Obstructive Sleep Apnea and Chronic Pulmonary Diseases
Received: 09-Oct-2017 / Accepted Date: 12-Oct-2017 / Published Date: 20-Oct-2017
Abstract
Sleep is associated with adaptive changes of the airways and the lungs. In patients with chronic pulmonary diseases such physiologic changes and the pathophysiologic changes induced by obstructive sleep apnea (OSA) may result in worsening of the pulmonary disease and can contribute to worsened outcomes. Pulmonologists need to be aware of the importance of screening patients with chronic pulmonary disease for OSA, as patients suffering from both chronic pulmonary disease and OSA, termed “overlap syndrome”, have increased morbidity and mortality. It is equally important to initiate appropriate treatment as CPAP treatment will improve quality of life, and prevent long-term respiratory and cardiovascular complications, with improved survival rates and decreased hospitalizations. The purpose of this review is to increase awareness of this association and provide pulmonologist with knowledge of how recent advances in sensor technology and computing now offer simple and cost-effective ambulatory methods to accurately screen for sleep disorders. This review should encourage more attention to symptoms of sleep apnea in patients with chronic pulmonary diseases.
Keywords: Obstructive sleep apnea; Chronic pulmonary diseases; Cardio pulmonary coupling; Cyclic variation of heart rate
Abbreviations
AASM: American Academy of Sleep Medicine; AHI: Apnea Hypopnea Index; CAP: Cyclic Alternating Pattern; CPC: Cardiopulmonary Coupling; CSA: Central Sleep Apnea; CNS: Central Nervous System; COPD: Chronic Obstructive Pulmonary Disease; CPAP: Continuous Positive Airway Pressure; CVHR: Cyclic Variation of Heart Rate; EDR: Electrocardiogram Derived Respiration; ECG: Electrocardiogram; EEG: Electroencephalogram; eLFCBB: Elevated Low Frequency Broad-band; eLFCNB: Elevated Low Frequency Narrow-band; HRV: Heart Rate Variability; HFC: High Frequency Coupling; NREM: Non-Rapid Eye Movement Sleep; OHS: Obesity Hypoventilation Syndrome; OSA: Obstructive Sleep Apnea; REM: Rapid Eye Movement; SA: Sleep Apnea; SAI: Sleep Apnea Indicator; SDB: Sleep Disordered Breathing; SQI: Sleep Quality Index; SWS: Slow-wave Sleep; vLFC: Very Low Frequency Coupling
Introduction
Medical providers are today managing an ever-growing number of medical conditions, encompassing all organ systems, many of which are associated with sleep disorders like compromised sleep quality and sleep apnea. In order to effectively manage these diseases, physicians must be educated about disease associations and how diligent and multi-disciplinary efforts to screen for comorbid conditions can inform or drive clinical decisions to aid therapy management. As the importance of sleep is increasingly being recognized, the implications of untreated sleep disorders on overall health are also being realized [1,2]. As chronic diseases have assumed an increasingly common role in premature death and illness, interest in the role of sleep health in the development and management of chronic diseases has grown and the Center for Disease Control and Prevention has stated that getting sufficient sleep is not a luxury – it is something people need for good health [3].
Recent advances in sensor technology now offer ambulatory methods to easily collect medically relevant physiological signals that can be analyzed to estimate sleep quality, sleep quantity and sleep pathology providing a unique insight into sleep regulation in health and disease [4].
Sleep apnea, characterized by paused breathing during sleep, disrupts a healthy sleep pattern and has been shown to adversely affect the overall health of these patients. The more common form of sleep apnea, Obstructive Sleep Apnea is characterized by repeated partial or complete obstruction of the upper airway during sleep, resulting in intermittent hypoxia and transient repetitive sympathetic arousals from sleep. Central Sleep Apnea is a less common form of sleep apnea associated with disordered respiratory control [5]. The hypoxia induced by repeated cessation of breathing during sleep has multisystem effects and is associated with increased risk and progression of diseases like hypertension and cardiovascular disease [6-8] obesity, [9,10] type 2 diabetes, [11] and various pulmonary diseases [12-14]. Activation of the sympathetic nervous system during sleep and the intermittent hypoxia may be the primary mechanisms behind the development and persistence of these comorbid diseases. The prevalence of OSA has been increasing over recent decades, most likely because of the rising prevalence of obesity [15]. Today it is estimated that 12% of adults suffer from sleep apnea in the United States and that 80% of the patient population is undiagnosed [16].
Prevalence of chronic pulmonary diseases like chronic obstructive pulmonary disease and asthma is high in the adult population and with the increased prevalence of obesity; obesity hypoventilation syndrome is becoming more prevalent. These patients commonly have complaints of compromised sleep quality, increased dyspnea, cough, and difficulty with maintenance of sleep, even without known coexistent primary sleep disorders [17].
Sleep has a number of adverse effects on breathing that include negative effects on respiratory control, respiratory muscle function, and lung mechanisms [18]. These changes have negligible adverse effects in healthy individuals but in patients with chronic pulmonary diseases, such physiologic changes as well as the pathophysiologic changes of sleep breathing disorders like OSA may result in acute and chronic adverse effects, including worsening of hypoxemia and hypercapnia and in the long term can contribute to worsened outcomes in these patients [19,20]. To describe the excessive risk for worsened sleep and wake related outcomes in patients suffering from both OSA and chronic pulmonary disease, compared to patients with only OSA or only chronic pulmonary disease, the term “overlap syndrome” was introduced [21,22].
Obstructive Sleep Apnea and Chronic Pulmonary Diseases
Chronic obstructive pulmonary disease
Prevalence of COPD is related to the prevalence of tobacco smoking and is estimated around 10% in the general population, and coexistence of both disorders, COPD and OSA, “overlap syndrome”, affects at least 1% of the adult population.
Sleep has significant effects on respiration in COPD patients as respiratory motor output and skeletal muscle function diminishes, particularly during rapid eye movement sleep and the reduced contribution of the intercostal muscles, increased upper airway resistance, due to a loss of tone in the upper pharyngeal muscles, and reduced efficiency of diaphragmatic contraction due to lung hyperinflation, all contributing to reduction in functional residual capacity augmenting ventilation-perfusion mismatch, hypoxemia, and hypercapnia in COPD patients [23].
OSA is characterized by increased upper airway resistance, reduced or blocked airflow causing paused breathing during sleep, sympathetic activation and intermittent hypoxia and hypercapnia [24]. During an apneic episode to overcome the upper airway resistance and to maintain adequate airflow to the lungs, increased diaphragmatic and abdominal muscle effort is required. This can be particularly difficult in COPD patients who already have increased intrathoracic airway resistance and lung hyperinflation. When COPD patients develop obstructive apnea episodes, the compensatory response of the respiratory center is slower, apneas are longer and hypoxemia and hypercapnia more intense compared with non-COPD apnea patients. Majority of patients with OSA alone do not develop significant sleeprelated hypercapnia because of inter-apnea hyperventilation but in COPD patients, due to abnormal mechanical and chemical ventilator responses, this can result in post-apnea hypercapnia levels that do not return to baseline. Over time, a progressive desensitization of the respiratory center in response to OSA-related hypoxic-hypercapnic episodes develops and patients with “overlap syndrome” can remain hypercapnic during sleep [23,25,26]. Number of additional factors have to be considered as they may influence the relationship between COPD and OSA which include cigarette smoking, contributing to upper airway inflammation and certain medications such as corticosteroids, frequently used to treat COPD, contributing to pharyngeal fat deposition. For several reasons patients with “overlap syndrome” may therefore have a worse prognosis compared to patients with only one of those diseases as they suffer from more frequent episodes that are also associated with more severe sleep-related hypoxemia than OSA patients without COPD, particularly during REM sleep [26]. Patients suffering from “overlap syndrome” are also more likely to develop daytime pulmonary hypertension and heart failure [26] and hypoxemia predisposes these patients to cardiac arrhythmias, that can lead to nocturnal death, often during REM sleep. The fact that COPD patients have reduced sleep quality, increased sleep fragmentation, and consequent reduction in slow-wave sleep (SWS) and REM sleep duration may though relatively protect them from the potential detrimental effects of REM sleep [27].
Management of sleep disorders in patients with COPD should address both sleep quality and disordered gas exchanges as patients with “overlap syndrome” clearly benefit from continuous positive airway pressure (CPAP) particularly for long-term survival [28-30].
Smoking and OSA
In 1964 the U.S. Surgeon General issued a groundbreaking report on smoking and health. Since that report was published the prevalence of smoking in the United States has dropped from 43% to 15% among adults over 19 years of age [31,32]. Even with this decrease, cigarette smoking still remains the leading cause of preventable morbidity and mortality; it is a major contributing factor causing chronic pulmonary diseases and a major contributing factor in cardiovascular disease progression and the mortality rate among smokers is almost three times that of those who have never smoked [33].
The degree to which smoking and OSA are related has not been well characterized, but despite conflicting evidence and a lack of longitudinal studies supporting the association between smoking and OSA, many researchers have proposed potential pathophysiology of OSA may include changes in sleep architecture and a “rebound effect” due to nightly short-term nicotine withdrawal, smoking-induced upper airway inflammation and a stimulant effects of nicotine on upper airway muscles, or all of the above.
Nicotine in cigarette smoke may attribute to both the immediate effects on sleep, causing increased sleep latency and/or the effects of nicotine withdrawal during the sleep period on sleep architecture. Nicotine affects the central nervous system (CNS) both directly and by stimulating release of several neurotransmitters affecting the central mechanism regulating the sleep-wake cycle and sleep architecture. Current smokers report less sleep quality with more difficulty falling asleep, staying asleep and experience more daytime sleepiness than non-smokers. Smokers have been found to have increased sleep latency and smoking has been linked to changes in NREM sleep with shift towards lighter sleep stages when compared with non-smokers but smoking does not seem to affect REM sleep [34,35].
Tobacco and other ingredients in cigarettes have also been found to contribute to the existing airway inflammation in OSA patients, which can then further narrow the upper airways and predispose to airway collapse contributing to more obstructive apneas than among nonsmokers. Impairment of the upper airway neuromuscular protective reflexes by nicotine is another potential explanation for the effect of smoking on sleep apnea, causing current smokers to have higher AHI than non-smokers, with longer episodes of upper airway collapse and with greater desaturation [36,37].
Despite the high prevalence of both OSA and smoking, available evidence does not conclusively establish a clinically significant relationship between the two diseases. Further research is needed to characterize the association between the two diseases as to clarifying the effect of one disorder on the other and the effect of treatment of one disorder on the other, as appropriate treatment recommendations may have significant implication in reducing the public health and economic burden of these two diseases. Smoking cessation is recommended when considering treatment for OSA.
Asthma
Prevalence of asthma, a chronic respiratory disorder associated with reversible air flow obstruction and bronchial hyper-responsiveness is about 1-18% of the general population among different regions of the world [38] and prevalence of “overlap syndrome” is not well documented but ranges from 38% up to as high as 70% in asthma patients [39].
Sleep has significant effects on respiration in asthma patients. Many patients with nonatopic asthma and most atopic asthmatics suffer from nasal obstruction due to rhinitis and chronic sinusitis, causing nasal congestion and airflow resistance and nasopharyngeal polyps which reduce airway caliber, promoting upper airway collapse during inspiration, snoring and obstructive apnea. Similarly in patients with chronic asthma, persistent mucosal inflammation affects the upper airway promoting upper airway collapse [40]. A potential asthma trigger is the chronic intermittent hypoxia in OSA that may induce a systemic inflammation in the upper airways, similar to those noted in asthma, that may reduce airway caliber and at the same time increase underlying bronchial hyper-responsiveness by stimulation of carotid body receptors [41].
Inhaled corticosteroids are the most effective and most widely used asthma drugs. Long-term effects of inhaled corticosteroids on the collapsibility of the pharynx remain unknown but effects of oral corticosteroids, including myopathy of the muscles of the pharynx and fatty infiltration of the pharyngeal wall, are documented [26].
CPAP treatment appears to have significant potential clinical benefits in the treatment of “overlap syndrome”, improving asthma symptoms, decrease needed use of rescue medication and improves quality of life [42,43]. In patients with nocturnal asthma whose quality of sleep does not improve with proper antiasthma treatment, the coexistence of OSA, “overlap syndrome” should be excluded and periodic OSA evaluation in asthma patients is recommended as asthma is associated with an increased risk of new-onset OSA [44,45].
Obesity hypoventilation syndrome (OHS)
Prevalence of OHS, the combination of obesity, daytime hypercapnia and sleep disordered breathing (SDB) in the absence of other known causes of hypercapnia, has markedly increased during the last three decades due to the global obesity epidemic.
The National Health and Nutrition Examination Survey (NHANES) reported that in 2013-2014 32.7% of adult Americans were overweight (body mass index (BMI) of 25.0-29.9 kg/m2) and 37.9% were obese (BMI >30.0 kg/m2), compared with 1960-1962 when a similar percentage of overweight Americans was 31%, but only 13% were obese, demonstrating a vast increase in obesity [46]. Although most OHS patients have OSA, approximately 10% have no evidence of nocturnal OSA [47,48]. The mechanism by which obesity leads to chronic daytime hypercapnia is complex and not fully understood but mechanisms that are believed to be involved are that higher body surface area in obese individuals is associated with an increase in carbon dioxide production, abnormal respiratory system mechanics due to obesity, impaired central chemo-responsiveness to hypercapnia and hypoxia, sleep-disordered breathing, and neurohormonal abnormalities such as leptin resistance [49].
Compared with patients with similar degrees of obesity and similar severity of OSA, hypercapnic patients have increased health-care expenses [50], higher risk of developing pulmonary hypertension [51], and serious cardiovascular diseases leading to early mortality [52].
Treatment with CPAP improves gas exchanges, functional status and quality of life in OHS patients. Therefore, early identification of OHS is important as treatment can reduce the burden of the significant morbidity and mortality associated with undiagnosed and untreated OHS [52,53].
Methods to screen and diagnose sleep disorders
Accepted clinical methods used to screen for sleep disorders have been limited to subjective questionnaires, mostly for reasons of convenience and cost. Questionnaires were originally created, as technology to objectively screen for sleep disorders as screening and diagnosis was not readily available outside of the sleep lab. They are relatively effortless to conduct but are based on respondents’ own subjective perception of their sleep rather than objective data. Even though questionnaires have historically been perceived as accurate, when compared to objective measures, the results have shown a level of inconsistency that makes them unreliable [54,55]. Subjective estimates undoubtedly can be useful to a degree, but sleepiness and day time functioning varies widely between patients as some patients subjectively report excessive day time sleepiness while others do not [56,57]. Patients with sleep complaints and sleep disorders may not accurately estimate sleep quality or duration as their perception depends heavily on extraneous factors including demographics and comorbidities, and they often tend to underestimate sleep time and quality [58]. Generally, if a questionnaire has a high sensitivity, it is at the expense of poor specificity, and vice versa, deeming them inaccurate primary tools to evaluate sleep disorders, and it is likely that most of the questionnaires will inaccurately classify a significant proportion of sleep disorder patients [54,55].
For patients identified to have insomnia based on subjective responses to a questionnaire, a diagnostic test such as a Polysomnography (PSG) is not used for routine evaluation, unless the screening test is inconclusive, or behavioral or pharmacologic treatment fails [59,60]. However, emerging evidence suggests that sleep-related breathing disorders like OSA, may be an underrecognized cause of insomnia complaints, even among individuals who deny symptoms of sleep apnea at initial presentation [61,62]. It is recommended that positive results for identifying OSA based on sleep questionnaires is followed up with a diagnostic test for OSA. PSG is the reference standard for diagnosis of OSA before treatment is initiated, and an attended study is recommended [63]. PSG tests can be challenging to obtain, as the procedure is time consuming, labor intensive, costly and not available to all at-risk patients. Home Sleep Testing (HST) is less expensive and can be somewhat less challenging to obtain, but lacks the sensitivity to rule out OSA diagnosis as it does not record sleep onset, sleep duration, sleep efficiency or wakefulness. If a patient suspected of having OSA has a negative HST, the current recommendation is to follow up with a PSG test [64]. When a therapy is recommended, it is expensive and burdensome to use PSG or HST to determine efficacy of prescribed therapy. This entire process is costly, time consuming and impractical in the population of at-risk patients suffering from comorbid disorders associated with OSA.
Cardio Pulmonary Coupling and Cyclic Variation of Heart Rate Analysis
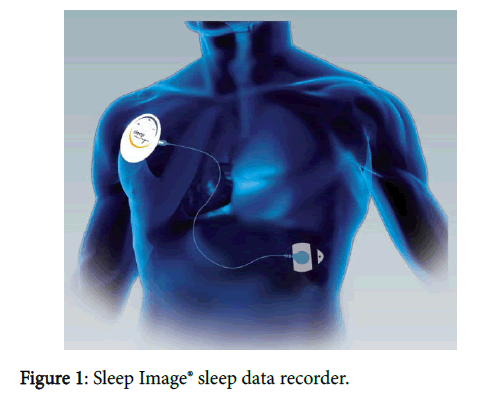
Recent advances in sensor technology now offer ambulatory methods to easily collect single lead ECG data [65,66]. The Sleep Image® system is FDA approved to aid in the evaluation of sleep disorders. Based on an objective measure of sleep duration, sleep quality and sleep pathology, it can inform or drive clinical management for sleep disorder patients. The Sleep Image® wearable sleep data recorder collects a continuous ECG signal. One electrode pad is attached to the device that then is connected to a second electrode using a thin cable across the chest. Activity and body position is measured by internal accelerometers and gyroscopes and snoring is detected by tissue vibration (Figure 1).
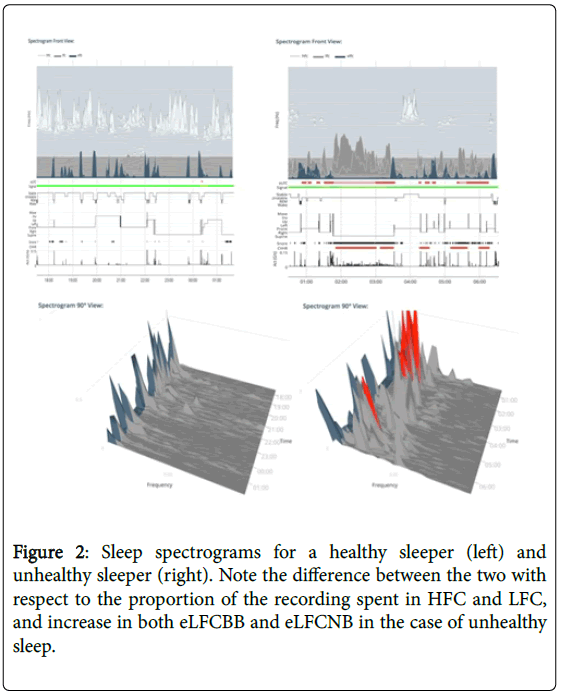
Collected data is uploaded to the Sleep Image® secure, cloud-based system for automatic analysis where the Cardiopulmonary Coupling and Cyclic Variation of Heart Rate (CPC-CVHR) algorithms automatically generate sleep metrics and spectrographic analysis of the sleep period (Figure 2). The patented and clinically validated algorithms analyze ECG data, where Heart Rate Variability (HRV) is coupled with derived respiration (EDR) to provide an objective measure of sleep duration, sleep quality and sleep pathology. The CPC analysis of the ECG signal was performed as described in detail [65,66,67]. Sleep apnea, the periodic cessation of breathing during sleep, alters heart rate dynamics, and during periods of prolonged SA, heart rate typically shows cyclic increases and decreases associated with apneic phase and resumption of breathing. The CVHR-analysis of the ECG signal was performed as described in detail [68,69]. Objective sleep metrics are presented through the sleep quality index, sleep apnea indicator, stable sleep, unstable sleep and the sleep pathology markers elevated low frequency coupling broad band and elevated low frequency coupling narrow band distinguishing between apneas caused by upper airway anatomical obstruction and respiratory dyscontrol [70].
The Sleep Quality Index is a summary index of an automated measure of sleep duration, sleep stability, sleep fragmentation, and sleep pathology. The SQI equation seeks to balance these sleep metrics and uses these variables to generate a number between 0 and 100. This score is meant to serve as a summary of the CPC results.
The Sleep Apnea Indicator is an automated measure of CVHR during unstable breathing detecting oscillations in cardiac intervals often associated with prolonged cycles of sleep apnea. Displaying this physiological reaction occurring in the cardiovascular system as a consequence of drop in oxygen saturation during apneas as an indicator, the SAI helps to identify patients suffering from sleep disordered breathing.
Using SAI together with SQI, eLFCBB, and eLFCNB, it is possible to identify the presence of SA and to categorize SA as obstructive, central or complex sleep apnea [70].
The automated analysis also presents an ECG-derived sleep spectrogram (Figure 2), revealing that non-rapid eye movement sleep has a distinct bimodal-type structure marked by distinct alternating and abruptly varying periods of high and low frequency Cardio Pulmonary Coupling. Stable sleep (High frequency coupling, HFC) occurs during part of stage N2 and all of stage N3, and is associated with periods of stable breathing, non-cyclic alternating pattern (non- CAP), electroencephalogram morphology, increased absolute and relative delta power, strong sinus arrhythmia, and blood pressure dipping. Conversely, unstable sleep (low frequency coupling, LFC) is characterized by temporal variability of tidal volumes, cyclic alternating pattern (CAP) EEG morphology, non-dipping of blood pressure and lower frequency cyclic variation in heart rate.
Fragmented rapid eye movement sleep has an LFC signature, while normal REM sleep and wake show very low frequency coupling signature (vLFC) [1,67]. Processes that fragment sleep like sleep apnea and fibromyalgia reduce the amount of HFC [71,72]. ECG-derived CPC metrics show an independence of absolute EEG amplitudes and are thus not constrained by the “loss” of slow wave sleep with age [67]. Specific spectrographic signatures of fragmented sleep are biomarkers of strong chemo reflex effects on sleep-respiration [70]. The CPC technique accurately identifies sleep apnea [66] and captures treatment effects in sleep apnea [73-77] and insomnia [78,79]. In this way, the NREM sleep phenotype extends beyond conventional scoring of AHI and its reliability on absolute delta power. These disparities are especially apparent in individuals over the age of 40-50 years, for whom stage N3 makes up less than 20% of the sleep period [80].
Discussion and Conclusion
Sensor technologies now offer additional ambulatory methods to easily collect bio-signals like ECG, which provides opportunities and possibilities to collect objective data to analyze and improve clinical diagnosis and treatment decisions.
While the relationship between chronic pulmonary diseases and untreated sleep disorders may not be the most immediate obvious observation, it should not be ignored or overlooked given the implications of how untreated sleep disorders can adversely affect progression of various chronic pulmonary diseases.
Therefore identifying the coexistence and severity of OSA in patients with comorbid pulmonary conditions, the presence of “overlap syndrome”, is highly important as these patients have worse quality of life and prognosis. Therefore for quality patient care, implementing simple, efficient, and accurate objective methods to identify OSA in patients with “overlap syndrome” is highly important and to initiate treatment when appropriate, as sleep disorder breathing therapy prevents long-term respiratory and cardiovascular complications, decreases hospitalizations and improves survival rate quality of life in these patients [29,30,33,36,38,43,53].
This improved ambulatory method to screen for sleep apnea will help clinicians to improve diagnosis accuracy for sleep apnea in patients with chronic pulmonary diseases. It can also provide objective evidence and feedback for these patients to assist in improving therapy management for both sleep apnea and related chronic pulmonary diseases. A crucial part of the disease management is to educate the patient on the association between OSA and chronic pulmonary diseases for them to understand that the treatment of the OSA not only benefits to improve their sleep symptoms but also their pulmonary disease and overall health and wellbeing. Regular tracking of therapy efficacy and adherence to treatment should also help to maximize medical benefits. Management of OSA and its associated conditions should involve the primary care physician as well as the pulmonologist and other specialists.
Using objective and medically accurate methods regularly to track physiology during sleep may be key to optimizing sleep health that can incentivize preventative wellness behavior and improve patient care with cost benefits to patients and payers.
Conflict of Interest
Magnusdottir, Solveig, MD MBA: Works as a medical director of MyCardio LLC and has partial ownership. Sleep Image is the brand name of MyCardio LLC, a privately held entity. MyCardio LLC is a licensee of the CPC+CVHR algorithms, a method to phenotype sleep and sleep apnea based on coupling of cardiovascular and respiratory data streams, from the Beth Israel Deaconess Medical Center, Boston, MA, USA.
References
- Gallicchio L, Kalesan B (2009) Sleep duration and mortality: A systematic review and meta-analysis. J Sleep Res 18(2): 148-158.
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, et al. (2008) Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohorts. Sleep 31: 1071-1078.
- Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, et al. (2014) Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 127: 95.e11-95.e17.
- Leung RS, Comodore VR, Ryan CM, Stevens D (2012) Mechanisms of sleep-disordered breathing: Causes and consequences. Pflugers Arch 463: 213-230.
- Beuters F, Reitzschel ER, Hertegonne KB, Chirinos JA (2015) The link between obstructive sleep apnea and cardiovascular disease. Curr Atheroscler Rep 18: 1.
- Wang D, Li W, Cui X, Meng Y, Zhou M, et al. (2016) Sleep duration and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Int J Cardiol 219: 231-239.
- Montag SE, Knutson KL, Zee PC, Goldberger J, Ng J, et al. (2017) Association of sleep characteristics with cardiovascular and metabolic risk factors in a population sample: The Chicago Area Sleep Study. Sleep Health 3: 107-112.
- Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, et al. (2004) The association between short sleep duration and obesity in young adults: A 13-year prospective study. Sleep 27: 661-666.
- Chaput J, Despres J, Bouchard C, Tremblay A (2007) Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity 15: 253-261.
- Heianza Y, Kato K, Fujihara K, Fujihara K, Tanaka S, et al. (2014) Role of sleep duration as a risk factor for Type 2 diabetes among adults of different ages in Japan: the Niigata Wellness Study. Diabet Med 31: 1363-1367.
- Lewis CA, Fergusson W, Eaton T, Eaton T, Zeng I (2009) Isolated nocturnal desaturation in COPD: Prevalence and impact on quality of life and sleep. Thorac 64: 133-138.
- Alkhalil M, Shulman E, Getsy J (2009) Obstructive sleep apnea syndrome and asthma: What are the links? J Clin Sleep Med 5: 71-78.
- Verbraecken J, McNicholas W (2013) Respiratory mechanics and ventilator control in overlap syndrome and obesity hypoventilation. Respir Res 14: 132.
- Peppard PE, Young TE, Barnet JH, Palta M, Hagen EW, et al. (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177: 1006-1014.
- McSarry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT (2012) Sleep quality in chronic obstructive pulmonary disease. Respirology 17: 1119-1124.
- Johnson MW, Remmers JE (1984) Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol Respir Envirion Exerc Physiol 57: 1011-7.
- Lewis C, Fergusson W, Eaton T, Zeng I (2009) Isolated nocturnal desaturation in COPD: Prevalence and impact on quality of life and sleep. Thorax 64: 133-138.
- Omachi TA, Balnc PD, Claman DM, Chen H, Yelin EH, et al. (2012) Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med 13: 476-483.
- McNicholas WT (2016) Chronic obstructive pulmonary disease and obstructive sleep apnoea-The overlap syndrome. J Thorac Dis 8: 236-242.
- Kent B, Mitchelli P, McNicholas W (2011) Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int J Chron Obstruct Pulm Dis 6: 199-208.
- Iber C, Ancoli-Israel S, Chesson AL (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine, Westchester, IL.
- Berger KI, Norman RG, Ayappa I, Oppenheimer BW, Rapoport DM, et al. (2008) Potential mechanism for transition between acute hypercapnia during sleep to chronic hypercapnia during wakefulness in obstructive sleep apnea. AdvExo Med Biol 605: 431-436.
- McNicholas WT (2009) Chronic obstructive pulmonary disease and obstructive sleep apnea: Overlaps in pathophysiology, systemic inflammation and cardiovascular disease. AM J Respir Crit Care Med 180: 692-700.
- Tirlapur VG, Mir MA (1982) Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airway disease. N Engl J Med 306: 125-130.
- Wang TY, Lo YL, Lee KY, Liu WT, Lin SM, et al. (2013) Nocturnal CPAP improves walking capacity in COPD patients with obstructive sleep apnoea. Resp Res 14: 66.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR (2010) Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: The overlap syndrome. Am J Respir Crit Care Med 182: 325-331.
- Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W (2013) Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: The overlap syndrome. J Clin Sleep Med 9: 767-772.
- Us Surgeon General’s Advisory Committee on Smoking and Health (1964) Smoking and Health. Public Health Service, Washington, DC.
- https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, et al. (2013) 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 368: 341-350.
- Wetter DW, Young TB (1994) The relation between cigarette smoking and sleep disturbance. Prev Med 23: 328-334.
- Zhang L, Samet J, Caffo B, Punjabi NM (2006) Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol 164: 529-537.
- Krishnan V, Dixon-Williams S, Thornton JD (2014) Where there is smoke…There is sleep apnea. Chest 146: 1637-1680.
- Conway SG, Roizenblatt SS, Palombini L, Castro LS, Bittencourt LR, et al. (2008) Effect of smoking habits on sleep. Braz J Med Biol Res 41: 722-727.
- Loftus PA, Wise SK (2016) Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg 24: 245-249.
- Abdul Razak MR, Chirakalwasan N (2016) Obstructive sleep apnea and asthma. Asian Pac J Allergy Immunol 34: 265-271.
- Young T, Finn L, Kim H (1997) Nasal obstruction as a risk factor for sleep disordered breathing. J Allergy Clin Immunol 99: 57-62.
- Collett PW, Bracatisano AP, Engel LA (1986) Upper airway dimensions and movements in bronchial asthma. Am Rev Respir Dis 133: 1143-9.
- Kauppi P, Bachour P, Maasilta P, Bachour A (2016) Long-term CPAP treatment improves asthma control in patients with asthma and obstructive sleep apnoea. Sleep and Breath 20: 1217-1224.
- Serrano-Pariente J, Plaza V, Soriano JB, Mayos M, Lopez-Vina A, et al. (2017) Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy 72: 802-812.
- Yim S, Fredberg JJ, Malhotra A (2007) Continuous positive airway pressure for asthma: Not a big stretch? Eur Respir J 29: 226-228.
- Teodorescu M, Barnet JH, Hagen EW, Palta M (2015) Association between asthma and risk of developing obstructive sleep apnea. JAMA 313: 156-164.
- https://www.cdc.gov/nchs/data/hestat/obesity_adult_13_14/obesity_adult_13_14.pdf
- Kaw R, Hernandex AV, Walker E, Aboussouan L, Mokhlesi B (2009) Determinants of hypercapnia in obese patients with obstructive sleep apnea: Systematic review and metanalysis of cohort studies. Chest 136: 787-796.
- Pepin JL, Borel JC, Janssens JP (2012) Obesity hypoventilation syndrome: An underdiagnosed and undertreated condition. Am J Respir Crit Care Med 186: 1205-1207.
- Mokhlesi B, Tulaimat A (2007) Recent advances in obesity-hypoventilation syndrome. Chest 132: 1322-1336.
- Berg D, Delaive K, Manfreda, J, Walld R, Kryger MH (2001) The use of health care resources in obesity-hypoventilation syndrome. Chest 120: 337-383.
- Kessler R, Chaouat A, Weitzenblum E, Oswald M, Ehrhart M, et al. (1996) Pulmonary hypertension in the obstructive sleep apnoea syndrome: prevalence, causes and therapeutic consequences. Eur Respir J 9: 787-794.
- Castro-Anon O, Perez de Llano LA, Fuente Sanchez SD, Golpe R, Marote LM, et al. (2015) Obesity hypoventilation syndrome: Increased risk of death over sleep apnea syndrome. PLoS One 10: e117808.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR (2008) Randomized trial of CPAP vs bi-level support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorac 63: 395-401.
- Westlake K, Plihalova A, Pretl M, Lattova Z, Polak J (2016) Screening for obstructive sleep apnea syndrome in patients with type 2 diabetes mellitus: a prospective study on sensitivity of Berlin and STOP-Bang questionnaires. Sleep Med 26: 71-76.
- Pereira E, Driver H, Stewart S, Fitzpatrick M (2013) Comparing a combination of validated questionnaires and level III portable monitor with polysomnography to diagnose and exclude sleep apnea. J Clin Sleep Med 9: 1259-1266.
- Vakulin A, Catcheside PG, Baulk SD, Antic NA, Banks S, et al. (2014) Individual variability and predictors of driving simulator impairment in patients with obstructive sleep apnea. J Clin Sleep Med 10: 647-655.
- Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B (2016) Obstructive sleep apnea in the general population: Highly prevalent but minimal symptoms. Eur Respir J 47: 194-202.
- Bianchi M, Thomas R, Westover B (2017) An open request to epidemiologist: Please stop querying self-reported sleep duration. Sleep Med 35: 92-93.
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M (2008) Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 4: 487-504.
- Littner M, Hirshkowitz M, Kramer M, Kapen S, Anderson WM, et al. (2003) Practical parameters of using polysomnography to evaluate insomnia: An update. Sleep 26: 754-760.
- Bianchi MT, Williams KL, McKinney S, Ellenbogen JM (2013) The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea. J Sleep Res 22: 557-558.
- Bianchi MT, Goparaju B, Moro M (2015) Sleep apnea in patients reporting insomnia or restless leg symptoms. Acta Neurol Scand 133: 61-67.
- Epstein L, Kristo D, Strollo P, Friedman N, Malhotra A, et al. (2009) Clinical Guideline for the Evaluation, Management and Long-term Care of Obstructive Sleep Apnea in Adults. J Clin Sleep Med 5: 263-276.
- Shayeb M El, Topfer L, Stafinski T, Pawluk L, Menon D (2014) Diagnostic accuracy of level 3 portable sleep test versus level 1 polysomnography for sleep disordered breathing: A systematic review and meta-analysis. CMAJ 186: 25-51.
- Thomas RJ (2016) Cardiopulmonary coupling sleep spectrograms. In: Principles and Practice of Sleep Medicine, pp: 1615-1623.
- Magnusdottir S, Hilmisson H (2017) Ambulatory screening tool for sleep apnea: Analyzing a single-lead electrocardiogram (ECG). Sleep Breath, pp: 1-9.
- Thomas RJ, Mietus JE, Peng CK, Guo D, Gozal D, et al. (2014) Relationship between delta power and the electrocardiogram-derived cardiopulmonary spectrogram: Possible implications for assessing the effectiveness of sleep. Sleep Med 15: 125-131.
- Mietus JE, Peng CK, Ivanov PC, Goldberger AL (2000) Detection of obstructive sleep apnea from cardiac interbeat interval time series. Comput Cardiol 27: 753-758.
- Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A (1984) Cyclical variation of the heart rate in sleep apnea syndrome: Mechanisms, and usefulness of 24h electrocardiography as a screening technique. Lancet 1: 126-131.
- Thomas RJ, Mietus JE, Peng CK, Gilmarin G, Daly RW, et al. (2007) Differentiation obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep 30: 1756-1759.
- Thomas RJ, Mietus JE, Peng CK, Goldberger AL, Crofford L, et al. (2010) Impaired sleep quality in fibromyalgia: Detection and quantification with ECG-based cardiopulmonary coupling spectrograms. Sleep Med 11: 497-498.
- Thomas RJ, Weiss MD, Mietus JE, Peng CK, Goldberger AL, et al. (2009) Prevalent hypertension and stroke in the sleep heart health study: Association with an ECG-derived spectrographic marker of cardiopulmonary coupling. Sleep 32: 897-904.
- Lee WH, Ahn JC, We J, Rhee CS, Lee CH, et al. (2014) Cardiopulmonary coupling analysis: Changes before and after treatment with mandibular advancement device. Sleep Breath 18: 891-896.
- Choi JH, Thomas RJ, Suh SY, Park IH, Kim TH, et.al. (2015) Sleep quality changes after upper airway surgery in obstructive sleep apnea: Electrocardiogram-based cardiopulmonary coupling analysis. Laryngoscope 125: 1737-1742.
- Lee WH, Hong SN, Kim HJ, Rhee CS, Lee CH, et al. (2016) A comparison of different success definitions in non-continuous positive airway pressure treatment for obstructive sleep apnea using cardiopulmonary coupling. J Clin Sleep Med 1: 35-41.
- Lee SH, Choi JH, Park IH, Lee SH, Kim TH, et al. (2012) Measuring sleep quality after adenotonsillectomy in pediatric sleep apnea. Laryngoscope 122: 2115-2121.
- Guo D, Peng CK, Wu HL, Mietus JE, Liu Y, et al. (2011) ECG-derived cardiopulmonary analysis of pediatric sleep-disorder breathing. Sleep Med 12: 384-389.
- Schramm PJ, Thomas R, Feige B, Spiegelhalder K, Riemann D (2012) Quantitative measurement of sleep quality using cardiopulmonary coupling analysis: A retrospective comparison of individuals with and without primary insomnia. Sleep Breath. 17: 713-721.
- Schramm PJ, Zobel I, Monch K, Schramm E, Michalak J (2016) Sleep quality changes in chronically depressed patients treated with mindfulness-based cognitive therapy or the cognitive behavioral analysis system of psycotherapy: A pilot study. Sleep Med 17: 57-63.
- Ancoli-Israel S (2009) Sleep and its disorders in aging populations. Sleep Med 1: 7-11.
Citation: Magnusdottir S (2017) Obstructive Sleep Apnea and Chronic Pulmonary Diseases. J Respir Med 1: 102.
Copyright: © 2017 Magnusdottir S. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4333
- [From(publication date): 0-2017 - Aug 25, 2025]
- Breakdown by view type
- HTML page views: 3443
- PDF downloads: 890