Pattern and Distribution of Lymph Node Metastases in Papillary Thyroid Cancer
Received: 09-Dec-2014 / Accepted Date: 26-Dec-2014 / Published Date: 03-Jan-2015 DOI: 10.4172/2161-0681.1000204
Abstract
Background: The indications and extent of lymph node dissection in the treatment of papillary thyroid carcinoma remains controversial, and benefit from therapy is debatable. This study was designed to identify the pattern and distribution of lymph node metastases and to establish an optimal strategy for neck dissection for those patients.
Methods: A total of 44 patients diagnosed with papillary thyroid cancer were treated from 2006 to 2013. All patients underwent total thyroidectomy, central neck dissection, and ipsilateral selective neck dissection removing lymphatic structures in levels II through V. The frequency of cervical lymph node metastases in each level, and the presence of capsular invasion were analysed. In addition, we investigated postoperative complications after total thyroidectomy and central lymph node dissection.
Results: Lymph node metastases were found in 18 patients (40.9%); all of them had ipsilateral level VI nodal involvement. 7 patients had level V involvement, 2 patients had level II affection, 3 patients had level III & IV affection and 2 patients had contralateral level VI lymph node affection. We also found extracapsular invasion in 6 (13.6%) patients and grade I, II, III in 2, 40, 2 patients respectively. The frequency of temporary hypocalcaemia, permanent hypocalcaemia and temporary vocal cord paralysis were 6.8%, 2.3% and 4.5%, respectively.
Conclusion: We recommend total thyroidectomy and central compartment lymph node dissection. If ipsilateral central lymph nodes are positive for metastases in frozen section, we proceed to ipsilateral selective neck dissection removing lymphatic structures in levels II through V even in the absence of clinically evident lymph node metastasis irrespective of tumor size. The technique had a low rate of complications; namely laryngeal nerve injury and hypoparathyroidism.
Keywords: Papillary thyroid carcinoma; Total thyroidectomy; Lymph node metastases; Neck dissection
311616Introduction
Papillary Thyroid Cancer (PTC) is the commonest type of thyroid cancer, representing about 75% of all thyroid malignancies and more than 90% of differentiated thyroid cancer [1]. PTC shows a mild biological behavior and has an excellent prognosis. Adequate management leads to a survival rate in excess of 90%. Death due to PTC is very rare [2]. However, cervical lymph nodal metastases are common in PTC and are associated with a significant probability for loco-regional recurrence of the disease. As a result, a rapid shift in patient care from a focus on overall survival to a focus on recurrencefree survival has recently noted. These considerations generated a strong interest in a more comprehensive preoperative evaluation of the neck and renewed the controversy about the role and the extent of lymphadenectomy at the time of thyroidectomy [3,4].
Subclinical nodal disease has been demonstrated in electively dissected necks that had no nodal disease detected clinically or by US. Clinical examination may detect lymph node involvement in 15-30% of patients [5].
Despite the very high incidence of cervical lymph node metastases in PTC, the reported rate of loco-regional recurrence ranges between 3% and 30% for low-risk PTC [6]. Even for high risk cases, the rate is only 59%; often in patients with evidence of macroscopically involved nodes [2].
The surgeon should recognize that local nodal recurrence is a significant problem for patients, associated with a poor prognosis and high morbidity and mortality rates, usually due to invasion of the trachea or the great vessels or to recurrent laryngeal nerve involvement [7]. Reoperation is a traumatic event and may be associated with unacceptably high complication rates, such as injury to the recurrent laryngeal nerve, hypoparathy-roidism, palsy of the spinal accessory nerve, and unsightly surgical scars [8].
Many questions remain unanswered regarding the optimal management of patients with cervical lymph node metastases. In selecting the optimal management, an in-depth understanding of the biological behavior of cervical lymph node metastases is required. Ideally, surgical treatment should be radical enough in order to achieve complete eradication of the disease, while -at the same timeminimizing treatment and disease-related morbidity. Routine total thyroidectomy with Cervical Lymph Node Dissection (CLND) would be theoretically the ideal operation. However, such an aggressive surgical approach will represent over-treatment in a large percentage of patients, associated with an unjustified increase of surgical complications [2]. The complications that may occur due to cervical lymph node dissection includes hypoparathyroidism (temporary or permanent), nerve injury (Recurrent laryngeal nerve, superior laryngeal nerve, spinal accessory, ramus mandibularis, sympathetic chain, phrenic nerve, brachial plexus, cutaneous cervical plexus), chyle leak due to thoracic duct injury, hemorrhage, seroma and wound infection [2]. These complications are very low with best hand or in high volume centers.
The aim of this study is to evaluate the frequency and pattern of regional lymph node metastasis in patients with papillary thyroid cancer and clinically negative lymph node metastases to establish the optimal strategy for neck dissection in these patients.
Patients and methods
A total of 44 patients diagnosed with papillary thyroid cancer with clinically or radiologically negative lateral lymph nodes metastases were treated in Menofia University and National Cancer Institute from 2006 to 2013; all were treated with intent to cure. The study included 11 males and 33 females. The mean age at initial treatment was 38.9 ± 11.9 years (range: 21-68 years).
Ethical approval: This study was approved by the ethical committee of Faculty of Medicine, Menofia University in accordance with the Helsinki guidelines for the protection of human subjects. Written informed consents were obtained from all participants involved in our study.
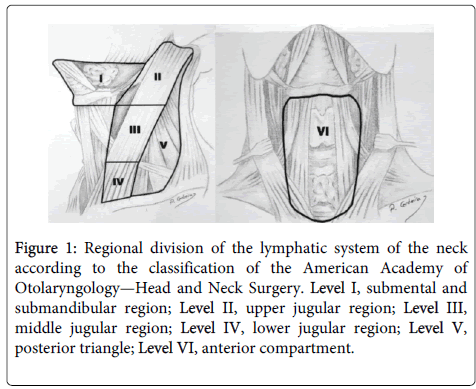
All patients underwent good clinical examination and neck ultrasound. All patients were diagnosed as papillary thyroid cancer by FNAC or by frozen section biopsy during surgery. Patients with nonpapillary cancer, bilateral disease and distant metastasis were excluded from this study. All patients underwent total thyroidectomy, bilateral central compartement lymph node dissection and ipsilateral selective neck dissection removing levels II through V. The central compartment was limited by the hyoid bone superiorly, the innominate vein inferiorly, the carotid sheaths in both sides laterally and the paravertebral fascia dorsally and was divided into 3 node sites: pretracheal, ipsilateral level VI and contralateral level VI lymph nodes. Neck levels were defined by nomenclature of Memorial Sloan- Kettering Cancer Center in the United States: level I, addressing submental and submandibular lymph nodes, levels II, III, IV addressing upper, mid and lower jugular lymph nodes respectively, and level V posterior triangle lymph nodes (Figure 1).
Figure 1: Regional division of the lymphatic system of the neck according to the classification of the American Academy of Otolaryngology—Head and Neck Surgery. Level I, submental and submandibular region; Level II, upper jugular region; Level III, middle jugular region; Level IV, lower jugular region; Level V, posterior triangle; Level VI, anterior compartment.
The neck dissection was performed in a standard fashion, sparing the internal jugular vein, spinal accessory nerve and sternomastoid muscle.
Technique of surgery
The procedure is performed in the operating room setting with the patient under general anesthesia. Total thyroidectomy with taking care to the recurrent laryngeal nerve and parathyroid glands with intact blood supply.
Central neck dissection
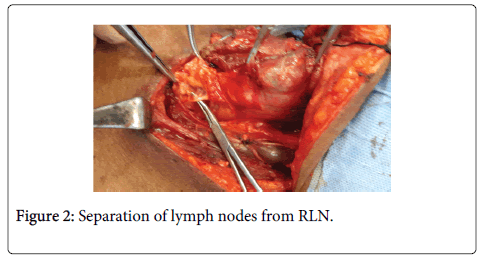
• The pretracheal lymph nodes usually are dissected off the trachea at the time of thyroidectomy (Figure 2).
• After identification of the RLN, the lateral side of the paratracheal lymph nodes is then separated from the carotid sheath, and the dissection line is extended to the innominate artery or brachiocephalic vein.
• Dissection for the right central compartment and left central compartment differ slightly as a result of the anatomical course of the nerve. Because the right RLN loops around the subclavian artery and ascends apart from the tracheoesophageal groove, it divides the right paratracheal lymph nodes into anterior and posterior compartments. The posterior compartment LNs are mobilized anteriorly and then divided along the right RLN. Then the fibrofatty tissue and lymph nodes inferior to the inferior thyroid artery and anterior to the RLN are then mobilized off the prevertebral fascia and esophagus. The dissection of this fibrofatty should be carried down to the level of the innominate artery or brachiocephalic vein to incorporate the anterior compartment LNs.
• In contrast, the left RLN loops around the aortic arch and travels along the tracheoesophageal groove. The esophagus is present immediately behind the nerve. Therefore, after the paratracheal lymph nodes are dissected from the carotid sheath, dissection of the lymph nodes medial and lateral to the left RLN–without division is usually done and is sufficient for the left side. Also the dissection should be carried down to the innominate artery or brachiocephalic vein.
• At the completion of central compartment dissection, the viability of the parathyroid glands should be assessed. Typically, the superior gland can be identified and remain in situ. The inferior parathyroid glands may have to be resected to allow a comprehensive lymphadenectomy within the central compartment or if they was adherent to a bulky nodal disease or disease with gross extracapsular spread. If any gland was removed with lymphadenectomy, or if any gland’s viability is in question, the gland should be minced into 1 mm cubes and implanted into the sternocleidomastoid muscle or brachioradialis muscle of the forearm and the site of implantation is marked with a surgical clip.
• After completion of the central dissection, we send the specimen for frozen section to know if the central lymph nodes are positive for metastases or not.
• If the central nodes were positive in frozen section, we extend the incision along the anterior border of the sternomastoid muscle to the mastoid process on both sides (U-shaped incision) to do the bilateral neck dissection.
• The flaps are elevated deep to the platysma muscle preserving the superficial layer of the cervical fascia exposing the posterior belly of digastic muscle on both sides superiorly, anterior border of trapezius muscle on both sides posteriorly and supra-clavicular fossa on both sides inferiorly.
Dissection of the posterior triangle
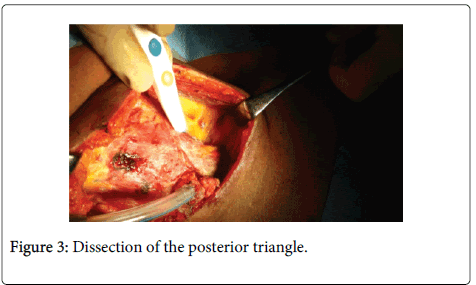
The region is approached posterior to the sternomastoid muscle. The loose fibrofatty tissue in this area is removed from the anterior border of the trapezius muscle in a medial direction including the lymphatic contents of this area. The omohyoid muscle is identified and transected at this stage and its fascia is removed with the contents of the posterior triangle. The transverse cervical vessels are identified deep to the omohyoid muscle. At the upper margin of this area, the spinal accessory nerve should be identified while exiting from the sternomastoid muscle to reach the trapezius muscle. The deep layer of the cervical fascia over the levator scapulae and scalene muscles is preserved protecting the brachial plexus and phrenic nerve. The dissection is continued medially to the sternocleidomastoid muscle which is retracted laterally and the contents are passed underneath to continue the dissection anterior to the muscle towards the carotid sheath (Figure 3).
• After that identification of the spinal accessory nerve anterior to the steromastoid muscle is done. The sternocleidomastoid muscle is retracted posteriorly and the posterior belly of the digastric muscle is pulled superiorly with a smooth blade retractor. At this level the nerve runs within the “lymphatic container” of the neck. In consequence, the tissue overlying the nerve is divided and the nerve completely exposed. Usually, the internal jugular vein lies immediately behind the proximal portion of the nerve. Once the spinal accessory nerve has been completely exposed, the tissue lying superior and posterior to the nerve must be dissected from the splenius capitis and levator scapulae muscles. The occipital artery may be ligated or cauterized here. When the dissected tissue reaches the level of the spinal accessory nerve it must be passed beneath the nerve to be removed in continuity with the main part of the specimen.
Dissection of carotid sheath
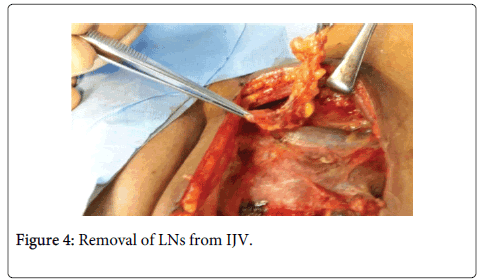
The surgical specimen is grasped with hemostats and retracted medially, while the surgeon uses one hand with a gauze pad to pull laterally over the deep cervical muscles. The fascia is then removed from the Internal Jugular Vein (IJV). This is achieved by using a head and neck scissor along the wall of the internal jugular vein up and down along its entire length (Figure 4).
When this is properly done and the traction exerted on the tissue is adequate, this maneuver is extremely safe and effective. The facial, lingual, and thyroid veins should be clearly identified, ligated, and divided to complete the isolation of the internal jugular vein. The traction exerted to facilitate the dissection of the fascial envelope produces a folding of the wall of the internal jugular vein that can be easily injured during dissection. So, the surgeon must be extremely cautious to avoid injuring the vein during dissection. Lower in the neck, the terminal portion of the thoracic duct on the left side, and the right lymphatic duct-when present also are within the boundaries of the dissection and must be preserved. Once the internal jugular vein is released from its covering fascia, the dissection continues medially over the carotid artery and released from it. The specimen is now completely freed from the neck.
We labeled the specimens of lymph nodes as ipsilateral level VI nodes, contralateral level VI nodes, level II nodes, levels III & IV nodes and level V nodes. All these specimens were evaluated histopathologically with the thyroid gland. The frequency and pattern of lymph node metastasis in the central and lateral compartments were analyzed. Postoperative hypocalcemia and recurrent laryngeal nerve injury were also evaluated. Postoperative hypocalcemia was defined as at least one event of hypocalcemia symptoms (perioral numbness, or parasthesia of hands and feet) or at least one event of biochemical hypocalcemia (ionized Ca level <1.0 mmol/L or total Ca level <8.0 mg/ dl). Ionized Ca level and / or total Ca level were checked on follow up. Permanent hypocalcemia was defined as persistent symptoms or persistent biochemical hypocalcemia >6 months. Postoperative recurrent laryngeal palsy was also investigated. Vocal fold mobility was evaluated with routine laryngoscopy immediately after thyroidectomy.Patients with postoperative vocal fold palsy were examined with laryngoscope at every follow up.
Statistical Methods
Data was analyzed using IBM SPSS Advanced Statistics version 20.0 (SPSS Inc., Chicago, IL). Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. A p-value <0.05 was considered significant.
Results
Table 1 shows description of the personal and clinical characteristics of the studied group (n=44). Level VI of ipsilateral lymph nodes was involved in 18 patients (40.9%). This level was the main level of lymph node metastases, followed by level V nodes (15.9%). Contralateral level VI nodes were only involved in 2 patients (4.5%). Table 2 shows significant association between extracapsular tumor invasion and ipsilateral level VI nodal metastases (p=0.003). Also, 6 of the 7 patients with level Apparently, higher grade seems to be more frequently associated with level VI nodal metastases, despite lack of statistical evidence owing to the small number of cases with grade I or III disease. V nodal involvement had Extra-capsular invasion (p<0.001).
| Variable | Description |
|---|---|
| Age, mean±SD (years) | 38.9±11.9 |
| Gender (male/female) | 11/33 |
| Grade (I/II/III) | 2/40/2 |
| Extracapsular invasion, No. (%) | 6 (13.6%) |
| Lymph node involvement | |
| Ipsilateral level VI, No. (%) | 18 (40.9%) |
| Ipsilateral level II, No. (%) | 2 (4.5%) |
| Ipsilateral level III & IV, No. (%) | 3 (6.8%) |
| Ipsilateral level V, No. (%) | 7 (15.9%) |
| Contralateral level VI, No. (%) | 2 (4.5%) |
| Temporary hypocalcemia, No. (%) | 3 (6.8%) |
| Permanent hypocalcemia, No. (%) | 1 (2.3%) |
| Temporary vocal cord dysfunction, No. (%) | 2 (4.5%) |
Table 1: Demographic and clinical characteristics of the studied group (n=44).
| Positive n = 18 |
Negative n = 26 |
p value | |
|---|---|---|---|
| Tumor size | |||
| <1 cm | 7 (53.8%) | 6 (46.2%) | 0.258 |
| ≥1 cm | 11 (35.5%) | 20 (64.5%) | |
| Grade | * | ||
| I | 0 (0.0%) | 2 (100.0%) | |
| II | 16 (40.0%) | 24 (60.0%) | |
| III | 2 (100.0%) | 0 (0.0%) | |
| Extracapsular Invasion | 0.003 | ||
| Positive | 12(100.0%) | 0 (0.0%) | |
| Negative | 6 (18.8%) | 26 (81.2%) | |
| * No p value due to the small number of cases in grade I and III | |||
Table 2: Relation between ipsilateral level VI lymph node involvement and tumor characteristics.
The primary tumor size does not affect the frequency of lymph node metastases, 11/31 patients (35.5%) with tumor size >1 cm had lymph node metastases have compared to 7/13 patients (53.8%) with tumor size ≤1 cm (p=0.258). It was evident that ipsilateral II, III & IV and V and contralateral level VI nodes are exclusively involved only in patients with level VI ipsilateral lymph nodal metastases (Table 3). Ipsilateral level V nodal involvement was significantly associated with level VI nodal metastases (p=0.001). Also, there is tendency to statistical significance (p=0.062) in the association between Ipsilateral level III & IV and level VI lymph node metastases.
| Positive n = 18 |
Negative n = 26 |
p value | |
|---|---|---|---|
| Ipsilateral level II (n = 2) | 0.162 | ||
| Positive | 2 (11.1%) | 0 (0.0%) | |
| Negative | 16 (88.9%) | 26 (100.0%) | |
| Ipsilateral level III & IV (n = 3) | 0.062 | ||
| Positive | 3 (16.7%) | 0 (0.0%) | |
| Negative | 15 (83.3%) | 26 (100.0%) | |
| Ipsilateral level V (n = 7) | 0.001 | ||
| Positive | 7 (38.9%) | 0 (0.0%) | |
| Negative | 11 (61.1%) | 26 (100.0%) |
Table 3: Relation between ipsilateral level VI lymph node involvement and other levels of lymph node involvement
Similar association was found between level V involvement and level III & IV nodal metastases (p=0.003). All patients with negative level V nodes (n=37) had also negative level III & IV nodes, while 3 patients (42.9%) of the former group had positive level III & IV nodes. The same was observed in patients with level II nodal metastases; the two patients with level II positive nodes had level V positive nodes (p=0.022). The two cases of vocal cord dysfunction did not have level VI nodal metastases (p=0.505). Similarly, there was no association between temporary hypocalcemia and level VI nodal metastases (p=0.558); two cases had positive nodes and one did not.
Table 4 shows the relation between between ipsilateral level VI lymph node involvement and contralateral level VI. Of the 18 patients with positive ipsilateral central nodes, we found only 2 patients having positive contralateral level VI. While all patients with no ipsilateral level VI lymph nodes affection were found to have no contralateral level VI lymph node affection.
| Positive n = 18 |
Negative n = 26 |
p value | |
|---|---|---|---|
| Contralateral level VI | 0.162 | ||
| Positive | 2 (11.1%) | 0 (0.0%) | |
| Negative | 16 (88.9%) | 26 (100.0%) |
Table 4: Relation between ipsilateral level VI lymph node involvement and contralateral level VI.
Discussion
The current study demonstrated that 40.9% of patients (18/44) have lymph node metastasis; within the central compartment, the ipsilateral level VI was involved in all of them. These results are similar to a study by Wada and colleagues [9], who found ipsilateral level VI nodal involvement in 36.3% of a larger series (n=259). Machens and colleagues [10] reported lower proportion of level VI lymph node involvement (29%).
In our study, we found that 4 patients with level V lymph node metastases have no metastases in the lateral group. This would be against the well-established literature, that level V affection does not occur without affection of the lateral group and this raises a concern about the proper nodal level labeling for examination, or the inclusion of part of level IV (which are posterior to the internal jugular vein) with the specimen of level V, thus causing an error in the frequency of the affected level V [11].
Our study demonstrated an association between lymph node metastases to level V and metastases to levels II or III & IV. This observation was similar to a recent study by Kupferman et al [12]. We consider performing a level V lymphadenectomy in patients in whom metastatic lymph nodes are suspected clinically in levels II, III, and IV. The ipsilateral level II is rarely involved (2/44 patients). The ipsilateral levels III & IV lymph nodes were involved in 3 patients (6.8%).We found that the contralateral central compartment lymph nodes were involved in only two patients (4.5%). Isolated contralateral central compartment lymph node metastasis was not found in our study. These results may be matched with results by Machens et al. [10], who found that the rate of contralateral level VI lymph node metastases for the primary and reoperative papillary thyroid cancer was 5% to 31%.
Koo et al. [13] reported higher rate contralateral level VI lymph node metastasis of 34%. We found that the primary tumor size did not affect the frequency of lymph node metastases (p=0.258). Kasai and Sakamoto [14], found a more significant difference in the frequency of lymph node metastases in relation to the primary size, 13% of tumors 0.5 cm or less and 95% of those >0.5 cm.
Six patients (13.6%) were found to have thyroid capsule invasion; all of them had lymph node metastasis (p=0.003). Also, 6/7 patients (85.7%) with level V nodal metastases had thyroid capsule invasion. This agrees with Ohshima et al. [15] who advocated higher incidence of lymph node metastasis with extracapsular invasion especially in the contralateral side.
In our patients, we did contralateral central lymph node dissection because in papillary thyroid cancer there is high incidence of multifocality which may be present in the contralateral thyroid lobe. Also we were afraid of nodal recurrence in the contralateral site which may be aggressive and needs a destructive operation for eradication.
Lymph node dissection remains controversial in papillary thyroid cancer. The current treatment is to complete the total thyroidectomy by the radio-iodine ablation without lymph node dissection in the absence of lymph nodes at ultrasound examination or during preoperative palpation. However, radioactive iodine treatment can be inefficient for some tumors without radioiodine uptake (25-30% of cases) [16]. It has been shown that neck lymph node metastases were present in 20 to 50% of cases, even if the tumor is small and located in the thyroid gland [17]. The frequency of central lymph node involvement is approximately 30% [18]. The ipsilateral jugulo-carotid lymph nodes involvement can be as frequent as the central involvement and is isolated in about 20% of cases [19]. Palpable lymph nodes are a predictive factor of recurrence [20].
The American Thyroid Association (ATA) recommends thyroidectomy with central Compartment Lymph Node Dissection (CLND) in cases of differentiated thyroid cancer and clinically positive central compartment metastatic lymph nodes. However, the role of prophylactic CLND in the presence of smaller tumors and no clinically evident central lymph node metastasis remains controversial [21]. This is due to lack of evidence for its benefit to overall and disease free survival in addition to increased risk of surgical complications as hypoparathyroidism and recurrent laryngeal nerve injury [22,23].
Therapeutic central lymph node dissection decreases the frequency of loco-regional recurrences, and lymph node metastases appear to be an independent variable affecting the prognosis of T1-T2 tumors [24]. Mortality seems to be higher in the presence of metastatic lymph nodes. Surgery for papillary thyroid cancer is not without morbidity. In our study, 3 patients (6.8%) developed temporary hypocalcemia, one patient (2.3%) developed permanent hypocalcemia, and 2 patients (4.5%) developed temporary vocal cord dysfunction. No permanent vocal cord dysfunction was developed. In a study by Gemsenjager and colleague [25], only one patient suffered from permanent hypocalcemia. Henry and colleagues [26] noted a 4% rate of permanent hypocalcemia after prophylactic central lymph node dissection, and they advised against its routine use. Our results concerning temporary hypocalcemia are different from a recent study by Moo and Fahey [4] who fond temporary hypocalcaemia in 31% of their series. So et al. [27] reported 3.8% rate of temporary vocal cord paralysis which resolved within 6 months.
To avoid unnecessary lateral neck dissection, we recommend frozen section to the ipsilateral central compartment and only if positive, lateral neck dissection should be done.
Conclusion
We recommend total thyroidectomy and central compartment lymph node dissection. If ipsilateral central lymph nodes are positive for metastases in frozen section, we proceed to ipsilateral selective neck dissection removing lymphatic structures in levels II through V even in the absence of clinically evident lymph node metastasis irrespective of tumor size. The technique had a low rate of complications; namely laryngeal nerve injury and hypoparathyroidism.
References
- Gosnell JE, Clark OH (2008) Surgical approaches to thyroid tumors. EndocrinolMetabClin North Am 37: 437-55.
- Sakorafas GH, Sampanis D, Safioleas M (2010) Cervical lymph node dissection in papillary thyroid cancer: Current trends, persisting controversies, and unclarified uncertainties. SurgOncol 19: e57-70.
- Cooper DS, Doherty GM, Haugen BR (2006) Management guidelines for patients with thyroid nodules and differentiated thyroid cancer Thyroid 16: 109-142.
- Moo TA, Fahey TJ 3rd (2011) Lymph node dissection in papillary thyroid carcinoma. SeminNucl Med Mar 41: 84-88.
- Shaha AR, Shah JP, Loree TR (1996) Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg 172: 692-694.
- Shaha AR, Shah J, Loree TR (1998) Patterns of failure in differentiated carcinoma of the thyroid based on risk groups. Head Neck 20: 26-30.
- Sivanandan R, Soo KC (2001) Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg 88: 1241-1244.
- Esnaola NF, Cantor SB, Sherman SI (2001) Optimal treatment strategy in patients with papillary thyroid cancer: a decision analysis. Surgery 130: 921-930.
- Wada N, Duh QY, Sugino K (2003) Lymph node metastasis from 259 papillary thyroid microcarcinomas. Ann Surg 273: 399-407.
- Machens A, Hinze R, Thomusch O, Dralle H (2002) Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg 26: 22-28.
- Lim YC, Choi EC, Yoon YH, Koo BS (2010) Occult lymph node metastasis in neck level V in papillary thyroid carcinoma. Surgery 147: 241-245.
- Kupferman ME, Wienstock YE, Santillan AA (2008) Predictors of level V metastasis in well-differentiated thyroid cancer. Head neck 30: 1469-1474.
- Koo BS, Choi EC, Park YH, Kim EH (2010) Occult contralateral central lymph node metastases in papillary thyroid carcinoma with unilateral lymph node metastasis in the lateral neck. J Am CollSurg 210: 895-900.
- Kasai N, Sakamoto A (1987) New subgrouping of small thyroid carcinomas. Cancer 60: 1767-1770.
- Ohshima A, Yamashita H, Noguchi S (2000) Indications for bilateral modified radical neck dissection in patients with papillary carcinoma of the thyroid. Arch Surg 135: 1194-1198.
- Bonnet S, Hartl D, Leboulleux S (2009) Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implication for radioiodine treatment. J ClinEndocrinolMetab 94: 1162-1167.
- Chow SM, Law SC, Chan JK (2003) Papillary microcarcinoma of the thyroid. Prognostic significance of lymph node metastasis and multifocality. Cancer 98: 31-40.
- Pellegriti G, Scollo C, Lumera G (2004) Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J ClinEndocrinolMetab 89: 3713–3720.
- Machens A, Holzhausen HJ, Dralle H (2004) Skip metastases in thyroidd cancer leaping the central lymph node compartment. Arch Surg 139: 43–45.
- Borson-Chazot F, Causeret S, Lifante JC (2004) Predictive factors for recurrence from a series of 74 children and adolescents with differentiated thyroid cancer. World J Surg 28: 1088-1092.
- Enyioha C, Roman SA, Sosa JA (2013) Central lymph node dissection in patients with papillary thyroid cancer: a population level analysis of 14,257 cases. Am J Surg 205: 655-661.
- Hughes DT, Doherty GM (2011) Central neck dissection for papillary thyroid cancer. Cancer Control 18: 83-88.
- Costa S, Giugliano G, Santoro L (2009) Role of prophylactic central neck dissection in cNO papillary thyroid cancer. ActaOtorhinolaryngolItal 29: 61–69.
- Passler C, Scheuba C, Prager G (2004) Prognostic factors of papillary and follicular thyroid cancer: differences in an iodine-replete endemic goiter region. EndocrRelat Cancer 11: 131–139.
- Gemsenjager E, Perren A, Siefert B (2003) Lymph node surgery in papillary thyroid carcinoma. J Am CollSurg 197: 182-190.
- Henry JF, Gramatica L, Denizot A (1998) Morbidity of prophylactic lymph node dissection in the central neck area in patients with papillary thyroid carcinoma. Langenbeck’s Arch Surg 383: 167-169.
- So YK, Son YI, Hong SD (2010) Subclinical lymph node metastasis in papillary thyroid microcarcinoma: A study of 551 resections. Surgery 148: 526-531.
Citation: El-Foll HA, El-Sebaey HI, El-Kased AF, Hendawy A, Kamel MM (2015) Pattern and Distribution of Lymph Node Metastases in Papillary Thyroid Cancer. J Clin Exp Pathol 5:204. DOI: 10.4172/2161-0681.1000204
Copyright: © 2015 El-Foll HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 27696
- [From(publication date): 2-2015 - Jul 01, 2025]
- Breakdown by view type
- HTML page views: 22794
- PDF downloads: 4902