more likely to be discharged in relation to patients with severe COVID-19, controlling for other variables (Table 2).
| Variables | OR | IC95% | p-Value | |
|---|---|---|---|---|
| MPG vs. SPG | 9.33 | 1.65 | 52.81 | 0.012 |
| Comorbidity | 0.1 | 0.01 | 0.89 | 0.039 |
| ITU | 1.11 | 0.19 | 6.25 | 0.9 |
| Sex | 0.62 | 0.13 | 2.84 | 0.53 |
| Age | 0.98 | 0.94 | 1.03 | 0.55 |
Table 2: Multivariate analysis, with the medical discharge variable as a response in relation to other variables.
According to Table 2, the presence of comorbidity decreases the chance of medical discharge by 10% in people with COVID-19 who received Equine Serum, controlling for other variables (OR<1). People with COVID-19 who were treated with Equine Serum and were in ICU equalled their chances of receiving medical discharge when the comparison was made by the interaction of other variables. The sex and age variables are independent in relation to the chance of receiving medical discharge (p>0.05), when they were treated in a comprehensive manner through interaction with other variables (p>0.05) (Table 3).
| Associated comorbidities | Deaths |
|---|---|
| HTA-obesity | 1 |
| HTA-coronary heart disease | 1 |
| HTA-DBT | 1 |
| HTA-DBT-pulmonary disease | 1 |
| HTA-DBT-coronary heart disease | 1 |
| HTA | 2 |
| Obesity/overweight | 2 |
| Pulmonary disease | 1 |
| Smoker/ex-smoker | 3 |
| Total | 13 |
Table 3: Patients with comorbidities who died after receiving 2 doses (N=13 out of a total of 82).
Discussion
The use of horses for producing therapeutics is likely to continue as long as nonanimal alternatives are unavailable or are significantly more expensive to produce. In this sense, in the Argentine Republic, equine hyperimmune serum has been advocated for treating SARSCoV2- infected patients [7]. However, in this work we have only addressed the in vitro effectiveness of equine hyperimmune serum in relation to the decrease in viral load compared to the effectiveness of AntiRBD antibodies extracted from the plasma of convalescent COVID patients. In relation to hyperimmune equine serum, to the best of our knowledge, a study is being carried out in Argentina, such as passive immunotherapy Equine polyclonal antibodies (EpAbs) F(ab´)2 fragments in patients with moderate to severe COVID-19 disease. In this paper, the authors reported a significant decrease in mortality in the group treated with equine serum [11]. Thus, the objective of this paper is to establish recommendations that could serve or guide the use of the equine serum that is already available on label from the Elea laboratory for use in Argentina. The recommendations we based on this observational study are based on a total of 84 patients, of which 35 were in moderate stage (MPG) and 49 were in severe stage (SPG). The main finding of this paper is that in relation to the primary objective no death was observed, unlike what was observed in the group of severe patients. This variance in the difference in proportions was significant for the moderate group. It should be noted, as shown in Table 1, that both groups were homogeneous in relation to the variables of age, weight, BMI, as well as in relation to the presence of comorbidities, both groups were homogeneous (Figure 3). We think that the contribution of the guidelines of the work with the hyperimmune equine serum in the province of Tucuman could result in the modification of the inclusion criteria for treatment, as a strong recommendation emanating from the work is that of greater use in moderate patients to avoid mortality, and the increase of medical discharge. In relation to medical discharge, we have also observed that it was much higher for moderate patients after 21 days of treatment. It should be noted that in both groups equine serum was indicated with the same FIS (date of symptom onset) which was 9-10 days after hospitalization. In relation to medical discharge, the bivariate analysis showed 8 times more chance of to receive of medical discharge in moderate patients versus severe patients. Finally, it should be noted that as shown in the logistic regression model, the variables of comorbidities, sex, age and admission to the ICU did not modify the probability of medical discharge in moderate patients, but the chance of obtaining clinical discharge or medical discharge increased by 1 more point, i.e., 9.33 times more likely to achieve medical discharge despite the presence of pre-existing comorbidities in both groups, strongly supporting the hypothesis of treatment with equine serum in moderate stage with early symptom onset date, always respecting a symptom onset date of no more than 10 days. Consistent with our work, other interventional therapies against COVID-19 in patients with moderate and severe Covid, such as treatment of convalescent plasma patients have been shown dissimilar results y major adverse events in the transfusion reaction [12]. This fact can be explained by the fact that unlike hyperimmune serum, the plasma of convalescent patients presents at the binding site of the IgG antibody the complete region of the fc fragment, which can lead to potential ADEs [13].
Conclusion
MPG participants received Equine Serum had a greater chance of medical discharge vs. SPG (p=0.004). The presence of comorbidity decreases the chance of medical discharge by 10%. This proposed treatment brings additional benefits in relation to the improvement in the patient's clinical condition, without this having an impact on adherence.
Declarations
Ethics approval and consent to participate
The people who agreed to participate in the study gave their informed consent before starting the study. The protocol was approved by Research Ethics Committee/Health Research Directorate, File Number 49/2020.
Consent for Publication
“Not applicable”
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The database collected for the study will be made available on request for scientific interests. Information available includes: investigation protocol, informed consent and database of trial with ID numbers to protect patient´s identity.
Conflict of Interests
The authors did not receive any monetary compensation for this work. They declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The Ministry of Public Health. Tucumán, Argentina participated in the design of the study and the collection, analysis and interpretation of the data, as well as in the drafting of the manuscript.
Authors’ Contributions
ESO supervised the database. ESO and DGG contributed with the data processing and contributed to the statistical analysis. ESO, DGG and MPB were responsible for writing the manuscript. MR, and MH contributed to data collection, and diagnosis. REC and LMR were the institutional managers to carry out the work. MPB supervised the project.
Acknowledgements
We wish to thank the Ministry of Public Health of Tucuman in whose centres the study was carried out.
References
- Roser M, Ritchie H, Ortiz-Ospina E, Hasell J, Ritchie H (2020) Our World in Data: Coronavirus Pandemic (COVID-19). Our World in Data.
- Manteca Vilanova X, De Briyne N, Beaver B, Turner PV (2019) Horse welfare during equine Chorionic Gonadotropin (eCG) production. Animals (Basel) 9:1053.
[Crossref] [Google Scholar] [PubMed]
- Grundbacher FJ (1992) Behring's discovery of diphtheria and tetanus antitoxins. Immunol Today 13:188-190.
[Crossref] [Google Scholar] [PubMed]
- Redwan ER (2009) Animal-derived pharmaceutical proteins. J Immunoassay Immunochem 30:262-290.
[Crossref] [Google Scholar] [PubMed]
- Zheng X, Wong G, Zhao Y, Wang H, He S, et al. (2016) Treatment with hyperimmune equine immunoglobulin or immunoglobulin fragments completely protects rodents from Ebola virus infection. Sci Rep 6:24179.
[Crossref] [Google Scholar] [PubMed]
- Pan X, Wu Y, Wang W, Zhang L, Xiao G (2020) Development of horse neutralizing immunoglobulin and immunoglobulin fragments against Junín virus. Antiviral Res 174:104666.
[Crossref] [Google Scholar] [PubMed]
- Zylberman V, Sanguineti S, Pontoriero AV, Higa SV, Cerutti ML, et al. (2020) Development of a hyperimmune equine serum therapy for COVID-19 in Argentina. Medicina (B Aires) 80:1-6.
[Google Scholar] [PubMed]
- Pan X, Zhou P, Fan T, Wu Y, Zhang J, et al. (2020) Immunoglobulin fragment F(ab’) 2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antiviral Res 182:104868.
[Crossref] [Google Scholar] [PubMed]
- WHO W Got C, Management of Ci (2020) A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 20:192-197.
[Crossref] [Google Scholar] [PubMed]
- Ministry of health secretariat of health quality (2021).
- Lopardo G, Belloso WH, Nannini E, Colonna M, Sanguineti S, et al. (2021) RBD-specific polyclonal F (ab) 2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. EClinicalMedicine 34:100843.
[Crossref] [Google Scholar] [PubMed]
- Janiaud P, Axfors C, Schmitt AM, Gloy V, Ebrahimi F, et al. (2021) Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. Jama 325:1185-1195.
[Crossref] [Google Scholar] [PubMed]
- Wu F, Yan R, Liu M, Liu Z, Wang Y, et al. (2020) Antibody-dependent enhancement (ADE) of SARS-CoV-2 infection in recovered COVID-19 patients: Studies based on cellular and structural biology analysis. MedRxiv.
Citation: Elena CR, Luis MR, Contreras MEF, Raya M, Herrera N, et al. (2022) Observational Study: Application of Therapy with Equine Serum for Patients Diagnosed with COVID-19 in Tucumán, Argentina. J Infect Dis Ther S2:004.
Copyright: © 2022 Elena CR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2575
- [From(publication date): 0-2022 - Dec 04, 2025]
- Breakdown by view type
- HTML page views: 1962
- PDF downloads: 613