Pleurodesis through Pleural Catheterization in Patients with Symptomatic Malignant Pleural Effusions: Which One is better? Talc, Bleomycin or Tetracycline?
Received: 03-Feb-2018 / Accepted Date: 14-Feb-2018 / Published Date: 19-Feb-2018 DOI: 10.4172/2165-7386.1000329
Abstract
Purpose: To analyze the results of pleurodesis through pleural catheterization using talc slurry, bleomycin, and tetracycline in patients with symptomatic malignant pleural effusion (MPE) and to compare the efficacy, reliability and outcomes of these agents.
Methods: Talc (4 g), bleomycin (60.000 U) or tetracycline (1 g) was used for chemical pleurodesis in 271 patients. Successful pleurodesis was defined as no fluid build up and lack of recurrence of symptoms within the first 30 days after treatment. Data were analyzed using SPSS 15.0 for Windows.
Results: Pleural catheterization was performed in a total of 368 patients. Eighteen patients were lost to follow-up. Seventy-nine patients were excluded due to either of the following factors; trapped lung syndrome or patient lost during the early post-catheterization period due to advanced disease. In 271 patients chemical pleurodesis was performed with talc slurry (17.3%), bleomycin (13.7%) or tetracycline (49.1%). In 19.9% of the patients, multiple chemical agents were used in different sessions as successful results were not obtained with one agent. Clinical and radiological success was achieved in 78.2% of patients. There was no significant difference among 4 groups (talc slurry, bleomycin, tetracycline and multiple agents) in terms of clinical success, complication rates and median symptom-free life periods.
Conclusion: Talc slurry, bleomycin, or tetracycline administration through percutaneous pleural catheterization have comparable efficacy rates and safety profiles. If pleurodesis failure with one agent occurs, the attempt with other agents may result in success.
Keywords: Malignant pleural effusion; Pleural catheterization pleurodesis; Talc slurry; Bleomycin; Tetracycline
Introduction
Malignant pleural effusion (MPE) is caused most commonly by lung, breast, ovarian and gastrointestinal tract cancer or lymphoma. The main goals of management are evacuation of the pleural fluid, prevention of its re-accumulation and clinical relief. Treatment options include thoracentesis, chest tube drainage, thoracoscopy followed by chemical and mechanical pleurodesis, tunneled indwelling pleural catheter placement and pleurectomy [1,2]. Thoracentesis helps symptomatic relief but it does not prevent re-accumulation of fluid and usually requires repeated sessions [3]. Chemical pleurodesis via pleural catheter is a commonly performed procedure to manage recurring symptomatic MPE. The choice of sclerosing agent for pleurodesis depends on efficacy, safety, accessibility, ease of administration and cost [4].
Based on the recent meta-analysis, talc poudrage appeared to be a more effective and reliable pleurodesis method than a number of other frequently used methods, and yet it was concluded that further data were required to confirm if it was more effective than other commonly used interventions with different agents [5]. As talc poudrage method likely necessitates general anesthesia, it can pose a collateral burden for patients with advanced malignancy. It is of importance to perform a pleurodesis in interventional radiology unit with the ideal agent through a pleural catheter in the setting of co-morbidities that preclude pleurodesis methods requiring general anesthesia. Thus, we aimed to retrospectively analyze the efficacy, adverse effects, complications and symptom-free life periods among talc slurry, bleomycin, tetracycline and multiple agent administration through pleural catheterization in patients with symptomatic MPE. The outcome of pleurodesis in different tumor types was also evaluated.
Materials and Methods
Patients and study design
Between January 2003 and January 2010, all symptomatic patients with MPE who were referred to the department of Interventional Radiology for pleurodesis and were followed up at least for 1 month were included in this retrospective study. Their clinical information was collected from our hospital database. This study was approved by the Institutional Review Board (B.30.2.HAC.0.70.00.01/431.10-1270).
Pleural Catheterization
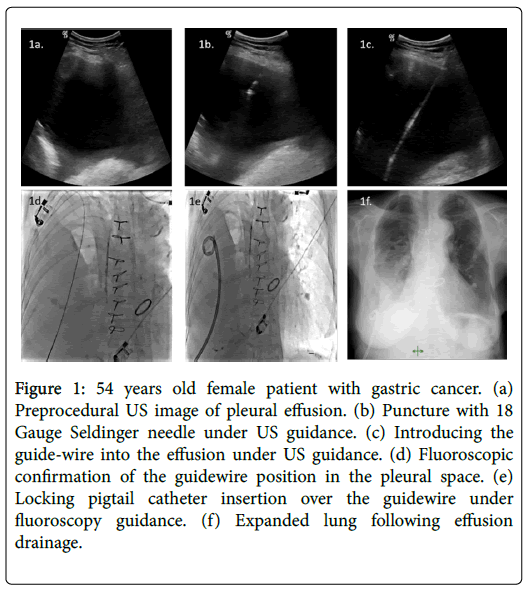
All procedures were performed in interventional radiology unit under local anesthesia with or without intravenous sedation. Any coagulopathy was appropriately corrected before the procedure. All catheters were placed by an interventional radiologist using the Seldinger technique (Figure 1).
Figure 1: 54 years old female patient with gastric cancer. (a) Preprocedural US image of pleural effusion. (b) Puncture with 18 Gauge Seldinger needle under US guidance. (c) Introducing the guide-wire into the effusion under US guidance. (d) Fluoroscopic confirmation of the guidewire position in the pleural space. (e) Locking pigtail catheter insertion over the guidewire under fluoroscopy guidance. (f) Expanded lung following effusion drainage.
Puncture of the pleural cavity was performed with an 18 G Chiba needle under ultrasonography (US) guidance. Entry site was chosen as medial to the posterior axillary line, just above the possible lowest rib. The puncture was angled toward the posterior part of pleural space. Following coiling of 0.035-inch stiff guide wire (Amplatz Super Stiff, Boston Scientific, USA) into the pleural cavity, the access site was crossed with fascial dilators and the 10-16 F pigtail drainage catheter (Skater, Angiotech, Denmark or Flexima APDL Boston Scientific, USA) was positioned under US with or without fluoroscopy guidance. At the time of placement, up to 1 L of fluid was initially evacuated, depending on patient comfort and symptoms; the catheter was then connected to an underwater seal drainage system. Catheter position and lung expansion were controlled by chest radiograph 24-48 hours after the procedure. All patients were hospitalized for the duration of the procedure, and catheter output was recorded daily. Patients were brought to interventional radiology suite for US examination and catheter repositioning or exchange if there was a catheter-related complication. If chest radiography or US showed evidence of any septa, 150,000-250,000 U of streptokinase or 1-5 U of t-PA in 30-50 mL of normal saline was injected through the catheter; the catheter was then clamped for 4 hours and it was unclamped at the end of 4 h. If tube output did not increase and the fluid remained loculated, an additional dose of the fibrinolytic agent was injected on each subsequent day until the tube output improved [6-8].
Chemical pleurodesis
Chemical pleurodesis was performed when;
1. No pleural effusion on posteroanterior (PA) chest radiograph, US and fluoroscopy was detected,
2. Adequate lung expansion was obtained, and
3. Drainage amount was less than 100 mL/day [7-9].
Pleurodesis was performed in the radiology department, by the interventional radiologist. Vital signs and pulse oximetry were monitored during the procedure. Talc (4 g), bleomycin (60.000 U) or tetracycline (1 g) was used for chemical pleurodesis. Bleomycin and tetracycline solutions were prepared in 50 mL of 0.9% saline solution, whereas sterile talc was prepared in 50 mL saline solution containing 1% lidocaine. The sclerosant was administered slowly through the catheter by the interventional radiologist. The catheter was clamped for 4 hours after instillation of selected chemical agent and the patient was asked to change position in order to establish a uniform distribution of the solution in the hemithorax. The catheter was then opened and reconnected to underwater seal drainage system. When daily drainage was less than 100 mL and no evidence of complication was detected, catheter was removed [7-9].
Assessment of response, and statistical analysis
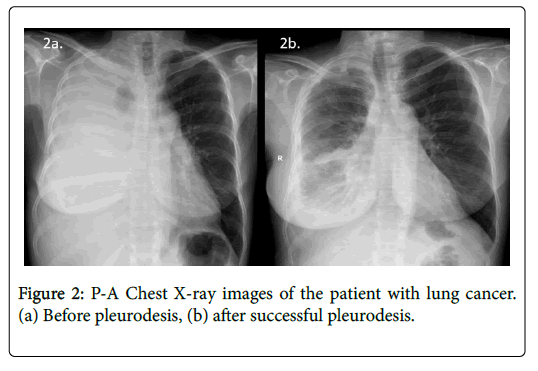
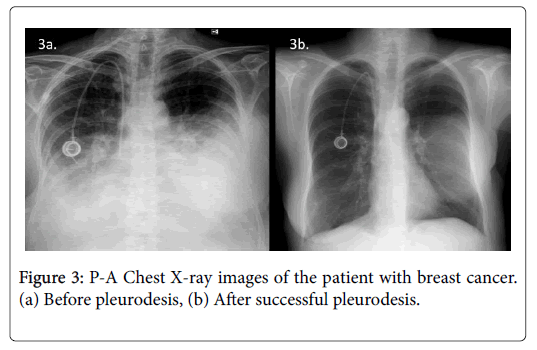
To assess the response to sclerotherapy, we compared the 30-day postsclerotherapy chest radiographs with the presclerotherapy baseline chest radiographs (Figures 2 and 3). Successful pleurodesis was defined as no recurrence of effusion within first 30 days after treatment on clinical and radiological follow-up. The procedure was also considered successful in symptom-free patients with small residual effusion not requiring new thoracentesis [7-10]. Complications were classified as major or minor according to the Society of Interventional Radiology guidelines [11].
Data were analyzed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL). Kruskal–Wallis test was used to compare more than two independent groups and Mann–Whitney U test was used to compare two independent groups for non-normally distributed variables. Percentages were compared by Pearson's chi-square test or Likelihood ratio chi-square test, depending on the frequencies. Descriptive statistics are reported as frequency and percent for qualitative variables and as mean and standard deviation or median and range (min-max) for quantitative variables. A p-value of <0.05 was accepted as significance.
Results
Pleural catheterization was performed in a total of 368 patients. Eighteen of them were excluded as their post-procedural follow-up information was not available. All patients were symptomatic, with either increasing dyspnea or chest discomfort, and all had an increasing effusion on chest radiographs. Before the procedure, diagnosis of MPE was confirmed by cytologically in 78% of the patients whereas it was confirmed clinically or radiologically in 22%. Pleural effusion was unilateral in 305 (87.1%), and bilateral in 45 (12.9%) of the patients. In patients with bilateral effusions, the larger effusion was targeted for treatment. All patients were treated as inpatient. Informed consent for the procedure was obtained. The technical success rate of pleural catheterization was 100%. Locking pigtail catheters such as 10, 12, 14 and 16 F were placed to 11%, 24%, 60% and 5% of the patients respectively. Catheters were inserted with US and fluoroscopy guidance in 32% and with only US guidance in 68% of the patients. No procedure related mortality or major complication occurred. Minor complications such as catheter site infection (n=15), catheter dislodgement (n=8), obstruction (n=21) and kink (n=28) were observed 72 times in 61 patients (17.4%). The majority of the minor complications were managed by catheter revision or exchange under fluoroscopy guidance. In three cases, a new puncture was needed due to accidental removal of the catheter. Catheter site infections were treated with oral and topical antimicrobials. Transcatheter streptokinase or t-PA injections were performed in 7.1% (n=25) of patients and clinical success was achieved in term of dissolving any septations.
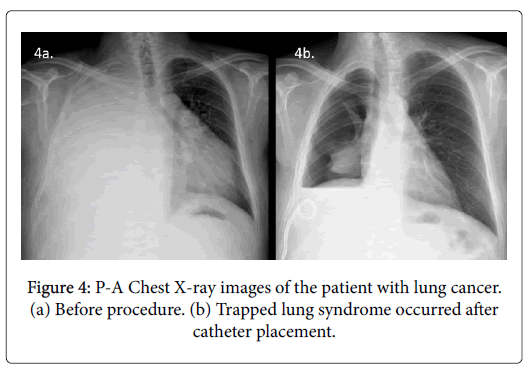
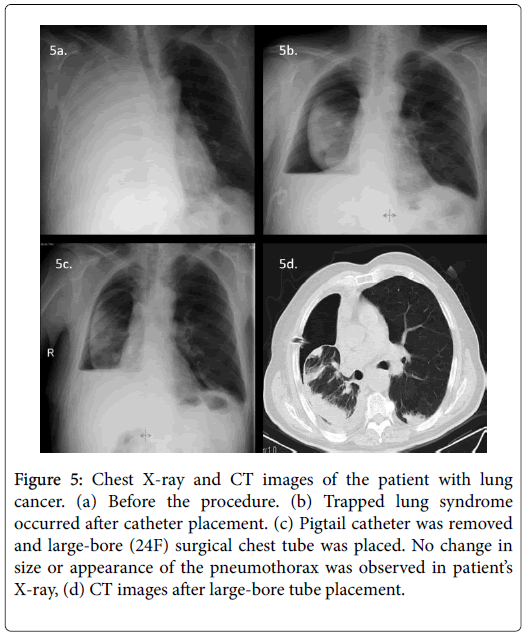
Chemical pleurodesis could not be performed in 79 (22.6%) of 350 patients because of trapped lung syndrome (17.7% n=62) (Figure 4) or mortality during the early post-procedural period due to preexisting advanced disease (4.9% n=17). These patients were excluded from statistical analysis. None of the patients with trapped lung syndrome developed acute symptoms or tension pneumothorax. All had their catheters checked for treatable causes of failure (i.e. kinking or malposition). In 10 of the 62 patients with trapped lung, although in our opinion it was useless, 12-16F catheters were removed and largebore (20-24F) surgical chest tubes were placed. No change in size or appearance of the pneumothorax was observed in any patient after large-bore tube placement. After removal of the chest tubes, these residual pleural spaces filled with fluid rather than increasing amounts of air (Figure 5).
Figure 5: Chest X-ray and CT images of the patient with lung cancer. (a) Before the procedure. (b) Trapped lung syndrome occurred after catheter placement. (c) Pigtail catheter was removed and large-bore (24F) surgical chest tube was placed. No change in size or appearance of the pneumothorax was observed in patient’s X-ray, (d) CT images after large-bore tube placement.
In 271 patients (87 male - 215 female, mean age: 56.4 ± 11.3 years) chemical pleurodesis was performed. The underlying malignancies were breast carcinoma (32.5%, n=88), lung carcinoma (22.1%, n=60), ovarian carcinoma (15.5%, n=42), stomach carcinoma (11.8%, n=32), lymphoma and others (18.1%, n=49). Chemical pleurodesis was performed with talc (n=47, 17.3%), bleomycin (n=37, 13.7%) or tetracycline (n=133, 49.1%). In 19.9% (n=54) of the patients multiple chemical agents were used in different sessions as successful results were not obtained with one agent.
There were no cases of respiratory failure or death that could be directly attributed to the pleurodesis agents. There were 10 patients (3.7%) with major complications (iatrogenic empyema treated with drainage and intravenous antibiotics). Minor adverse reactions to sclerotherapy were observed 60 times in 44 patients (16.2%). These were fever lasting less than 24 h (24 times), nausea-vomiting (11 times) and mild to moderate pleuritic chest pain or shortness of breath (25 times).
Clinical success was achieved in 78.2% (n=212) of patients with sclerotherapy. All of these patients were discharged without a catheter. For these patients mean catheter duration was 8 days (range: 4-22 days) and median symptom-free life period was 5 months (between 2.2 and 9.3 months). Clinical success was not achieved in 59 of the 271 patients (21.8%). Of those patients, 19 (7%) had low lung reserve without pleural fluid, 10 (3.7%) had resistant fluid re-accumulation, however symptoms were regressed, 10 (3.7%) had iatrogenic empyema treated with intravenous antibiotics, the remaining 20 (7.4%) had recurrence within 1 month of the procedure. Mean catheter duration was 24 days (range: 14-66 days) and median symptom-free life period was 2.1 months (between 1 and 3.4 months) in these patients.
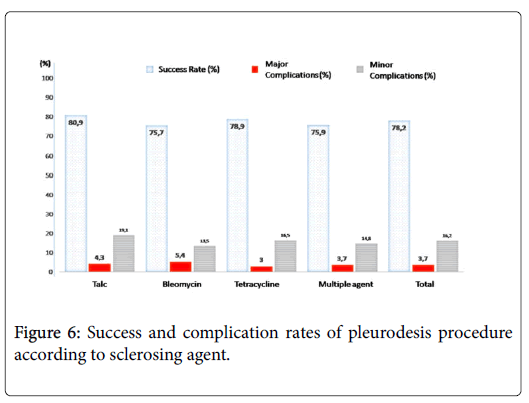
There was no significant difference among 4 groups (talc, bleomycin, tetracycline and success rates, minor-major complication rates and median symptom-free life periods after pleurodesis (Figure 6). Mean catheter duration in multiple agent group (13.3 days) was significantly longer than other groups (7.2 days for talc group, 7.8 days for bleomycin group and 8.3 days for tetracycline group, P <0.001). There was no significant difference among 5 groups (breast cancer, lung cancer, ovarian cancer, stomach cancer, lenfoma and other cancer groups) in terms of mean ages, clinical success rates, minor-major complication rates, mean catheter durations, and median symptomfree life periods after pleurodesis. Statistical analysis results are summarized in Tables 1 and 2.
| Sclerosing Agent | Age (Year) Median (min-max) | Gender (%) Male/Female | Primary Disease (%) | Success Rate (%) | Complications (Minor)(%) | Complications (Major)(%) | Symptom free duration (month) Median (min-max) |
|---|---|---|---|---|---|---|---|
| Talc 17.3% (n=47) | 52 (34-82) | 38.3 /61.7 | Breast: 31.9 | 80.9 | 19.1 | 4.3 | 4.7 (1.3-9.3) |
| Lung: 12.8 | |||||||
| Over: 19.1 | |||||||
| Stomach: 12.8 | |||||||
| Lymphoma, etc:23.4 | |||||||
| Bleomycin 13.7% (n=37) | 53 (40-77) | 32.4/ 67.6 | Breast: 29.7 | 75.7 | 13.5 | 5.4 | 4.0 (1.9-9.0) |
| Lung: 27 | |||||||
| Over: 16.2 | |||||||
| Stomach: 5.4 | |||||||
| Lymphoma etc: 21.6 | |||||||
| Tetracycline 49.1% (n=133) | 54 (35-80) | 31.6/68.4 | Breast: 31.6 | 78.9 | 16.5 | 3 | 4.0 (2.0-8.6) |
| Lung: 21.8 | |||||||
| Over: 15 | |||||||
| Stomach: 12.8 | |||||||
| Lymphoma etc: 18.8 | |||||||
| Multipl agent 19.9% (n=54) | 52 (37-80) | 27.8/72.2 | Breast: 37 | 75.9 | 14.8 | 3.7 | 4.0 (2.4-8.2) |
| Lung: 27.8 | |||||||
| Over: 13 | |||||||
| Stomach: 13 | |||||||
| Lenfoma etc: 9.3 | |||||||
| TOTAL 100% (n=271) | 53 (34-82) | 32.1/67.9 | Breast: 32.5 | 78.2 | 16.2 | 3.7 | 4.5 (1.3-9.3) |
| Lung: 22.1 | |||||||
| Over: 15.5 | |||||||
| Stomach: 11.8 | |||||||
| Lymphoma etc: 18.1 | |||||||
| p value | 0.533 | 0.727 | 0.701 | 0.91 | 0.9 | 0.92 | 0.884 |
Table 1: Clinical outcomes of the study population after pleurodesis procedure according to sclerosing agent.
| Primary Disease | Age (year) Median (min-max) | Gender (%) Male/Female | Agent (%) | Success Rate (%) | Complications (%) (Minor) | Complications (%) (Major) | Sympthom free duration (month) Median (min-max) |
|---|---|---|---|---|---|---|---|
| Breast 32.5% (n=88) | 53 (34-82) | 0/100 | Talc: 17 | 73.9 | 18.2 | 3.4 | 3.7 (1.3-9.3) |
| Bleomycin: 12.5 | |||||||
| Tetracycline: 47.7 | |||||||
| Multipl: 22.7 | |||||||
| Lung 22.1% (n=60) | 52 (36-80) | 76.7/23.3 | Talc: 10 | 76.7 | 16.7 | 0 | 3.8 (1.3-8.6) |
| Bleomycin: 16.7 | |||||||
| Tetracycline: 48.3 | |||||||
| Multipl: 25% | |||||||
| Ovarian 15.5% (n=42) | 55 (38-80) | 0/100 | Talc: 21.4 | 76.2 | 14.3 | 7.1 | 4.1 (1.5-8.7) |
| Bleomycin: 14.3 | |||||||
| Tetracycline: 47.6 | |||||||
| Multipl: 16.7 | |||||||
| Stomach 11.8% (n=32) | 54.5 (40-80) | 53.1/46.9 | Talc: 18.8 | 84.40% | 12.5 | 6.3 | 5.1 (1.8-8.3) |
| Bleomycin: 6.3 | |||||||
| Tetracycline: 53.1 | |||||||
| Multipl: 21.9 | |||||||
| Lenfoma and others 18.1% (n=49) | 54 (36-78) | 9/51 % | Talc: 22.4 | 85.7 | 16.3 | 4.1 | 4.7 (1.7-9.1) |
| Bleomycin: 16.3 | |||||||
| Tetracycline: 51 | |||||||
| Multipl: 10.2 | |||||||
| TOTAL 100% (n=271) | 53 (34-82) | 32.1/67.9 | Talc: 17.3 | 78.2 | 16.2 | 3.7 | 4.0 (1.3-9.3) |
| Bleomycin: 13.7 | |||||||
| Tetracycline: 49.1 | |||||||
| Multipl: 19.9 | |||||||
| P value | 0.527 | <0.001 | 0.616 | 0.479 | 0.951 | 0.188 | 0.532 |
Table 2: Clinical outcomes of the study population after pleurodesis procedure according to primary malignancies.
Discussion
The results of this study suggest that talc slurry, bleomycin, and tetracycline as agents for chemical pleurodesis have similar efficacy and reliability, with clinical success rates of 80.9%, 75.7%, and 78.9%, respectively. The overall major and minor complication rates related with pleurodesis (3.7% and 16.2% respectively) in the present study were comparable to previous reports in the literature [7,8,12-16]; more importantly, no procedure-related deaths or respiratory failure were observed.
There is no consensus regarding the ideal sclerosant for MPE’s due to the lack of high quality, comparative trials. A survey shows a wide variety in selecting sclerosants [17]. The basic choices of sclerosant for chemical pleurodesis are talc, tetracycline, and bleomycin in clinical practice.
Talc is one of the oldest sclerosing agents used in the treatment of MPE. Its mechanism of action is the triggering of intense pleuritis [4,18,19]. Sterile asbestos-free talc is a relatively inexpensive agent which is readily available. It may be administered in two ways; during thoracoscopy using an atomizer called ‘talc poudrage’ or via a pleural catheter in the form of a suspension called ‘talc slurry’ [4]. Two extensive reviews showed that talc poudrage had the highest efficacy for preventing MPE recurrence in comparison with other commonly used sclerosants [16,20]. Talc poudrage appeared to be a more effective and reliable pleurodesis method than a number of other frequently used methods based on the recent metaanalysis, and yet it was concluded that further data were required to indubitably confirm if it was more effective than other commonly used interventions with different agents [5]. The possibility of the need for general anesthesia to perform a talk poudrage can pose a collateral burden for patients with advanced malignancy. At this point, administration of talc slurry by pleural catheter is of importance for patients who are not able to tolerate general anesthesia. Although talc provides a very satisfactory successful pleurodesis rates (71 to 96%), the concerns about the risks of adverse effects of talc limit its universal adoption [7,8,10,12,14,17]. Complications include dyspnea, fever, chest pain, atelectasis, pneumonia, arrhythmias, empyema, and acute respiratory failure, which may progress to the acute respiratory distress syndrome (ARDS) [4,16]. Furthermore, talc increases 18F-fluorodeoxyglucose uptake on positron emission tomography, therefore might pose diagnostic difficulties of pleural malignancies [21]. In the present study, chemical pleurodesis was performed with talc slurry through a pleural catheter in 17.3% of the patients and clinical success was achieved in 80.9% of them. Minor and major complication rates related to talc were 19.1% and 4.3%, respectively.
Bleomycin is another effective sclerosant with high success rates ranging from 58% to 85% with a mean of 61% after a single administration [4,12-15,19]. These are comparable to success rates observed with talc. The side effect profile of bleomycin is acceptable which includes fever, chest pain, and nausea being the most common ones [12-15,19,22,23]. It causes minimal or no myelosuppression and therefore can be safely used in patients with compromised immune system [23]. The major disadvantage of bleomycin is the relatively high cost of treatment [4,15,23]. In the present study, chemical pleurodesis was performed with bleomycin in 13.7% of the patients and clinical success rate was found to be 75.7% for these patients. Minor and major complication rates related with bleomycin were 13.5% and 5.4% respectively.
Until recently, tetracycline has been one of the most widely used sclerosing agent. Unfortunately, production of parenteral tetracycline has ceased and it is no longer available in many countries [4,24]. Oxytetracycline, however, was available in our country by 2010. The major advantage of tetracycline is its low cost of treatment. Moreover, treatment-associated side effects, including localized pain and fever are rarely observed [23]. Producing local antibacterial activity, which helps to reduce the risk of infection is the additional advantage of tetracycline [23,25]. The success rates for tetracycline ranges from 15% to 92%, with an average of 75% in patients with MPE [23,26]. In the present study, chemical pleurodesis was performed with tetracycline in 49.1% of the patients and clinical success rate was calculated as 78.9% for these patients. Minor and major complication rates related with tetracycline were 13.5% and 5.4% respectively.
A randomized study by Lynch et al. [27] compared talc slurry with bleomycin and tetracycline. Although the study was terminated early due to the absence of tetracycline, analysis of the data revealed no differences between the three treatment groups 1 month after pleurodesis and this result is similar to the result of our study. In another randomized trial comparing talc slurry and bleomycin, 90% of patients treated by talc achieved a complete response at 2 weeks, while the success rate was 79% in patients treated with bleomycin [28]. The difference was however statistically insignificant.
In this study, approximately 20% of the patients (n=54) didn’t give a response to initial pleurodesis procedure which was performed either with talc, bleomycin or tetracycline. In such condition, we continue to perform pleurodesis procedure by other agents and we obtain 75.9% success rate in this group similar to other groups. But mean catheter duration in this patient group was longer than mean catheter duration in other groups. In this study, a special criterion was not taken into consideration during the determination of another agent when the first agent failed in apposition of pleural surfaces. The selection was performed according to the presence of the agent in the hospital. In a case of MPE, if the agent chosen for pleurodesis is not sufficient enough for apposition of the pleural surfaces, the procedure should not be accepted as a failure and other agents should be tried.
Pleurodesis outcome may be influenced by various factors, but whether tumor type is one of them is a question of debate [29]. In a meta-analysis, Heffner et al. [30] described that certain high-risk tumors (lung, soft tissues, renal, ovary, gastrointestinal, prostate, and oropharynx) are associated with shorter survival as compared with other cell types (breast, mesothelioma, lymphoma, uterine/cervical, sarcoma, and skin). Stefani et al. [31] reported that talc has the highest success rate in patients with breast cancer (82.1%), the intermediate in lung cancer patients (71.4%), and the poorest in mesothelioma patients (70%) with no statistically significant difference. In the same way in our study, there is no significant difference among malignancy groups in terms of clinical success rates after pleurodesis.
After drainage of the pleural cavity, the most important requirement for successful pleurodesis is satisfactory apposition of the parietal and visceral pleura [4]. However in cases with MPE, the situation could be more problematic, as the visceral pleura can become very thick, the lung parenchyma can be very stiff secondary to diffuse tumor involvement or central mass may be present. This, in turn, leads to incomplete lung re-expansion, a phenomenon called as trapped lung syndrome [18,32,33]. Full lung reexpansion can’t be achieved in patients with trapped lung despite complete drainage of the pleural effusion. Up to 30% of patients who are evaluated for pleurodesis are unsuitable candidates because of trapped lungs [32,33]. Patients evaluated for pleurodesis, therefore, require a careful radiographic evaluation in order to identify the likelihood of lung reexpansion [17]. In a patient with MPE, the observation of pneumothorax (pneumothorax ex vacuo) after a large-volume thoracentesis or after placement of chest catheter concerned as trapped lung syndrome, especially if the configuration of the pneumothorax space simulates the distribution of pleural fluid before thoracentesis [34,35]. Once a lung has become noncompliant or stiff, any space in the pleural cavity is partially filled with a pleural effusion. If the pleural effusion is removed, the lung is unable to reexpand fully. A pneumothorax will, therefore, develop not from a puncture and air leak but from the vacuum induced in the pleural space [32,33]. Two hypotheses have been suggested for the failure of the lung to reexpand including underlying restriction by pleural disease and depletion of pulmonary surfactant preventing aeration and lung reexpansion [32,33,35]. Gas originates either from pulmonary venules or from pleural blebs or subpleural bullae [32,33]. Whatever the exact mechanism of pneumothorax, most patients with trapped lung are asymptomatic; some patients might experience dyspnea because of significant restriction. These patients represent a different population than those who develop a pneumothorax after chest biopsy or other transpleural interventions such as ablation procedures [32,33]. When the pneumothorax does not resolve over several days, pleurodesis does not appear to be indicated because the pleural surfaces are not opposed. The drainage tube should be removed, with no apparent risk of the pneumothorax growing larger. After a varying time period, the pleural effusion will recur, leading initially to a hydropneumothorax and finally to complete replacement of the residual pleural space by pleural effusion [32,33]. It should be kept in mind that placement of a pleural catheter or surgical tube thoracostomy are useless in these cases. Longterm tunneled indwelling catheter (Pleur-X) can be used in patients with trapped lung for palliation of symptoms [36]. In the present study, chemical pleurodesis was not performed in 17.7% (62) of the 350 patients because of trapped lung syndrome.
There are some potential limitations of our study. Even though all patients referred to our unit from the same center (medical oncology department) consecutively, the retrospective character of the study may have some limitations. Some factors (such as mediastinal irradiation, concomitant chemotherapy or performance status of the patient) affecting the outcome of the procedure couldn’t be evaluated. Other potential prognostic factors for successful pleurodeses, such as pleural fluid pH, were not evaluated due to lack of information in some of the patients’ records. Although there were no cases of respiratory failure that could be directly attributed to the usage of talc, we don’t know the mean size of the talc particles which may have consequences on its side effect.
Based on the recent meta-analysis, talc poudrage appears to be a more effective and reliable pleurodesis method than a number of other frequently used methods although there is lack of definitive evidence to conclude its certain superiority, yet there is also no evidence of suggesting large differences between bedside pleurodesis agents [5]. Since possible need for general anesthesia for talc poudgare limits its use, utilization of pleurodesis agents in interventional radiology suits under local anesthesia with or without sedoanalgesia will be worthwhile in the patients with advanced disease who cannot tolerate the extra burden of general anesthesia. Pleural catheterization and subsequent pleurodesis provide symptomatic palliation with high efficacy in patients with malignant pleural effusion. In addition, the use of inexpensive agents with highest efficacy may be important in the palliation of patients with malignant pleural effusion in centers with limited resources [37]. Our results show no conclusive superiority of a particular sclerosing agent in efficacy rates or safety profiles. The local availability, experience with a particular agent and its adverse effects and observed clinical outcomes should determine the local approach to pleurodesis. If a particular agent is not sufficient enough for apposition of the pleural surfaces, the procedure shouldn’t be accepted as a failure and other agents should be tried as success rates may be increased with the use of multiple agents.
Main points
• Pleural catheterization and subsequent pleurodesis provide symptomatic palliation with high efficacy in patients with malignant pleural effusion.
• Talc slurry, bleomycin and tetracycline can be safely used for pleurodesis as no superiority among them was observed.
• In case of initial pleurodesis procedure failure with any sclerosant agent, it is important to try another agent as close success rates were demonstrated in multiple agent groups.
References
- Porcel JM, Salud A, Nabal M, Vives M, Esquerda M, et al. (2006) Rapid pleurodesis with doxycycline through a small-bore catheter for the treatment of metastatic malignant effusions. Support Care Cancer 14: 475-478.
- Neragi-Miandoab S (2008) Surgical and other invasive approaches to recurrent pleural effusion with malignant etiology. Support Care Cancer 16: 1323-1331.
- Heffner JE, Klein JS (2008) Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 83: 235-250
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ, et al. ( 2010) Management of a malignant pleural effusion: British Thoracic society pleural disease guideline. Thorax 65: 32-40.
- Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N (2016) Interventions for the management of malignant pleural effusions: A network meta-analysis. Cochrane Database Syst Rev.
- Akhan O, Ozkan O, Akinci D, Hassan A, Ozmen M, et al. (2007) Image-guided catheter drainage of infected pleural effusions. Diagn Interv Radiol 13: 204-209.
- Marom EM, Patz EF Jr, Erasmus JJ, McAdams HP, Goodman PC, et al. (1999) Malignant pleural effusions: treatment with small-bore-catheter thoracostomy and talc pleurodesis. Radiology 210: 277-281.
- Marom EM, Erasmus JJ, Herndon JE, Zhang C, McAdams HP (2002) Usefulness of imaging-guided catheter drainage and talc sclerotherapy in patients with metastatic gynecologic malignancies and symptomatic pleural effusions. Am J Roentgenol 179: 105-108.
- Seaton KG, Patz EF Jr, Goodman PC (1995) Palliative treatment of malignant pleural effusions: value of small-bore catheter thoracostomy and doxycycline sclerotherapy. Am J Roentgenol 164: 589-591.
- Bloom AI, Wilson MW, Kerlan RK, Gordon RL, LaBerge JM, et al. (1999) Talc pleurodesis through small-bore percutaneous tubes. Cardiovasc Intervent Radiol 22: 433-436.
- Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol 14: 199-202.
- Haddad FJ, Younes RN, Gross JL, Deheinzelin D (2004) Pleurodesis in patients with malignant pleural effusions: Talc Slurry or bleomycin? results of a prospective randomized trial. World J Surg 28: 749-754.
- Moragón EM, Aparicio J, Rogado MJ, Sanchis J, Sanchis F, et al. (1997) Pleurodesis in malignant pleural effusions: A randomized study of tetracycline versus bleomycin. Eur Respir J 10: 2380-2383.
- Ong KC, Indumathi V, Raghuram J, Ong YY (2000) A comparative study of pleurodesis using talc slurry and bleomycin in the management of malignant pleural effusions. Respirology 5: 99-103.
- Patz EF, McAdams HP, Erasmus JJ, Goodman PC, Culhane DK, et al. (1998) Sclerotherapy for malignant pleural effusions: A prospective randomized trial of bleomycin vs doxycycline with small-bore catheter drainage. Chest 113: 1305-1311.
- Shaw PHS, Agarwal R (2004) Pleurodesis for malignant pleural effusions. Cochrane Database of Syst Rev.
- Heffner JE (2010) Management of the patient with a malignant pleural effusion. Semin Respir Crit Care Med 31: 723-733.
- Panadero FR, Worboys AM (2012) Mechanisms of Pleurodesis. Respiration 83: 91-98.
- Hartman DL, Gaither JM, Kesler KA, Mylet DM, Brown JW, et al. (1993) Comparison of insufflated talc under thoracoscopic guidance with standard tetracycline and bleomycin pleurodesis for control of malignant pleural effusions. J Thorac Cardiovasc Surg 105: 743-747.
- Tan C, Sedrakyan A, Browne J, Swift S, Treasure T (2006) The evidence on the effectiveness of management for malignant pleural effusion: A systematic review. Eur J Cardiothorac Surg 29: 829-838.
- Murray JG, Erasmus JJ, Bahtiarian EA, Swift S, Treasure T, et al. (1997) Talc pleurodesis simulating pleural metastases on 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol 168: 359-360.
- Alberts DS, Chen HS, Mayersohn M, Perrier D, Moon TE, et al. (1979) Bleomycin pharmacokinetics in man. Cancer Chemother Pharmacol 2: 127-132.
- Kaifi JT, Toth JW, Gusani NJ, Kimchi ET, Staveley-O'Carroll KF, et al. (2012) Multidisciplinary management of malignant pleural effusion. J Surg Oncol 105: 731-738.
- Heffner JE, Unruh LC (1992) Tetracycline pleurodesis. Adios, farewell, adieu. Chest 101: 5e7.
- Gebbia N, Mannino R, Di Dino A, Maxhouni L, Bellone N, et al. (1994) Intracavitarytreatment of malignant pleural and peritoneal effusions in cancer patients. Anticancer Res 14: 739-745.
- Ruckdeschel JC, Moores D, Lee JY, Einhorn LH, Mandelbaum I, et al.(1991) Intrapleural therapy for malignant pleural effusions. A randomized comparison of bleomycin and tetracycline. Chest 100: 1528-1535.
- Lynch TJ (1996) Optimal therapy of malignant pleural effusions: Report of a randomized trial of bleomycin, tetracycline, and talc and a meta-analysis. Int J Oncol 8: 183e90.
- Zimmer PW, Hill M, Casey K, Harvey E, Low DE, et al. (1997) Prospective randomized trial of talc slurry vs bleomycin in pleurodesis for symptomatic malignant pleural effusions. Chest 112: 430e4.
- Bielsa S, Hernández P, Rodriguez-Panadero F, Taberner T, Salud A, et al. (2011) Tumor type influences the effectiveness of pleurodesis in malignant effusions. Lung 189: 151-155.
- Heffner JE, Nietert PJ, Barbieri C (2000) Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 117: 79-86.
- Stefani A, Natali P, Casali C, Morandi U (2006) Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg 30: 827-832.
- Boland GW, Gazelle GS, Girard MJ, Mueller PR (1998) Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR AJR Am J Roentgenol. 170: 943-946.
- Chang YC, Patz EF Jr, Goodman PC (1996) Pneumothorax after small-bore catheter placement for malignant pleural effusions. AJR Am J Roentgenol 166: 1049-1051.
- Heidecker J, Huggins JT, Sahn SA, Doelken P (2006) Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 130: 1173-1184.
- Galvis AG, Bowen A, Oh KS (1981) Nonexpandable lung after drainage of pneumothorax. AJR Am J Roentgenol 136: 1224-1226.
- Pollak JS, Burdge CM, Rosenblatt M, Houston JP, Hwu WJ, et al. (2001) Treatment of malignant pleural effusions with tunneled long-term drainage catheters. J Vasc Interv Radiol 12: 201-208.
- Figueiredo I, Cossa A, Hassane A, Sousa J, Pondo J, et al. (2017) Pleurodesis: A comparison of two sclerosing agents for pleural effusions in Mozambique. J Thorac Dis 9: 3132- 3137.
Citation: Çiftçi T, Aksoy S, Topel C, Akinci D, Idilman IS, et al. (2018) Pleurodesis through Pleural Catheterization in Patients with Symptomatic Malignant Pleural Effusions: Which One is better? Talc, Bleomycin or Tetracycline?. J Palliat Care Med 8: 329. DOI: 10.4172/2165-7386.1000329
Copyright: ©2018 Çiftçi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9638
- [From(publication date): 0-2018 - Nov 28, 2025]
- Breakdown by view type
- HTML page views: 8564
- PDF downloads: 1074