Research Article Open Access
Passive Cycling Limits Myofibrillar Protein Catabolism in Unconscious Patients: A Pilot Study
| Jean-Charles Preiser1*, Christophe De Prato1, Amélie Harvengt1, Lauriane Peters1, Marie-Hélène Bastin1, Vincent Fraipont2, Pierre Damas1, Jean-Michel Crielaard3 and Gianni Biolo4 | |
| 1Department of Intensive Care Erasme University Hospital, Belgium | |
| 2Centre Hospitalier Régional de La Citadelle, Liège, Belgium | |
| 3Department of Motricity Sciences, Centre Hospitalier Universitaire de Liège, Belgium | |
| 4Division of Internal Medicine, Department of Medical, Technological and Translational Sciences, University of Trieste, Trieste, Italy | |
| Corresponding Author : | Jean-Charles Preiser Department of Intensive Care Erasme University Hospital 808 route de Lennik 1070 Brussels, Belgium Tel: +3225554445 Fax: +32 25554698 E-mail: Jean-Charles.Preiser@erasme.ulb.ac.be |
| Received June 24, 2014; Accepted September 22, 2014; Published September 24, 2014 | |
| Citation: Preiser JC, Prato CD, Harvengt A, Peters L, Bastin MH, et al. (2014) Passive Cycling Limits Myofibrillar Protein Catabolism in Unconscious Patients: A Pilot Study. J Nov Physiother 4:225. doi: 10.4172/2165-7025.1000225 | |
| Copyright: © 2014 Preiser JC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
Background: To test the effects of passive cycling on muscle protein metabolism in unconscious patients.
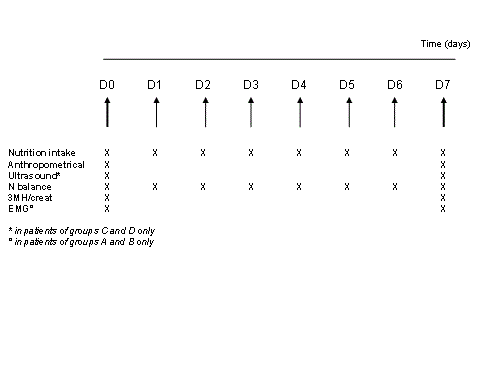
Materials and Methods: Twenty-seven patients (age 61.0 ± 16.4 years) admitted for coma (n=21) or with respiratory insufficiency requiring prolonged sedation were randomized to standard care (n=8) or passive cycling (2×30 minutes/day for 7 days, n=7). Longer-duration cycling (2×60 minutes/day, n=6) or passive cycling plus a hypercaloric hyperprotein diet (n=6) were assessed in separate groups. Ultrasound, biochemical and electrophysiological data were collected for 7 days. The thicknesses of the rectus femoris and of the vastus intermedius were measured by ultrasound. Myofibrillar protein catabolism was assessed by the urine 3- methylhistidine/creatinine ratio (3MH/creat).
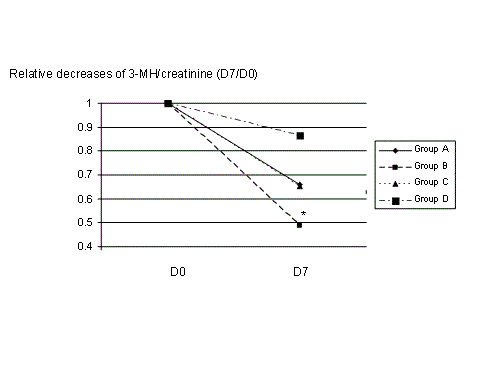
Findings: Passive cycling was well tolerated and resulted in a faster decrease in 3MH/creat and a slightly less negative nitrogen balance than standard care. These changes were not influenced by a longer duration of passive cycling or by a hypercaloric hyperprotein diet. There were no differences in muscle thicknesses or electromyographic data between standard care and passive cycling groups.
Conclusions: Passive cycling in comatose or sedated patients was associated with less myofibrillar proteolysis. If confirmed in larger trials, this approach could help to prevent the long-term muscular consequences of prolonged inactivity in critically ill patients.
Materials and Methods: Twenty-seven patients (age 61.0 ± 16.4 years) admitted for coma (n=21) or with respiratory insufficiency requiring prolonged sedation were randomized to standard care (n=8) or passive cycling (2×30 minutes/day for 7 days, n=7). Longer-duration cycling (2×60 minutes/day, n=6) or passive cycling plus a hypercaloric hyperprotein diet (n=6) were assessed in separate groups. Ultrasound, biochemical and electrophysiological data were collected for 7 days. The thicknesses of the rectus femoris and of the vastus intermedius were measured by ultrasound. Myofibrillar protein catabolism was assessed by the urine 3-methylhistidine/creatinine ratio (3MH/creat).
Findings: Passive cycling was well tolerated and resulted in a faster decrease in 3MH/creat and a slightly less negative nitrogen balance than standard care. These changes were not influenced by a longer duration of passive cycling or by a hypercaloric hyperprotein diet. There were no differences in muscle thicknesses or electromyographic data between standard care and passive cycling groups.
Conclusions: Passive cycling in comatose or sedated patients was associated with less myofibrillar proteolysis. If confirmed in larger trials, this approach could help to prevent the long-term muscular consequences of prolonged inactivity in critically ill patients.
The development of muscle weakness in ICU patients is associated with multiple factors, including bed rest [4], multiple organ failure, muscle inactivity, hyperglycemia, and use of corticosteroids and neuromuscular blockers [5,6]. The combination of these factors likely results in increased muscular proteolytic activity [7], largely exceeding the protein synthesis rate [8].
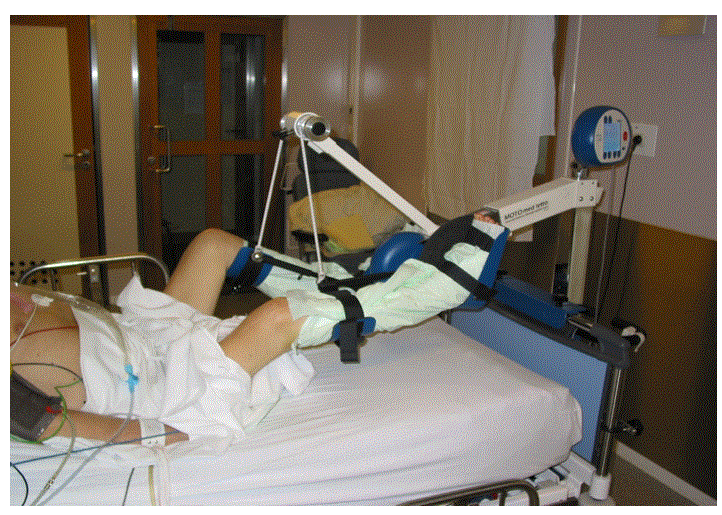
Several therapeutic approaches to limit ICU-acquired muscle weakness have been investigated. Early exercise and mobilization (physical and occupational therapy) during periods of daily interruption of sedation were associated with better functional outcomes at hospital discharge, evaluated by the ability to perform six activities of daily living and to walk independently [9]. Resistance exercise increased the acute amplitude of mitochondrial and sarcoplasmic protein synthesis and resulted in a robust, but delayed stimulation of myofibrillar protein synthesis [10]. Burtin et al. reported that passive or active exercise training sessions using a bedside ergometer were associated with improvements in the 6-min walking distance, the isometric quadriceps force, and the subjective feeling of functional and physical well-being (SF-36 questionnaire) at hospital discharge [11]. These effects of passive cycling could be partially related to an increase in muscle blood flow and to the prevention of joint stiffness.
When active techniques cannot be used, passive techniques may provide some benefit. In one study, passive stretching of one leg was reported to preserve the architecture of muscle fibers, although prevention of muscle wasting was uncertain [12]. Passive mobilization, such as cycling, can trigger muscular contraction after stimulation of stretch receptors, even in the absence of neural stimulation.
The present study aimed to define the intrinsic effects of passive cycling on muscle metabolism and function in patients with prolonged minimal muscular activity. Specifically, this pilot trial aimed to assess: 1) the effects of mobilization by passive cycling on muscle thickness, electrophysiological features and myofibrillar protein catabolism; 2) whether there was a dose-effect relationship between the intensity and duration of exercise and the magnitude of changes in muscle catabolism and thickness; and 3) whether there was a synergistic effect of increased calorie and protein intake with passive mobilization.
Inclusion criteria were the following: Adult (> 18 years at admission); period of unconsciousness and/or sedation and mechanical ventilation anticipated to last at least 7 days; and hemodynamic stability (i.e., no requirement for increasing doses of vasopressor agents). Exclusion criteria were: No signed consent; systemic treatment with steroids (intravenous or oral); contra-indication to mobilization of the lower limbs, including pelvic instability, leg fractures, or recent deep venous thrombosis. Standard care included manual mobilization of the limbs and passive turning twice daily by the nurse and the physiotherapist, mechanical ventilation, and nutritional support (Nutrison® Standard, Nutricia, Zoetermeer, NL) preferentially provided continuously by the enteral route, with target calories and nitrogen set at 25 kcal/kg/day and 1.2 kcal/kg/day, respectively. Enteral nutrition was considered as successful when at least 60% of the prescribed amount was delivered. The study was discontinued in patients who recovered spontaneous motor activity, could be weaned from mechanical ventilation, or died.
The model used in this clinical study is unique, as only patients with minimal muscular activity for a period of at least 7 days were studied. The increased rate of myofibrillar protein catabolism was confirmed by the supra-normal values of the 3-MH-to creatinine ratio. Admittedly, pure passive stretch was not investigated when neuromuscular blockade was not used. Therefore, these results may be confounded by the reflex arc and upper neuronal influences on tone and spasticity. However, the design of this study is a reflection of the current standard of care, since muscle paralysis is rarely used in the type of patient included in this study. The effects of standard physiotherapy only (passive mobilization and turning twice a day) on protein catabolism are probably minimal, as suggested by the slight decrease in the 3-MH-to creatinine ratio observed in group A. No potentially confounding factors, such as new sepsis, surgery or hypoxemia, were noted during the study period. However, in patients with ongoing sepsis or recent surgery, acute inflammatory changes in muscle metabolism may differ from those present in long-stay neurological patients. These patients were probably representative of a typical ICU population of long-stayers, in whom acquired weakness is a major issue. Nevertheless, as most patients were admitted for a neurological diagnosis, whether the findings of this study can be applied to non-neurological patients is unclear.
The use of the 3-MH-to creatinine ratio as an index of myofibrillar protein catabolism, was described in 1978 [13] and validated in 1981 for critically ill patients with sepsis or trauma [14]. The 3-MH-to creatinine ratio has been shown to decrease over time after surgical injury [15]. The faster decrease in the 3-MH-to creatinine ratio in patients treated with passive mobilization compared to standard care (sub-study 1) reflects the decreased myofibrillar protein catabolism, and is consistent with the slight decrease in nitrogen balance. Admittedly, some unrecorded changes in renal function may have influenced the 3-MH-to creatinine ratio [14,16]. The influence of changes in non-muscular protein metabolism, including changes in the protein turnover of the gastrointestinal tract, can also not be assessed from the present data. Indeed, the rate of muscle loss follows a logarithmic curve, implying a decreased rate of muscle loss after the acute inflammatory phase. The differences in time interval between admission in the ICU and study inclusion may have introduced a risk of ‘lead-time bias’.
Increased protein turnover involving an increased rate of protein synthesis followed by increased breakdown cannot be excluded. To evaluate the influence of these factors, more sophisticated or more invasive techniques, such as stable isotope methods or muscle biopsy, would be needed.
The absence of any effect of passive cycling on muscle thickness could be related to changes in the water and fat content of the muscles and surrounding tissues, perhaps related to changes in the muscle glycogen content. Muscle strength cannot anyway be deduced from measurements of muscle thickness, unlike muscular cross-sectional area. Others have shown slow changes in limb perimeters in critically ill patients [17]. Computerized tomography would probably have been ideal for accurate quantification of muscle mass [18], although this technique has not been widely used in ICU patients. Instead, we used echography with a 10-Hz probe, a technique that has been validated to assess the magnitude of ICU-acquired muscle wasting [19]. Variance in ultrasound measurements was minimized because the same, experienced radiologist collected these data and the technique used was standardized. The changes in muscle thicknesses seen in critically ill patients have been reported to be very heterogeneous [17]. Of note, after spinal cord injury in children, passive cycling increased muscle bulk only when associated with electrical stimulation of the quadriceps [20]. In the ICU, preliminary data suggest that neuromuscular electrical stimulation (NMES) provides some muscular activity even very early during critical illness, potentially helping preserve muscle mass [21] and increase muscle strength [22].
The lack of association between the interventions tested in sub-studies 2 and 3 (increased duration of cycling and increased intake of calories and proteins, respectively) and the ultrasound estimates of muscle thickness could also be related to the relatively short time interval (7 days) between the two measurements. In particular, the slope of the 3-MH-to creatinine ratio over time tended to be steeper in group C (long-duration cycling, decrease of 34 % from baseline) than in group B (27% from baseline), implying that the inclusion of a larger number of patients would allow a more accurate assessment of a dose-response effect, which was not the primary aim of the present study. The lack of a synergistic effect of increased calories and nitrogen with passive mobilization could be related to the amino-acid composition of the enriched formula, and/or to an excessive caloric load [23].
Interpretation of these data is limited because of the small number of patients who could be studied until the end of the 7-day period. The comparability of the groups at baseline is not established, because the proportion of primarily neurological patients differed, as did the time from ICU admission to study inclusion. However, the aim of the study was to assess the effects of passive cycling in all types of immobile patient at any time during the ICU stay. Differences in the concentrations of 3MH-to-creatinine ratio between sub-study 1 and sub-studies 2 and 3 could reflect lower muscle protein catabolism in groups C and D. Nonetheless, the relative change over time is probably a better reflection of the effect of the intervention than absolute values. For this reason, we preferred to use paired values (day 0 and day 7) to show the relative changes in the 3MH/creatinine ratio measured in samples processed simultaneously. The delay between admission to the ICU and inclusion in the study was also variable, in relation with the course of the disease: Some patients were awake at the time of admission and en developed a complication (loss of consciousness or requirement for sedation). This could confound our assessment of the effect of passive cycling, because muscle loss/protein catabolism could have begun well before the intervention. Unfortunately the doses of sedative used in some patients, the mean blood glucose level and the organ failure score during the study period were not recorded. No neuromuscular blocking agent was given during the study. However, regardless of the effects of passive mobilization, the set-up of the present trial might be helpful to evaluate and score the severity of muscular weakness.
Despite these limitations, we believe that our observations in this pilot “proof-of-concept” study, i.e., a decrease in myofibrillar protein catabolism and tolerance to passive cycling in patients with a prolonged period of minimal muscular activity, opens the way for further investigations. Changes in muscle blood flow, water/fluid shifts, stretching of the connective tissue or muscular contractions should be recorded in future evaluations. Obviously, larger study samples, longer periods of observation and intervention, and more frequent or longer duration sessions will be needed to fully evaluate the effects of passive mobilization on muscle function and recovery.
References
- Schefold JC, Bierbrauer J, Weber-Carstens S (2010) Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock.J Cachexia Sarcopenia Muscle 1: 147-157.
- Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, et al. (2009) A framework for diagnosing and classifying intensive care unit-acquired weakness.Crit Care Med 37: S299-308.
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, et al. (2011) Functional disability 5 years after acute respiratory distress syndrome.N Engl J Med 364: 1293-1304.
- Allen C, Glasziou P, Del Mar C (1999) Bed rest: a potentially harmful treatment needing more careful evaluation.Lancet 354: 1229-1233.
- de Jonghe B, Lacherade JC, Sharshar T, Outin H (2009) Intensive care unit-acquired weakness: risk factors and prevention.Crit Care Med 37: S309-315.
- Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, et al. (2007) Neuromuscular dysfunction acquired in critical illness: a systematic review.Intensive Care Med 33: 1876-1891.
- Klaude M, Hammarqvist F, Wemerman J (2005) An assay of microsomal membrane-associated proteasomes demonstrates increased proteolytic activity in skeletal muscle of intensive care unit patients.ClinNutr 24: 259-265.
- Streat SJ, Beddoe AH, Hill GL (1987) Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients.J Trauma 27: 262-266.
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, et al. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial.Lancet 373: 1874-1882.
- Burd NA, Andrews RJ, West DW, et al (2012) Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol 590: 351-362
- Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, et al. (2009) Early exercise in critically ill patients enhances short-term functional recovery.Crit Care Med 37: 2499-2505.
- Griffiths RD, Palmer TE, Helliwell T, MacLennan P, MacMillan RR (1995) Effect of passive stretching on the wasting of muscle in the critically ill.Nutrition 11: 428-432.
- Young VR, Munro HN (1978) Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview.Fed Proc 37: 2291-2300.
- Long CL, Birkhahn RH, Geiger JW, et al. (1981) Urinary excretion of 3-methylhistidine: an assessment of muscle protein catabolism in adult normal subjects and during malnutrition, sepsis, and skeletal trauma. Metabolism 30:765-776
- Bérard MP, Pelletier A, Ollivier JM, Gentil B, Cynober L (2002) Qualitative manipulation of amino acid supply during total parenteral nutrition in surgical patients.JPEN J Parenter Enteral Nutr 26: 136-143.
- Sjölin J, Stjernström H, Henneberg S, Hambraeus L, Friman G (1989) Evaluation of urinary 3-methylhistidine excretion in infection by measurements of 1-methylhistidine and the creatinine ratios.Am J ClinNutr 49: 62-70.
- Reid CL, Campbell IT, Little RA (2004) Muscle wasting and energy balance in critical illness.ClinNutr 23: 273-280.
- Prado CM, Birdsell LA, Baracos VE (2009) The emerging role of computerized tomography in assessing cancer cachexia.CurrOpin Support Palliat Care 3: 269-275.
- Gruther W, Benesch T, Zorn C, Paternostro-Sluga T, Quittan M, et al. (2008) Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer.J Rehabil Med 40: 185-189.
- Johnston TE, Modlesky CM, Betz RR, Lauer RT (2011) Muscle changes following cycling and/or electrical stimulation in pediatric spinal cord injury.Arch Phys Med Rehabil 92: 1937-1943.
- Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, et al. (2009) Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study.Crit Care 13: R161.
- Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, et al. (2010) Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial.Crit Care 14: R74.
- Biolo G, Agostini F, Simunic B, Sturma M, Torelli L, et al. (2008) Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest.Am J ClinNutr 88: 950-958.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 14524
- [From(publication date):
September-2014 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 9887
- PDF downloads : 4637
