A Review: Pharmaceutical and Pharmacologic Treatments Used In Covid-19
Received: 04-Jan-2022 / Manuscript No. CPB-22-51215 / Editor assigned: 06-Jan-2022 / PreQC No. CPB-22-51215 (PQ) / Reviewed: 19-Jan-2022 / QC No. CPB-22-51215 / Revised: 24-Jan-2022 / Manuscript No. CPB-22-51215 (R) / Accepted Date: 24-Jan-2022 / Published Date: 31-Jan-2022 DOI: 10.4172/2167-065X.1000246
Abstract
Importance: The pandemic of coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents an unprecedented challenge to identify effective drugs for prevention and treatment. Given the rapid pace of scientific discovery and clinical data generated by the large number of people rapidly infected by SARS-CoV-2, clinicians need accurate evidence regarding effective medical treatments for this infection. Vaccination is currently the best bet for the prevention of COVID-19. Still, in its absence, there has been considerable interest in repurposing existing therapeutic agents to reduce the severity of the illness and ease the burden on the already strained healthcare systems. This review outlines the current evidence regarding proposed treatments experimental or repurposed, for COVID-19, and gives an insight into the clinical trial landscape for drugs as well as vaccines.
Keywords
Angiotensin converting enzyme inhibitors; Coronavirus disease 2019 (covid-19); Hydroxychloroquine; Non-steroidal antiinflammatory drugs; Remidisivir; Severe acute respiratory syndromne coronavirus 2 ( SARS-CoV 2); Clinical trials.
Introduction
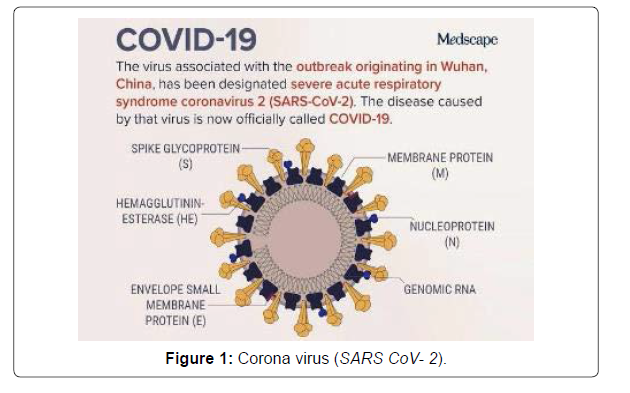
The global pandemic of novel coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV) began in Wuhan, China in December 2019, and has since spread worldwide [1]. As of April 5, 2020, there have been more than 1.2 million reported cases and 69000 deaths in more than 200 countries. This novel Betacoronavirus is similar to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV); based on its genetic proximity, it likely originated from bat derived coronaviruses with spread via an unknown intermediate mammal host to humans [1]. The viral genome of SARS-CoV-2 was rapidly sequenced to enable diagnostic testing, epidemiologic tracking, and development of preventive and therapeutic strategies. Currently, there is no evidence from randomized clinical trials (RCTs) that any potential therapy improves outcomes in patients with either suspected or confirmed COVID-19. There are no clinical trial data supporting any prophylactic therapy. More than 300 active clinical treatment trials are underway. This narrative review summarizes current evidence regarding major proposed treatments, repurposed or experimental, for COVID-19 and provides a summary of current clinical experience and treatment guidance for this novel epidemic coronavirus (Figure 1).
SARS-CoV-2: Virology and Drug Targets
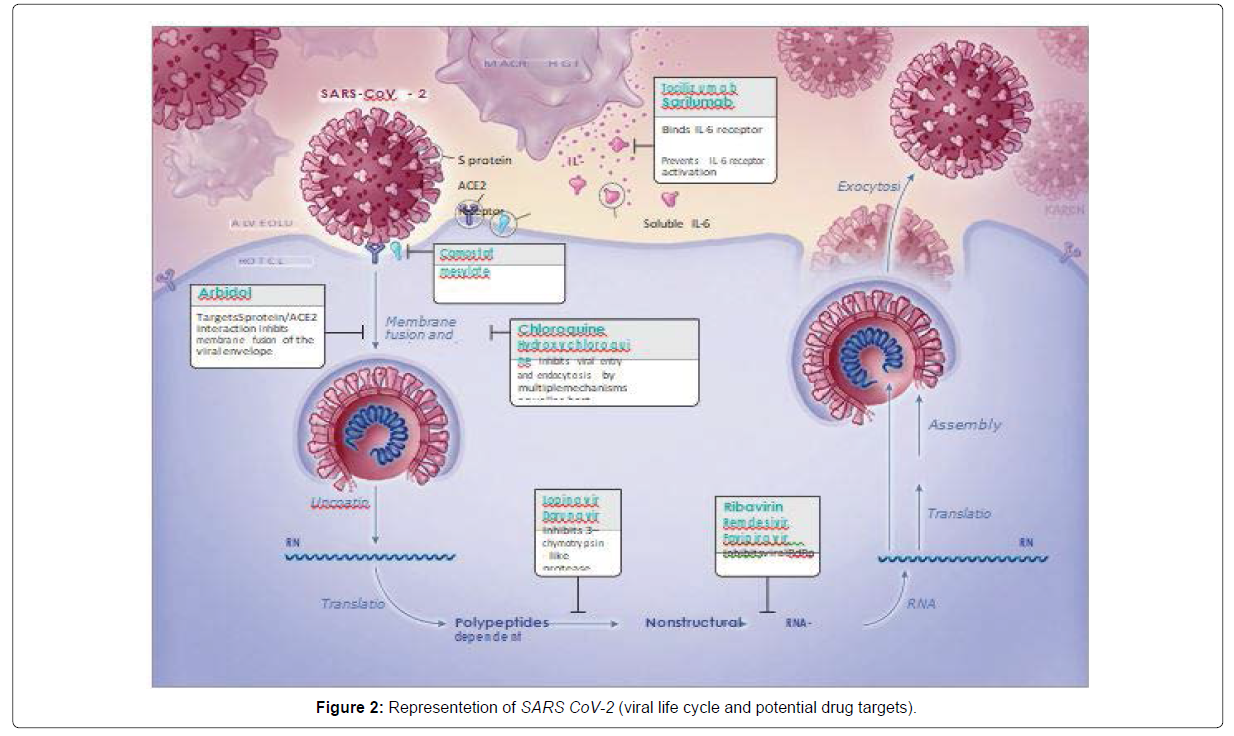
SARS-CoV-2 , a single staranded RNA enveloped virus , target cells through the viral structural spike (S) protein that binds to the Angiotensin Coverting Enzyme 2 (ACE2) receptor following receptor binding, the virus particle uses host cell receptors and endosomes to enter cells. A host type 2 transmembrane serine protease, TMPRSS2, facilitates cell entry via the S protein [2,3]. Once inside the cell, viral polyproteins are synthesized that encode for the replicase-transcriptase complex. The virus then synthesizes RNA via its RNA-dependent RNA polymerase. Structural proteins are synthesized leading to completion of assembly and release of viral particles. These viral lifecycle steps provide potential targets for drug therapy (Figure). Promising drug targets include nonstructural proteins (eg, 3-chymotrypsin-like protease, papain-like protease, RNA-dependent RNA polymerase), which share homology with other novel coronaviruses (nCoVs). Additional drug targets include viral entry and immune regulation pathways (Figure 2) [2].
Clinical features and susceptibility
People of all age groups are susceptible to COVID-19. The symptoms, which have been found to resemble those of the seasonal flu (Influenza), include fever, cough, sore throat, headache, fatigue, and myalgia, loss of smell and taste, and dyspnea. Up to 80% of cases are asymptomatic or have mild disease. In some patients, underlying co-morbidities can further exacerbate the diseased condition, leading to pneumonia, acute respiratory distress syndrome (ARDS), and multiorgan dysfunction by the end of the first week, eventually proving fatal.
Transmission
Inhalation of respiratory droplets generated by symptomatic patients’ cough and sneeze is the main mode of transmission of COVID-19. Infected droplets can spread to distances of up to 2m and deposit on surfaces, which can act as potential fomites for transmission of the virus to seemingly healthy individuals who touch their mucosa (mouth, nose) and conjunctiva (eyes) without proper sanitization. Asymptomatic or pre-symptomatic people can also spread the infection. The virus is also believed to be present in the stool, and transmission via the feco-oral route cannot be ruled out. There is currently no evidence of transmission of the virus from a pregnant mother to her fetus via transplacental route [3].
Physiochemical properties
SARS-CoV-2 can remain viable on surfaces like plastic and stainless steel for up to 72 h in favorable atmospheric conditions but is susceptible to common disinfectants like sodium hypochlorite, hydrogen peroxide, diethyl ether, 75% ethanol, chloroform etc. Soap is found to be equally effective since it readily dissolves the lipid bilayer of the virus. SARS-CoV-2 can also be inactivated by UV or when heated at 60℃ for 30 min.
Review of Selected Re proposed Drugs
Chloroquine
• Classification: Antimalarial
• Rationale for Use: Chloroquine has in vitro activity against SARS-CoV-2 and may have immune modulating properties.
• Mechanism of Action: Mechanisms may include inhibition of viral enzymes or processes such as viral DNA and RNA polymerase, viral protein glycosylation, virus assembly, new virus particle transport, and virus release. Other mechanisms may also involve ACE2 cellular receptor inhibition, acidification at the surface of the cell membrane inhibiting fusion of the virus, and immunomodulation of cytokine release.
• FDA Emergency Use Authorization (EUA):
Chloroquine is not FDA-approved for the treatment of COVID-19.
On June 15, 2020, the FDA revoked the EUA for chloroquine stating that it is unlikely to be effective in treating COVID-19. Also, in light of ongoing serious cardiac adverse events and other serious side effects, the known and potential benefits of chloroquine no longer outweigh the known and potential risks for the authorized use. The EUA was issued in March 2020 and previously stated that treatment was for adult and adolescent patients weighing 50 kg or more who were hospitalized with COVID-19.
• Evidence / Experience:
The NIH COVID-19 treatment guidelines recommend against the use of chloroquine (with or without azithromycin) for the treatment of COVID-19 in hospitalized patients. In non-hospitalized patients, guidelines recommend against the use of chloroquine (with or without azithromycin) for the treatment of COVID-19 outside of clinical trials. The NIH recommends against the use of high-dose, twice-daily chloroquine due to a higher risk of toxicities.
Pre-clinical data in vitro suggest chloroquine has activity has activity against SARS- CoV-2.
• Seriously ill patients with comorbidities have an increased risk of serious arrhythmias.
• Avoid other QT prolonging agents whenever feasible.
• Risk of retinal damage, especially with long term use.
• Caution in patients with G6PD deficiency.
• Caution in diabetics.
• Significant drug interactions [3]
Hydroxychloroquine
• Classification: Antimalarial
• Rationale for Use: Hydroxychloroquine has in vitro activity against SARS-CoV-2 and may have immunomodulating properties. • Mechanism of Action: Mechanisms may include inhibition of viral enzymes or processes such as viral DNA and RNA polymerase, viral protein glycosylation, virus assembly, new virus particle transport, and virus release. Other mechanisms may also involve ACE2 cellular receptor inhibition, acidification at the surface of the cell membrane inhibiting fusion of the virus, and immunomodulation of cytokine release.
• FDA Emergency Use Authorization (EUA):
• Hydroxychloroquine is not FDA-approved for the treatment of COVID-19.
• On June 15, 2020, the FDA revoked the EUA for hydroxychloroquine stating that it is unlikely to be effective in treating COVID-19. Also, in light of ongoing serious cardiac adverse events and other serious side effects, the known and potential benefits of hydroxychloroquine no longer outweigh the known and potential risks for the authorized use [2].
• The EUA was issued in March 2020 and previously stated that treatment was for adult and adolescent patients weighing 50 kg or more who were hospitalized with COVID-19.
• Evidence / Experience:
The NIH COVID-19 treatment guidelines recommend against the use of hydroxychloroquine (with or without azithromycin) for the treatment of COVID-19 in hospitalized patients. In non-hospitalized patients, guidelines recommend against the use of hydroxychloroquine (with or without azithromycin) for the treatment of COVID-19 outside of clinical trials.
• Early in vitro data and data from small trials are limited and inconclusive.
• In a multicenter, parallel, open-label, randomized trial in 150 adult hospitalized patients, hydroxychloroquine (n = 75) was added to standard therapy.
• The majority of patients (n = 148) had mild to moderate disease.
• The overall 28-day negative viral conversion rate was not different between the two groups (85.4% hydroxychloroquine vs. 81.3% control).
• Hydroxychloroquine-treated patients were more severely ill at baseline.
• Due to wide confidence intervals and the observational nature of the trial, the authors stated that the results should not be utilized to rule out either benefit or harm of hydroxychloroquine and suggested further randomized clinical trials to test efficacy.
• An observational study of 1,438 hospitalized patients assessed mortality in patients receiving hydroxychloroquine (n = 271), azithromycin (n = 211), or both (n = 735) compared to patients who received neither of these agents (n = 221).
• There was no difference in mortality in patients treated with hydroxychloroquine (HR 1.08; 95% CI, 0.63 to 1.85), azithromycin (HR 0.56; 95% CI, 0.26 to 1.21), or both (HR 1.35; 95% CI, 0.76 to 2.4) compared with no use of these agents.
• In logistic models, cardiac arrest was significantly more likely in patients receiving hydroxychloroquine plus azithromycin (OR 2.13; 95% CI, 1.12 to 4.05) compared to patients receiving neither drug; however, this was not the case in patients receiving either drug alone.
• In adjusted logistic regression models, there were no significant differences in the relative likelihood of abnormal electrocardiogram findings.
Chloroquine and hydroxychloroquine are relatively well tolerated as demonstrated by extensive experience in patients with SLE and malaria. However, both agents can cause rare and serious ad- verse effects (<10%), including QTc prolongation, hypoglycemia, neuropsychiatric effects, and retinopathy. Baseline electrocardiography toevaluate for prolonged QTcis advisable prior to and following initiation of these medications because of the potential for arrhythmias, especially in critically ill patients and those taking concomitant QT-interval prolonging medications such as azithromycin and fluoroquinolone. No significant adverse effects have been reported for chloroquine at the doses and durations proposed for COVID-19. Use of chloroquine and hydroxychloroquine in pregnancy is generally considered safe. A review of 12 studies including 588 patients receiving chloroquine or hydroxychloroquine during pregnancy found no overt infant ocular toxicity.
Mechanism of action of chloroquine and hydroxy chloroquine
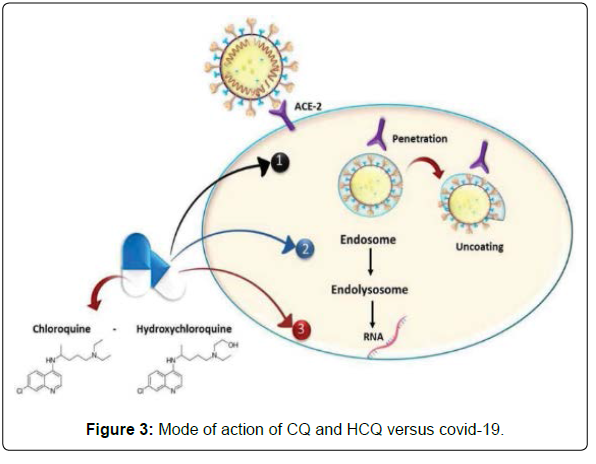
Both CQ and HCQ are weak bases that increase the pH of acidic intracellular organelles like lysosomes/endosome that require low pH for maturation and function. CQ showed elevation in pH of lysosomes from nearly 4.5 to 6.5 at 100 μM. However, the effect of HCQ on pH values of lysosomes/endosomes is not known due to the lack of studies in this regard [4].
Moreover, CQ was found to cause changes in the glycosylation of ACE2 spike protein and receptor that ultimately inhibits the entry step and the post-entry phase of SARS-CoV.
-2.12 HCQ in the time-of-addition experiment showed its ability to exert the same mechanism.
In addition to the previously known mechanism, a novel mechanism of action for CQ and HCQ on COVID-19 was discovered in 2020 by Fantini, et al. as it is known that SARS-CoV-2 starts its replication by attaching to the spike (S) viral protein of respiratory cells. The S protein utilizes sialic acids and ACE-2 receptor connected to host cell surface gangliosides for entry. The study showed that CQ (or its more active derivative, HCQ) has a high affinity for binding to gangliosides and sialic acids. The study also distinguished a novel ganglioside-binding domain (111-158) at the tip of the N-terminal domain of the SARS CoV-2 S protein. It is expected that this domain can ease attachment with the ACE-2 receptor and enhance contact of the virus to lipid rafts [1] (Figure 3).
Mechanism of action in table form:
• Interference with the terminal glycosylation of cellular receptor angiotensin-converting enzyme 2 (ACE-2) leads to obstructing virus-receptor attachment.
• Increasing the pH of acidic cellular organelles lead to prevention of endocytosis with adverse influences on posttranslational modification of recently synthesized viral RNA and virion transport.
• Blocking of viral protein synthesis and virion assembly.
Ritonavir, lopinavir
• Classification: HIV Protease Inhibitor
• Rationale for Use: In vitro and animal model studies show potential activity for other coronaviruses (SARS-CoV and MERS-CoV) [5].
• Mechanism of Action: Lopinavir and ritonavir may bind to Mpro, a key enzyme for coronavirus replication. This may suppress coronavirus activity.
• Evidence / Experience:
Due to unfavorable pharmacodynamics and negative clinical trial data, the NIH COVID- 19 treatment guidelines recommend against the use of lopinavir; ritonavir or other HIV protease inhibitors outside of clinical trials. Similarly, ESICM and SCCM Surviving Sepsis Campaign recommendations suggest against the routine use of lopinavir; ritonavir in critically ill adults with COVID-19.
Early reports of lopinavir/ritonavir for the treatment of COVID-19 are mostly case reports and small retrospective, nonrandomized cohort studies, making it difficult to ascertain the direct treatment effect of lopinavir/ritonavir. More recently, Cao and colleagues reported the results of an open-label RCT comparing the efficacy of lopinavir/ ritonavir vs standard care in 199 patients with COVID-19 [5].
SARS-CoV-2 -> Host entry -> Translation of viral RNA Genome -> Protease Inhibitor; Lopinavir/Ritonavir -> Cleavage of poly proteins PP1a and PP1ab by viral proteases
Ivermectin
• Classification: Antiparasitic
• Rationale for Use: Inhibits the replication of SARS-CoV-2 in cell cultures; however, pharmacokinetic and pharmacodynamic studies suggest that doses up to 100-fold higher than those approved in humans would be necessary to achieve the plasma concentrations necessary for the antiviral effect detected in vitro.
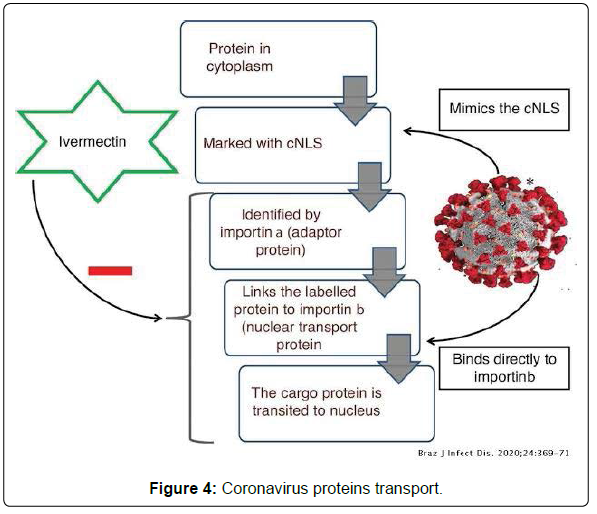
• Mechanism of Action: Ivermectin inhibits the host alpha/ beta-1 nuclear transport proteins, which are a part of a key intracellular transport process that viruses use to enhance infection by suppressing the host antiviral response.
• Evidence / Experience:
• The NIH COVID-19 treatment guidelines recommend against the use of ivermectin, except in a clinical trial.
• The available clinical data on the use of ivermectin to treat COVID-19 are limited.
• Mechanism of Ivermectin induced inhibition of Importin mediated coronavirus proteins transport (Figure 4) [6].
Remdesivir (GS-5734)
• Classification: Nucleoside Analogue.
• Rationale for Use: Remdesivir is a broad-spectrum antiviral with in vitro activity against coronaviruses.
• Mechanism of Action: Remdesivir is a monophosphoramidate prodrug of remdesivir- triphosphate (RDV-TP), an adenosine analog that acts as an inhibitor of RNA-dependent RNA polymerases (RdRps). Remdesivir-TP competes with adenosine-triphosphate for incorporation into nascent viral RNA chains. Once incorporated into the viral RNA at position i, RDV-TP terminates RNA synthesis at position i+3. Because RDV-TP does not cause immediate chain termination (i.e., 3 additional nucleotides are incorporated after RDV-TP), the drug appears to evade proofreading by viral exoribonuclease (an enzyme thought to excise nucleotide analog inhibitors) [5].
• Availability:
Remdesivir is FDA-approved for use in adults and pediatric patients (12 years and older and weighing at least 40 kg) to treat COVID-19 requiring hospitalization.
Although not FDA-approved for use in patients younger than 12 years or weighing less than 40 kg, the FDA has issued an Emergency Use Authorization (EUA) which allows the drug to be used to treat suspected or laboratory-confirmed COVID-19 in hospitalized pediatric patients weighing 3.5 to 39 kg or hospitalized pediatric patients less than 12 years of age weighing at least 3.5 kg.
Similar market approvals and restricted market authorizations have been issued by other nations (Figure 5) [5].
Supportive therapy
Azithromycin:
• Classification: Macrolide Antibacterial
• Rationale for Use: Azithromycin may prevent bacterial super infection, and macrolides may have immune modulatory properties to work as adjunct therapy.
• Mechanism of Action: Macrolides may have immune modulatory properties in pulmonary inflammatory disorders. They may down regulate inflammatory responses and reduce the excessive cytokine production associated with respiratory viral infections; however, their direct effects on viral clearance are uncertain. Immuno modulatory mechanisms may include reducing chemotaxis of neutrophils (PMNs) to the lungs by inhibiting cytokines (i.e., IL-8), inhibition of mucus hyper secretion, and decreased production of reactive oxygen species, accelerating neutrophil apoptosis, and blocking the activation of nuclear transcription factors [7].
Broncodilators
Most patients with COVID-19 do not need inhaled bronchodilator therapy. There is no role for inhaled bronchodilators in the management of COVID-19 unless the patient has underlying asthma or chronic obstructive pulmonary disease (COPD).
MDIs are preferred due to the potential for generation of aerosols that may increase the risk of viral transmission with nebulized therapy [6].
Bruton’s Tyrosine Kinase (BTK) Inhibitors:
• Rationale for Use: Cytokine release syndrome may be a component of severe disease in COVID-19 patients.
• Mechanism of Action: Bruton’s tyrosine kinases are macrophage signaling molecule. When stimulated by viruses such as SAR-CoV-2, BTK activates NF-kB, resulting in production of inflammatory cytokines and chemokines as well as phagocytosis. BTK also activates NLRP3 inflammasome, resulting in maturation and secretion of IL-1 beta. BTK inhibitors form a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity [1].
• Evidence / Experience:
Due to the broad immunosuppressive effect, the NIH COVID-19 treatment guidelines recommend against the use of BTK inhibitors outside of clinical trial [7,8].
Colchicine
• Classification: Anti-inflammatory Agent
• Rationale for Use: Cytokine release syndrome may be a component of severe disease in COVID-19 patients.
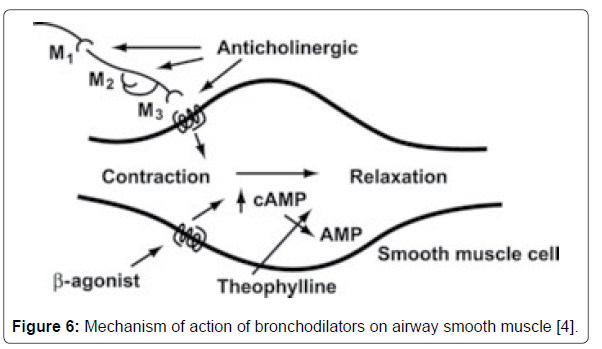
• Mechanism of Action: Colchicine down regulates multiple pro-inflammatory pathways and increases levels of antiinflammatory mediators. It also prevents microtubule assembly and thereby disrupts inflammasome activation, microtubulebased inflammatory cell chemotaxis, phagocytosis, and generation of leukotrienes and cytokines (including interleukin-1 beta). Consequently, colchicine prevents the activation, degranulation, and migration of neutrophils (Figure 6) [9].
Cortecosteroids
• The NIH COVID-19 treatment guidelines recommend the following regarding use of corticosteroids:
• For patients with mild to moderate COVID-19 (i.e., nonhospitalized patients or recommended unless the patient has another clinical indication for steroid therapy.
• For hospitalized patients who require supplemental oxygen BUT NOT on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or ECMO, dexamethasone in combination with remdesivir is recommended. If remdesivir cannot be used, dexamethasone may be given as monotherapy.
• For hospitalized patients who require oxygen through a highflow device, noninvasive ventilation, mechanical ventilation, or ECMO, dexamethasone may be given alone or in combination with a remdesivir.
• If dexamethasone is not available, it is recommended to use an alternative corticosteroid such as prednisone, methylprednisolone, or hydrocortisone; however, it is unclear if these alternatives will provide the same benefit as dexamethasone.
• Clinicians are advised to review the patient’s medical history and assess the potential risks and benefits before initiating dexamethasone.
• Oral and inhaled corticosteroids that were used prior to COVID-19 diagnosis for another underlying condition should not be discontinued [8].
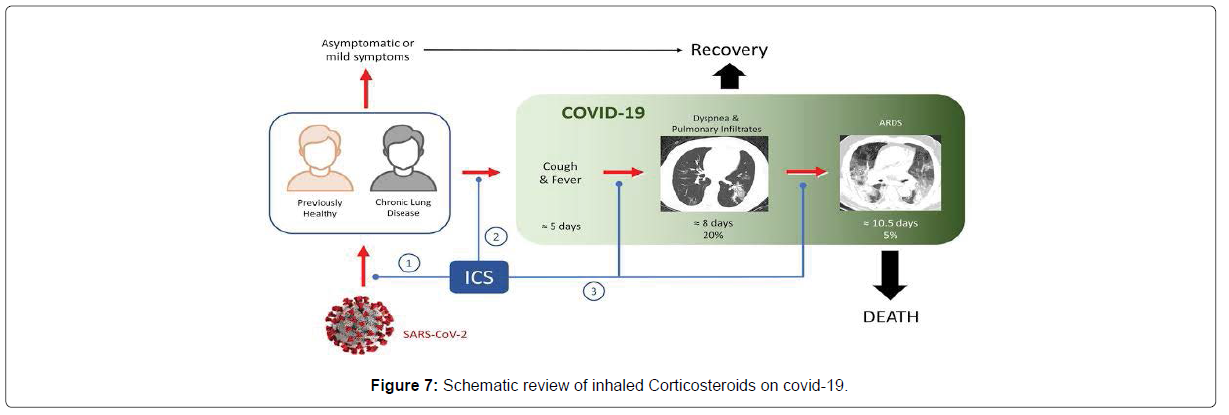
• The World Health Organization (WHO) strongly recommends use of systemic corticosteroids (for 7 to 10 days) to treat patients with severe or critical COVID-19; but suggests against use of corticosteroids in patients with non-severe COVID-19.
• Critical COVID-19 is defined by the criteria for ARDS, sepsis, septic shock, or other conditions that require life-sustaining therapies.
• Severe COVID-19 is defined as oxygen saturation less than 90% on room air, increased respiratory rate, or signs of severe respiratory distress (Figure 7).
Covid-19 Convalesent Plasma: (Plasma Therapy)
Classification: Plasma collected from persons who have recovered from COVID-19 that may contain antibodies to SARS-CoV-2 [4].
Possible mechanism of action
Convalescent plasma therapy is a passive antibody therapy that involves the administration of antibodies against a given pathogen to a susceptible individual for the purpose of preventing or treating an infectious disease. Neutralizing antibodies have been considered essential in the effects of convalescent plasma therapy, and the efficacy of this therapy was associated with the titer of neutralizing antibodies in the convalescent plasma. In addition to the antiviral mechanisms of neutralizing antibodies, immune modulatory effects of plasma components could have additional benefits. During apheresis, in addition to neutralizing antibodies, other proteins such as antiinflammatory cytokines, clotting factors, natural antibodies, defensins, pentraxins, and other undefined proteins are obtained from donors. In this sense, transfusion of convalescent plasma in infected patients may provide further benefits such as immunomodulation via amelioration of severe inflammatory response [8].
Mode of plasma therapy infusion and its association with Covid-19
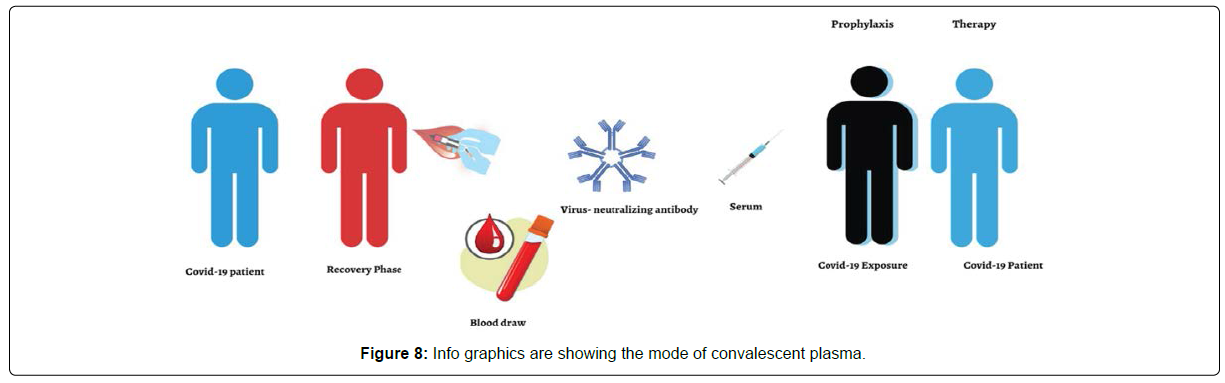
As illustrated patients who recovered from COVID-19 had been regarded as a valuable donor source of CPT, and especially those recovered COVID-19 individuals with a high neutralizing antibody titer at or over 1:640 dilution. Subsequently there is a need to obtain more clarity to maximize the potential clinical benefits and minimize risks associated with convalescent blood products of the COVID-19. The use of CPT for patients with COVID-19 has been attributed to either preventing infection or reducing the disease severity. The convalescent serum is beneficial in a prophylactic mode as it can minimize virulence of ailments and illnesses by neutralization of antigen by specific antibodies that may arise in individuals who are at high risk of acquiring infections, thereby reducing the rate of mortality. Individuals with multiple comorbidities, elderly susceptible patients, and healthcare providers are the classic example of convalescent serum recipients for the COVID-19 current global pandemic with the intention of minimizing virulence and intensity of infection, symptoms, and mortality. Research has justified the above mentioned use of convalescent serum, for example, with rabies viruses and hepatitis B treated with rabies immunoglobulin and hepatitis B immunoglobulin, respectively. Critics claim this approach’s efficacy should be questioned, especially when there is no clinical trial evidence to support this concept. However, through historical facts and with clinical shreds of evidence, it is often predicted that the administration of convalescent antibodies is likely to show effectiveness in the prevention of diseases as opposed to the treatment of already established disease.
Figure 4 depicts an individual who had COVID-19 but has recovered from this condition. Blood is drawn and then screened for virus-neutralizing antibodies. Those with high titers of neutralizing antibodies are identified. Thereafter an administration of serum containing these virus-neutralizing antibodies is given prophylactically to slow down progress or prevent infection in high-risk cases through neutralization of antigen by specific antibodies. As a last resort to improve survival with SARS-infection, the use of CPT has been widely accepted in recent times. Even though the use of passive antibody treatments has been recognized, there is a limited opinion that they should be used as initial therapies as opposed to their use against emerging and pandemic infectious threats. In terms of additional value added by CPT, research claims that while its antibodies contribute to restrictions of viral replication, its other plasma components are beneficial in replenishing coagulation factors, especially for those patients with concurrent coagulopathy when they are given to patients with hemorrhagic fevers like Ebola. Notwithstanding these trends, individual CPT shows a donor-dependent variation in antibody specificities and titers.
Considering that CPT is already being used in several diseases in general alongside its simplicity without adverse reactions, the evidence supplements the ideology that it can easily be used to treat individuals infected with COVID-19. Due to the increasing incidence, the quest to find a treatment for the SARS-Cov-2 pandemic remains a daunting challenge. Nevertheless, those patients who have recovered from COVID-19 are well suited to be capable of serum donors who can then be used for treating SARS-Cov-2 patients. The sample obtained can be stored and then used later when needed. Although it is often suggested that the potency of convalescent serum antiviral effects may not be completely reliable, they often show an increasingly significant variation. Overall, in the absence of specific therapy, CPT’s use is likely to reduce disease severity positively.
Safety concerns
Known side effects associated with plasma transfusion include transmitting infection, allergic or anaphylactic reaction, febrile nonhemolytic reactions, hemolytic reactions, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), post transfusion purpura, hypothermia, and metabolic complications (Figure 8) [9].
NSAIDs
• The NIH COVID-19 treatment guidelines recommend there be no difference in the use of antipyretic treatments (e.g., acetaminophen or NSAIDs) between patients with or without COVID-19. Patients taking NSAIDs for comorbid conditions should continue therapy as previously directed by their prescriber.
• ESICM and SCCM Surviving Sepsis Campaign recommendations suggest acetaminophen for temperature control in critically ill adults with COVID-19 who develop fever.
• The FDA continues to investigate the use of NSAIDs in patients with COVID-19 symptoms.
• Concern for potential worsening of COVID-19 symptoms has been suggested, but confirmatory clinical data is lacking at this time.
• There is an anecdotal published letter that suggests a link between ibuprofen and increased ACE2 expression that may lead to worse outcomes in COVID-19 patients (Figure 9) [7].
Vaccine Treatment in Covid-19
The US Food and Drug Administration, (FDA) has granted emergency use authorization for treatment with the Pfizer/ BioNTech and Moderna COVID-19 vaccines. Ma- ny people have a history of a significant allergic reaction to a specific food, medicine, or vaccine; hence, people all over the world have great concerns about these two authorized vaccines. This article compares the pharmacology, indications, contraindications, and adverse effects of the Pfiz- er/ BioNTech and Moderna vaccines [10].
Data extraction and ethics statement
The findings were documented by using a standardized form including a full description of the study characteristics. In this study we recorded the publicly available database literature on coronavirus, SARS-CoV-2, COVID-19 vaccine, Pfizer/BioNTech and Moderna vaccines; hence, Ethical approval was not required.
Pfizer/BioNTech vaccine
MOA: The vaccine is formulated in lipid particles, which enable delivery of the RNA into host cells to allow expression of the SARSCoV- 2 S antigen. It elicits animmune response to the S antigen, which protects against COVID-19 [8].
Moderna vaccine
MOA: The nucleoside-modified mRNA vaccine is formulated in lipid particles. It enables delivery of the nucleoside-modified mRNA into host cells to allow expression of the SARS-CoV-2 S antigen. The vaccine elicits an immune response to the S antigen, which protects against COVID-19. The antibodies are specific to the SARS-CoV-2 virus, to protect against a future infection [8].
Both authorized vaccines use modified RNA to encode the SARSCoV- 2 spike protein along with mutations in the mRNA added to lock the spike proteins into a three-dimensional shape that it naturally assumes just before it binds to the human ACE-2 receptors on cells with which elicited virus-neutralizing antibodies must in- teract. Both also use lipid nanoparticle (LNP) delivery system. These represent a new class of vaccine, which offer potential advantages, com- pared to traditional replicating or non-replicating viral vector vaccines, in those RNA vaccines are very potent and can be manufactured rapidly and at a relatively low cost. They have a good safety profile, compared to viral vaccines, since they are not made with an actual pathogen and do not integrate into host DNA. Both are administered in two doses into the deltoid muscle. A disadvantage of this class of vaccines is that because RNA is unstable, they require extreme refrigeration for stability during distribution.
Covi shield
Mechanism of action
Covi shield is a monovalent vaccine composed of a single recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1) vector encoding the S glycoprotein of SARS-CoV-2. Following administration, the S glycoprotein of SARS-CoV-2 is expressed locally stimulating neutralizing antibody and cellular immune responses [7].
Immunogenicity
Following vaccination with COVID-19 Vaccine AstraZeneca, in participants who were sero negative at fold increase from baseline in S-binding antibodies was demonstrated in 98% of participants at 28 days after the first dose and >99% at 28 days after the second. Higher S-binding antibodies were observed with increasing dose interval. Generally similar trends were observed between analyses of neutralising antibodies and S-binding antibodies. An immunological correlate of protection has not been established; therefore, the level of immune response that provides protection against COVID-19 is unknown (Figure 10).
Delivery Route of Vaccine
Most vaccines are injected intramuscularly because the traditional subcutaneous route for vaccines that include an aluminum salt adjuvant resulted in severe adverse effects. A clinical trial for a diphtheria toxin (DT) vaccine with a booster shot demonstrated that an intramuscular vaccination gave significantly fewer adverse effects than a subcutaneous injection. Intramuscular injections of the H3N2 and H1N1 influenza vaccines were more immunogenic than a subcutaneous injection. One vaccine study in rhesus macaques using the HIV-1 envelope glycoprotein (Env) in synthetic liposomes gave comparable immune responses for intramuscular and subcutaneous delivery. The authors of a systematic review of delivery methods recommended intradermal rather than subcutaneous or intramuscular injection, when appropriate, because an intradermal injection requires a lower dose of the vaccine.
The route of immunization may induce different mechanisms of protection. An aerosol vaccination of the DNA prime-Ad5 booster vaccine for Simian immunodeficiency virus (SIV) antigens induced IgA-driven neutrophil-mediated phagocytosis, while intramuscular injection induced IgG-driven antibody-dependent monocyte-mediated phagocytosis. However, these varied results came from different vaccine platforms suggesting that the efficacy of the vaccination route might be related to the platform technology, such as mRNA, DNA, or an adenoviral vector vaccine.
The discovery of tissue-resident memory T cells (Trm) showed that memory T cells residing in nonlymphoid tissue can respond immediately to pathogens. The prime and pull strategy, which comprises a conventional parenteral vaccination and recruitment of activated T cells to the local area, establishes long-lasting local immune memory and effective protection against vaginal herpes simplex virus type 2 (HSV-2) infection . Enhanced Trm accumulation requires repeated local antigen recognition. Although circulating IgG can protect the host from mucosal infection, local IgA from plasma cells inhibits infection of local target cells. After influenza infection, local plasma cells secrete IgA to protect type I mucosal cells of the upper respiratory tract while IgG protects lung cells. CD4 Trm cells recruit circulating memory B cells to secrete IgG and IgA in the type II mucosa. These results suggest that local vaccination might be more effective than peripheral vaccination for mucosal infection.
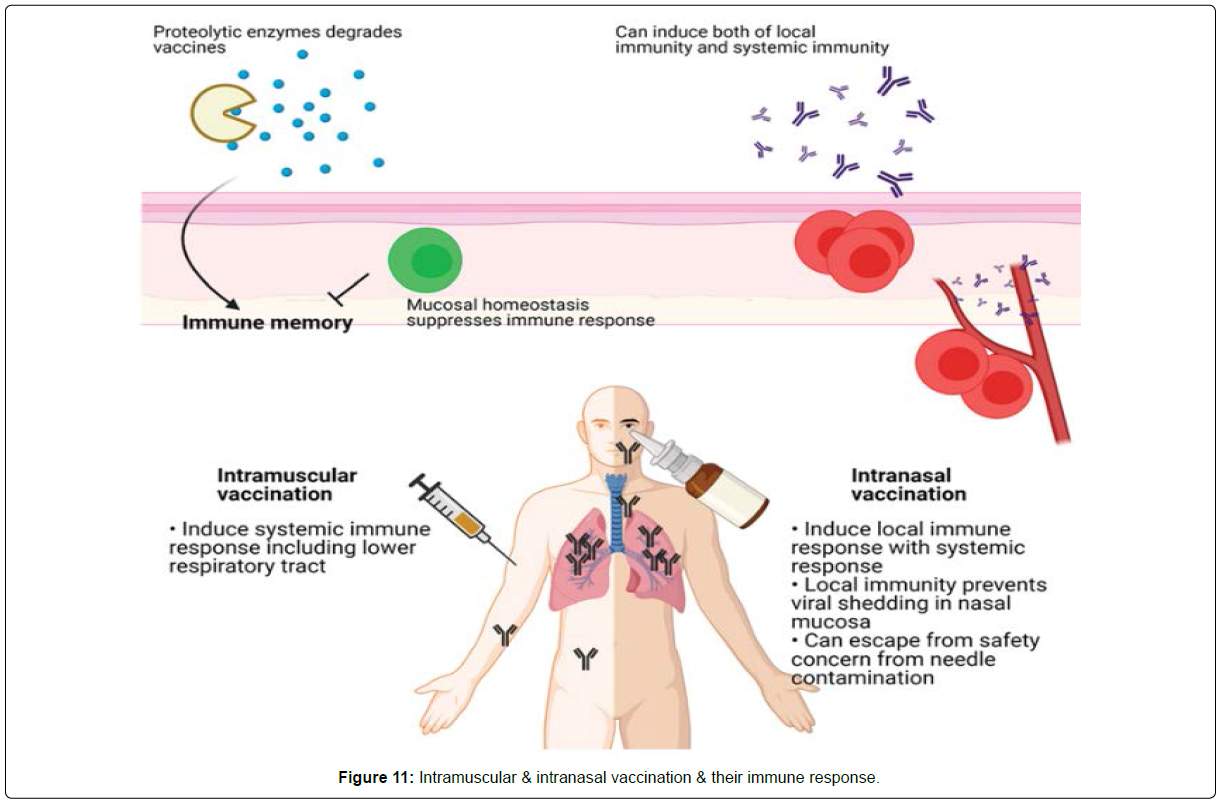
In addition to efficacy, mucosal vaccination is safer than peripheral vaccination because it avoids possible blood-borne infections from contaminated needles. While mucosal vaccination can induce a systemic immune response, the development of mucosal vaccines is difficult because little is known about mucosal immunity. The mucosal vaccine response could be attenuated since the mucosa maintains homeostasis in response to foreign invasion, and absorption of antigens through the mucosal barrier might be difficult as the antigens could be destroyed by proteolytic enzymes. Additionally, many vaccine platforms such as subunit and inactivated virus vaccines need adjuvants for an effective immune response. However, common adjuvants, including alum, could not induce the IgA and IgG class-switching and the recruitment of T and B cells to the mucosal area. Thus, infectious viral vectors that can trigger pathogen-associated molecular patterns (PAMPs) might be more suitable for mucosal vaccines (Figure 11) [11].
Immune Respone Against Covid-19
Innate immune response After entry of SARS-CoV-2, multiple pattern recognition receptors (PRRs) including Toll-like receptor 3 (TLR3), TLR7, retinoic acidinducible gene 1 (RIG-I), and melanoma differentiation-associated gene 5 (MDA5) sense viral infection. PRR recognition triggers phosphorylation of Interferon Regulatory Factor 3 (IRF3) and IRF7 which regulate type I interferon (IFN) and interferon-stimulated genes (ISGs). SARS-CoV-2-derived non- structural proteins suppress type I IFN production. As a result, SARS-CoV-2 induces low-level type I and II IFNs alongside high-level induction of pro inflammatory cytokines and chemokines. Although a normal immune response protects the host from infection, cytokine release syndrome (CRS), the so-called “cytokine storm”, is frequently observed in patients with severe COVID-19.
Adaptive immune response
Consistent with other viral infections, T cells and B cells are a critical component of COVID-19, and T cells are phenotypically different depending on the severity of the disease. COVID-19 patients show lymphopenia, with decreased CD4 T cells, CD8 T cells, and B cells, as well as a preference for natural killer cells over CD8 T cells. Many viral infections induce transient lymphopenia, but SARS-CoV-2 induces a severe, persistent lymphopenia that may be associated with proinflammatory cytokines or activation-induced expression of proapoptotic molecules. CD8 T cells from COVID-19 patients express more inhibitory receptors, such as PD-1 and TIM-3, which correlate with terminal differentiation and functional cell exhaustion. SARS-CoV-2-specific CD8 T cells are CD38+PD-1+. While it is not clear whether these markers represent cell exhaustion or activation, one study suggests that PD-1-expressing CD8 T cells are functional in COVID-19 patients. CD4 T cells show tendencies similar to CD8 T cells. T helper 1 (Th1) cells correlate with mild disease and CCR6+ CD4 T cells correlate strongly with severe disease. Subsets of T cells, including follicular helper T cell (Tfh) which supports B cell responses, correlate with normal antibody-mediated protection. Interestingly, some people who have not been exposed to SARS-CoV-2 have SARSCoV- 2-specific CD4 T cells, which cross-react with common cold viruses. While this protective immunity disappears within 12 months of infection, the potential benefits of this cross-reactivity should be examined [8].
Discussion
The novel coronavirus, SARS-CoV-2 infection is an emerging global health concern and has infected a significant portion of the world’s population [2]. During the COVID-19 pandemic, people worldwide are facing major health care challenges, anxiety and stress4, because there has been no specific treatment, and until December 2020 no vaccination for this pandemic. In mid-December 2020, all medications to covid-19 are specified along with the protocols and vaccinations started to end the pandemic [12].
Limitations
This review has several limitations to note. First, the tremendous volume and fast pace of published literature on the treatment of COVID-19 means that research findings and recommendations are constantly evolving as new evidence arises. Thus, findings from the studies might not always be generalizable to the pediatric population. Second, the search was limited to only English-language articles which might have been a bottle- neck because much of the early data on COVID-19 came from studies which were based in China.
Conclusion
The COVID-19 pandemic represents the greatest global public health crisis of this generation and, potentially, since the pandemic influenza outbreak of 1918. The speed and volume of clinical trials launched to investigate potential therapies for COVID-19 highlight both the need and capability to produce high-quality evidence even in the middle of a pandemic. No therapies have been shown effective to date. Animal studies and clinical trials on therapeutics directed against immune pathologic host responses are needed to elucidate the full spectrum of damage caused by this pathogen. Repurposing of existing drugs and the use of non-pharmacological therapies such as Convalescent Plasma are currently the best treatment avenues until a safe and efficacious vaccine is dis- covered. Although certain prospective agents listed in this review are promising, definitive evidence regarding their effectiveness remains inconclusive. COVID-19 has emerged to be a formidable adversary to the very existence of life as we know it. Overcoming these tough times requires the concerted efforts of people from all walks of life. It necessitates extensive international collaboration between academia, healthcare professionals, pharmaceutical companies, regulators, policymakers, public health organizations and philanthropic associations. The onus on coordinated technical and financial resource mobilization has never been higher, and is indispensable if we are to get the better of this pandemic.
References
- Wang Z, Yang B, Li Q, Wen L, Zhang R (2020) Clinical Features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 71:769-777.
- Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, et al. (2017) Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem 60:1648-1661.
- Al-Tawfiq JA, Al-Homoud AH, Memish ZA (2020) Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis 34:101615.
- Lammers AJJ, Brohet RM, Theunissen REP, Koster C, Rood R, et al. (2020) Early hydroxychloroquine but not chloroquine use reduces ICU admission in COVID-19 patients. Int J Infect Dis 101:283-289.
- Rajasingham R, Bangdiwala AS, Nicol MR, Skipper CP, Pastick KA, et al. (2020) Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial. Clin Infect Dis 72:835-843.
- Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, et al. (2020) Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 181:41-51.
- https://covid19.who.int/
- https://www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003
- https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1
- Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, et al. (2021) Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 384:80-82.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, et al. (2020) An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med 383:1920-1931.
- Keller MJ, Kitsis EA, Arora S, Chen J-T, Agarwal S, et al. (2020) Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med 8:489-493.
Citation: Mendhi SM, More SM, Nagbhidkar AP, Sarode M (2022) A Review: Pharmaceutical and Pharmacologic Treatments Used In Covid-19. Clin Pharmacol Biopharm 11: 246. DOI: 10.4172/2167-065X.1000246
Copyright: © 2022 Mendhi SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2551
- [From(publication date): 0-2022 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 1919
- PDF downloads: 632