Pain and Placebo
Received: 18-Feb-2021 / Accepted Date: 26-Mar-2021 / Published Date: 02-Apr-2021 DOI: 10.4172/2167-0846.1000370
Abstract
Expectation of pain relief can reduce pain and prior positive experiences increase the analgesic responses to subsequent placebo. Placebo effect is the positive beneficial response after receiving a placebo. Nocebo effects are the negative responses after receiving a placebo, which are usually minor, but can be life threatening. Endogenous neuropeptides such as opioids, dopamine, serotonin and cannabinoids are released in placebo analgesia. Part of the placebo response is mediated by intrinsic cognitive factors, alone or in combination with extrinsic environmental factors.
Keywords: Pain; Placebo; Nocebo
Introduction
Medical literature has been describing placebos and their effects for more than two hundred years, and the quest to understand the mechanisms and explore the applications is still expanding. Placebo for pain relief is perhaps the most well understood model with clearly defined neurobiological mechanisms [1].
The placebo effect describes ‘the positive response some patients / participants experience after receiving a placebo’. Placebo is in turn defined as ‘any therapy or component of therapy used for its nonspecific, psychological, or psychophysiological effect, or that is used for its presumed specific effect, but is without specific activity for the condition being treated’ [2]. This response has a beneficial effect, measurable either subjectively or objectively. These effects are related to intrinsic factors (e.g., personal expectations) either alone or in combination with extrinsic factors (e.g., environment, relationship with healthcare provider) [3].
Conversely, the Nocebo effect is ‘the negative response some patients / participants experience after receiving a placebo’. These effects are usually minor (e.g., headache, nausea) but can extend to life threatening (e.g., cardiac arrest) [3].
History
In medieval times, mourners were hired to attend funerals, but because their emotions were considered insincere, they were called ‘Placebos’, from the Latin for ‘I will please’ [4]. Nocebo derives from the word nocere, meaning ‘I shall harm’. For centuries placebos were perceived as morally useful and therapeutically precious, though they continued to evoke mixed feelings among clinicians. Indeed in 1803, Thomas Jefferson famously condemned the cynical use of placebo as a ‘pious fraud’ [4].
Despite the scepticism of Jefferson and others, the use of placebo was widespread in medicine until the first half of the twentieth century. After World War two, the respect for patient autonomy encouraged a debate over the ethical use of placebos in clinical practice. Moreover, an emphasis on transparency and candour in clinical practice meant that the traditional application of placebo was increasingly challenged [5].
In 1955, Henry Beecher, an eminent anaesthetist and medical ethicist, published a paper titled ‘The powerful placebo’, in which he stated, ‘Placebos have a high degree of therapeutic effectiveness in treating subjective responses’ [6]. This paper, in which he noted that placebos are effective 30% of the time, has been cited over a thousand times. Although in retrospect Beecher’s paper has been criticised for methodological flaws, at that time it had a profound effect on clinical research by advocating the use of placebo in the control group of randomised trials [4].
Two decades after Beecher, in a seminal paper, Levine and colleagues investigated the role of endorphins within the placebo effect. Following a dental procedure, patients were randomised to receive either morphine (treatment) or saline (placebo) injection. Patients from the placebo arm of the study were then separated into placeboresponders and non-responders. Both groups were then administered intravenous naloxone. The placebo responders reported increased pain following the naloxone injection, whereas the placebo non-responders showed no change in their pain scores, hence proving that endogenous endorphin production was the likely cause for the positive placebo effect in the group that initially responded to the saline injection [7]. Currently, many theories are postulated as possible explanations for the placebo effect.
Mechanisms of Placebo Analgesia
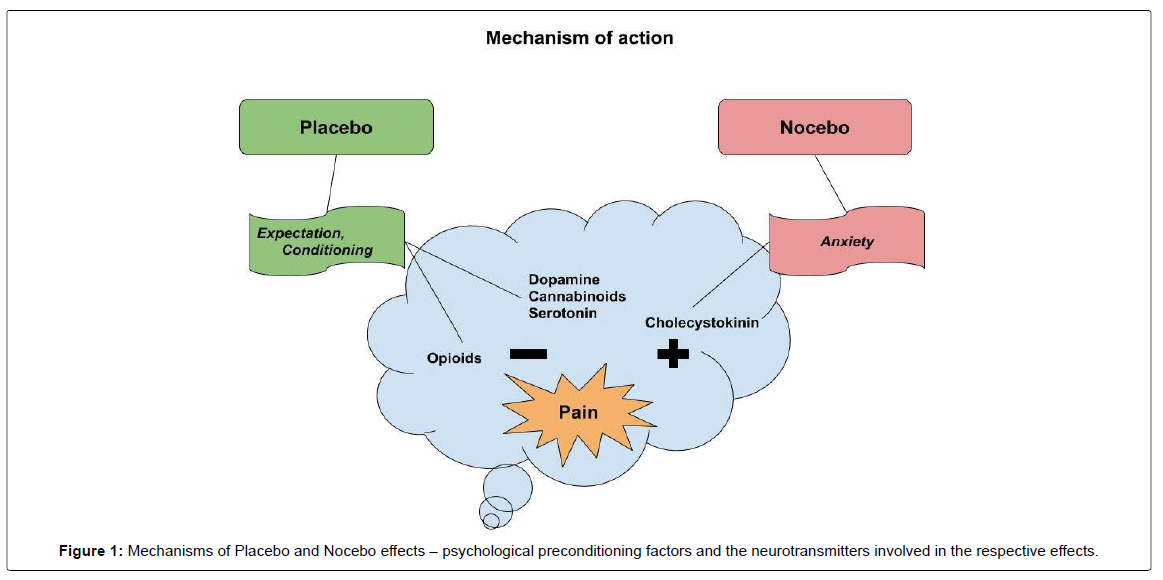
Pain relief by placebo is explained by psychological mechanisms (e.g., expectation, conditioning) and neurobiological mechanisms (e.g., endorphins, dopamine, serotonin, cannabinoids). The nocebo hyperalgesic response may be due to activation of the cholecystokininergic systems and is predisposed by psychological mechanisms such as anxiety and expectation of pain [8]. (Figure-1). When compared to the placebo effect, studies investigating the nocebo effect are relatively few due to the ethical difficulties involved, since nocebos trigger negative outcomes.
Psychological Mechanisms
Expectation of pain relief can induce placebo analgesia. Positive previous experiences can accentuate the placebo induced analgesic responses. Similarly, negative previous experiences attenuate the response to a subsequent placebo [9]. When these conditioning techniques are repeatedly combined with analgesic treatments, they can evoke a clinical effect similar to the response produced by the analgesic medications. For example, a placebo given after a repetitive administration of opioids produces opioid-like effects such as reducing the intensity of pain, and also opioid-induced adverse events, such as nausea [10]. Traits such as empathy, optimism, and altruism demonstrated by the clinician have been linked to reinforcing the effectiveness of placebo analgesia [10].
Neurobiological Mechanisms
Placebo analgesic effects have been demonstrated to activate different brain areas including the Dorsolateral Prefrontal Cortex, the rostral Anterior Cingulate Cortex and subcortical regions including the Hypothalamus, Amygdala and the Periaqueductal Gray. The Dorsolateral Prefrontal Cortex initiates the placebo analgesic response. The rostral Anterior Cingulate Cortex is connected to the Periaqueductal Gray and correlates with the modulation of placebo analgesia. These then connect to the descending pain inhibitory system involved in pain modulation [10]. It has been demonstrated that placebo analgesia is mediated by the release of endogenous neuropeptides such as opioids, dopamine, serotonin and cannabinoids [11].
Placebo and Brain Imaging
Although supraspinal cognitive and affective structures are thought to be involved in processing pain signals, the biological mechanisms are yet to be adequately elucidated. Brain imaging techniques such as functional magnetic resonance imaging (fMRI) and positron emission topography (PET) are beginning to provide an understanding of the central opioidergic system, and the changes produced by placebo effect on the central processing of pain [12]. Brain imaging studies suggest that placebo activates the same opioid receptor system to which remifentanil, a specific mu receptor agonist, binds [13]. Other fMRI studies identified that placebo analgesia is associated with reduced brain activity in pain sensitive brain areas like thalamus, thereby providing evidence that placebo alters the experience of pain [14]. FMRI studies have also shown that placebo analgesia reduces nociceptive processing in the spinal cord, suggesting that top-down mechanisms suppress pain processing in the central nervous system at the earliest stages.
Genetics and the Placebo Response
The study of the genomic effects on placebo response is termed the ‘placebome’. Several recent studies have explored the possibility that placebo responders share specific genetic and personality traits [15]. The analgesic effects of placebo have been shown to be mediated through activation of endogenous opioid as well dopaminergic and serotonergic mechanisms. However, genetic polymorphism in the expressing of these mechanisms may mean that placebo responses vary by genotype. Hall and others have identified 28 genes that are associated with 54 proteins, which can result in meaningful variation in the outcomes of the placebo arms of clinical trials. This genetic variation particularly relates to analgesics and antidepressants [16].
In randomised controlled trials, the placebo arm is commonly considered to be an adequate control for outcomes in the active treatment arm. But, if the placebo response varies by genotype, the potential ‘genedrug- placebo effect’ modification can make it challenging to interpret the results. Therefore, in order to improve the reliability and reproducibility of placebo controlled trials, a greater understanding of the role of the genetic influences in predicting placebo effect is essential.
Ethical Considerations
The current ethical debate over the clinical use of placebos hinges on whether it is permissible for clinicians to deceive patients in order to benefit them. While some authorities categorically reject the use of deceptive placebos, others argue that their therapeutic value justifies their use in certain circumstances. The American Medical Association’s position on placebo is that ‘physicians may use placebos for diagnosis or treatment only if the patient is informed of and agrees its use’ [17]. Guidelines have been developed for ethical use of placebo in the clinical and research environment [18, 19]. (Table 1)
| Placebo in clinical practice | Placebo in clinical trials |
|---|---|
| • The physician’s intentions must be benevolent. Financial, professional, or emotional interests should not influence clinical decisions. • The placebo should not be offered to placate or silence the patient. • If the placebo is not effective, it should be swiftly discontinued. • The placebo should not be given in place of another treatment that is considered to be more effective. • If asked, the physician should be open and transparent about the nature and effects of the placebo treatment. • It would be unethical to discontinue the placebo if the patient finds it helpful and there is no better alternative. |
• Placebo is justified in the absence of an effective and validated treatment for a condition. • When withholding standard therapy does not expose the participants to a higher risk. • When the study design mandates use of placebo and withholding standard therapy does not expose the participants to a higher risk. • When the study design mandates use of placebo, and the study intends to develop interventions, and it does not deprive patients of standard care for their condition. |
Table 1: Guidelines for ethical use of placebo.
While these guidelines may appear to be rigid and discourage the use of placebo in clinical and research arenas, in practice they offer a framework upon which ethical placebo use can be based.
Whenever appropriate, in randomized controlled trials, it is good practice to compare a new therapy or intervention with established treatments, rather than placebo. For example, when investigating the effectiveness of erector spinae plane block for post-thoracotomy pain, it is good practice to compare this technique with thoracic epidural analgesia rather than a sham procedure.
Recent Developments
‘Open Label Placebo’ is an interesting idea that breaks from the received wisdom that clinical administration of a placebo requires deception or double-blind conditions to be effective. Several studies have directly tested the effect of an Open Label Placebo prescription, and all indicated that patients reported benefits after taking pills presented honestly as placebos. A recent study found that adding Open Label Placebo to Treatment as Usual resulted in significantly greater reductions in chronic low back pain and pain related disability than Treatment as Usual alone. The amount of additional pain reduction produced by Open Label Placebo was approximately 30% of baseline pain and disability ratings [20]. Similar results have been replicated in studies for migraine, suggesting that taking a pill may have beneficial effects even if that pill is not deceptively presented as an effective medication [21].
The question then arises how a treatment openly labeled as placebo actually produces benefit without ‘fooling’ the mind, as randomized controlled trials do. The authors postulate “engendering hope when participants feel hopeless about their condition can be therapeutic” [21]. The Hawthorne (or Observer) effect may also be contributory: a person who is aware of being studied /observed may feel better, even if nothing has actually changed [4].
An intriguing phenomenon recently identified is that placebo responses are raising only in the United States. In 1996, patients in clinical trials described that drugs relieved their pain by 27% more than a placebo. By 2013, that gap had reduced to just 9% in United States clinical trials, largely due to an increase in the placebo effect. No significant change in placebo responses was observed in similar trials in Europe, Asia and elsewhere. One possible explanation is that direct-to-consumer drug advertising is allowed in the United States and this has increased people’s expectations of the benefits of drugs, creating stronger placebo effects. Moreover, big, well-funded United State trials, and the glamour and gloss of their presentation might indirectly augment patients’ expectations, leading to an increase in the placebo response. As a consequence, pharmaceutical companies are finding it harder to conduct placebo controlled clinical trials for new analgesic medications. In the last decade, more than 90% of potential drugs for treatment of cancer and neuropathic pain have failed at advanced phases of clinical trials [22].
Applications of Placebo
Translating placebo research into better patient care by ‘harnessing the placebo effect has been an ongoing topic of discussion. Placebo effects have an important role in at least three areas [23]. As controls in experimental studies to determine specific effects and to enable blinding, in placebo research to study placebo effects, As a tool in clinical practice.
Whereas the role of placebo in research is well established, the use of therapeutic placebo in clinical practice remains controversial. The Institute of Medicine (USA) opines that ‘Placebo can conceivably be a form of treatment of pain, especially in light of the shortcoming of other modalities or benefits they bring in their (own) right’ [20]. Historically, research on the factors that increase placebo response has focused on attributes of the treatment, showing that more invasive treatment modalities or specific pill colours are associated with higher placebo response rate, supporting the view that structured manipulation of physicians’ verbal and non-verbal performance may have a significant beneficial effect on the size of response to placebo analgesia [24] Physicians can produce a placebo-like effect through the skillful use of reassurance, encouragement and mutual respect, thereby improving health outcomes [17]. Studies have also demonstrated that patient education enhances perceptions of placebo knowledge, effectiveness and acceptability, even in deceptive treatment contexts [1]. It is possible that if physicians understood the mechanisms underlying placebo treatments, they may be more open with their patients when using this modality, particularly when used in conjunction with existing pain interventions to improve overall treatment effectiveness.
Barrett and others have proposed certain practical principles that clinicians can use to elicit placebo effects in order to enhance the feeling of being cared for, reduce anxiety, and reinforce positive expectations. These principles include: speaking about treatments in a positive light, fostering trust, providing reassurance and encouragement, building relationships, treating each patient as unique, exploring their values, and “creating ceremony” [25].
Conclusion
Over the last three decades, understanding of the complex mechanisms behind the placebo effect has improved considerably, with a clearer understanding of the alterations in central pain processing caused by placebo. Ethical concerns continue to persist, however; placebo is therefore less likely to be promoted as a stand-alone intervention if there is an evidence-based alternative. Scientific advances elucidating placebo’s psychological and neurobiological mechanisms have reinforced continued exploration of ethically acceptable placebo applications to facilitate better health outcomes. A better understanding of nocebo may also help in developing preventive measures by identifying factors that lead to maladaptive responses. Structured training in enhancing patient-doctor interactions is also likely to have a beneficial effect by potentiating the placebo response. r prevention of disease progression in osteoarthritis knee patients.
References
- Kisaalita N. R, Hurley R. W, Staud R, Robinson M. E. (2016). Placebo use in pain management: a mechanism-based educational intervention enhances placebo treatment acceptability. The Journal of Pain; 17(2): 257-269.
- Patterson CH. (1985). What is the placebo in psychotherapy? Psychotherapy: Theory, Research, Practice, Training; 22(2): 163-169
- Arnstein P, Broglio K, Wuhrman E, Kean MB. (2011). Use of Placebos in Pain Management. Pain Management Nursing ; 12(4): 225-229
- De Craen, AJ Kaptchuk TJ, Tijssen JG, Kleijnen J. (1999). Placebos and placebo effects in medicine: historical overview. Journal of the Royal Society of Medicine ; 92(10): 511-515
- Annoni M, Miller FG. (2014). Placebos in clinical practice: an ethical overview. Douleur et analgésie; 27.4: 215-220
- Beecher HK, Boston MD. (1955). The Powerful Placebo. JAMA ; 159(17): 1602–1606
- Levine JD, Gordon NC, Fields HL. (1978). The mechanism of placebo analgesia. Lancet; 2(8091): 654-7
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. (2005). Neurobiological mechanisms of the placebo effect. Journal of Neuroscience ; 25(45): 10390-10402
- Colloca L, Miller FG. (2011). Role of expectations in health. Current opinion in psychiatry; 24(2): 149-155
- Medoff ZM, Colloca L. (2015). Placebo analgesia: understanding the mechanisms. Pain Manag ; 5(2): 89–96
- Eippert F, Bingel U, Schoell ED. (2009).  Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron ; 63, 533–543
- Balasubramanian S, Morley-Forster P, Bureau Y. (2006) Opioids and Brain Imaging: Review. Journal of Opioid Management ; 2(3): 147-154
- Petrovic P, Kalso E, Petersson KM, Ingvar M. (2002). Placebo and opioid analgesia- imaging a shared neuronal network. Science ; 295:1737-40
- Wager TD, Rilling JK, Smith EE et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 2004; 303(5661): 1162-1167
- Hall KT, Loscalzo J, Kaptchuk TJ. (2015). Genetics and the Placebo Effect: the Placebome. Trends Mol Med; 21(5): 285–294
- Hall KT, Loscalzo J, Kaptchuk T. (2018). Pharmacogenomics and the placebo response. ACS Chem Neurosci ; 9(4): 633–635
- Bostick NA, Sade R, Levine MA, Stewart DM. (2008). Placebo use in clinical practice: report of the American Medical Association Council on Ethical and Judicial Affairs. Journal of Clinical Ethics: 19(1); 58
- Lichtenberg P, Heresco-Levy U, Nitzan U. (2004).The ethics of the placebo in clinical practice
- Millum J, Grady C. (2013).The Ethics of Placebo-controlled Trials: Methodological Justifications. Contemp Clin Trials ; 36(2).
- Carvalho C, Caetano JM, Cunha L. (2016). Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain ; 157: 2766–2772
- Kam-Hansen S, Jakubowski M, Kelley J. (2014). Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Translat Med ; 6:218ra5
- Tuttle AH, Tohyama S, Ramsay T. (2015). Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain ; 156(12): 2616–26
- Linde K, Fässler M, Meissner K. (2011). Placebo interventions, placebo effects and clinical practice. Phil. Trans. R. Soc. B. ; 366: 1905–1912
- Czerniak E, Biegon A, Ziv A. (2016). Manipulating the placebo response in experimental pain by altering doctor’s performance style. Frontiers in psychology ; 7: 874
- Barrett B, Muller D, Rakel D. (2006). Placebo, meaning and health. Perspectives in biology and medicine ; 49:178–198
Citation: Kapur S, Dunham R, Balasubramanian S (2021) Pain and Placebo. J Pain Relief 10: 370. DOI: 10.4172/2167-0846.1000370
Copyright: © 2021 Kapur S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3582
- [From(publication date): 0-2021 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 2653
- PDF downloads: 929