Point of Care Platform for Detection of SARS-CoV-2/COVID-19
Received: 31-Dec-2021 / Manuscript No. JIDT-22-50971 / Editor assigned: 04-Jan-2022 / PreQC No. JIDT-22-50971 (PQ) / Reviewed: 14-Jan-2022 / QC No. JIDT-22-50971 / Revised: 14-Jan-2022 / Manuscript No. JIDT-22-50971 (R) / Accepted Date: 16-Jan-2022 / Published Date: 23-Jan-2022 DOI: 10.4172/2332-0877.1000486
Abstract
COVID-19, a disease resulting from the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has caused more than 287 million infections and 5.5 million deaths worldwide. To mitigate the spread of the virus, highly specific, selective, and rapid Point of Care (POC) diagnostics are required to diagnose COVID-19 at all stages of infection. Here, we have developed a rapid, lightweight, portable, sensitive, and flexible paper-based microfluidic platform for singleplex and multiplex detection of biomarkers (antibodies and antigenic viral proteins) for COVID-19. Our singleplex platform, which individually tests for the SARS-CoV-2 Spike Protein (SP) and Nucleocapsid Protein (NP), demonstrates high sensitivity in the 100 pg/mL limit of detection range with a detection time of 5 minutes. In addition, our platform demonstrates high specificity to the SARS-CoV-2 SP when challenged in the presence of other coronavirus and pathogenic viruses. The detection capability for the SARS-CoV-2 SP in various specimen types (saliva, nasal fluid, blood, and urine) was also examined in this platform. As proof of concept, our platform was also evaluated for multiplex detection of antibodies against the SARS-CoV-2 SP (IgM and IgG) and the SP viral antigen in a single device. The platform demonstrates the capability for rapid multiplexing within 10 minutes.
Keywords: COVID-19; Paper-based test; Point-of-care diagnostics; Immunoassay
Introduction
In December of 2019, an outbreak of a new pathogenic virus (SARS-CoV-2), which causes COVID-19, spread to almost every country in the world, and the resulting pandemic severely affected daily life. Social distancing requirements, travel restrictions, and mandatory quarantines were put into place to mitigate the spread of the virus, and these actions disrupted socioeconomic activity around the world. To date, SARS-CoV-2 has caused more than 70 million infections and more than 1.6 million deaths worldwide [1]. The scientific community has worked diligently to develop various diagnostic tests to efficiently diagnose COVID-19. These tests include molecular assays for nucleic acid detection of the SARS-CoV-2 genome such as reverse transcriptase polymerase chain reaction (qRTPCR, gold standard) and Loop-mediated Isothermal Amplification (LAMP). Currently, these assays have several drawbacks such as long testing times, expensive implementation, the need for highly trained medical personnel, and susceptibility to false results due to low specificity [1-4]. In an effort to overcome some of these limitations, other methods for SARS-CoV-2/COVID-19 detection have been developed for simple, portable, rapid, efficient, and reliable diagnosis of this disease. A number of rapid diagnostic tests for SARS-CoV-2/ COVID-19 detection have been made available including Chemiluminescence Immunoassays (CLIA), Lateral Flow Immunoassays (LFIA), and Enzyme-linked Immunosorbent Assays (ELISA). These tests, however, have several limitations related to the quality of the diagnostic performance including sensitivity, specificity, and Limit of Detection (LOD) [5]. A comparison of CLIA, FLIA, and ELISA assay performances revealed that CLIA had the highest sensitivity at 97.8%. Sensitivities of only 84.3% and 66% were observed for ELISA and LFIA assays, respectively [6]. The CLIA approach, however, is limited by high cost, complex instrumentation requirements, and the need for well-trained technicians to perform the tests. Furthermore, even though the sensitivity and specificity of ELISA for the detection of SARS-CoV-2 are significantly higher than those for LFIA, commercially available ELISA test kits are typically intended to be used for research applications. Although LFIA produces rapid test results which are easy to interpret with the naked eye, this assay presents limitations regarding the velocity of the fluid and the capillary forces during the process of antigen capture. These hurdles are related to the pore size of the porous media, leading to sensitivity issues [6-8]. In addition, hook effect problems that could potentially compromise the detection efficiency of a diagnostic test is another possible drawback of this assay type. The limitations of the currently available approaches call for improvements in the development of a rapid and low-cost Point of Care Test (POCT) with enhanced sensitivity and specificity for more reliable diagnosis of SARSCoV-2/COVID-19. To overcome the poor sensitivity of LFIA, Vertical Flow Immunoassays (VFIA) can potentially be used as a rapid diagnostic test to detect SARS-CoV-2/COVID-19 [5,8,9]. This method has been utilized as a POCT for many different pathogenic diseases [10,11]. The principle of this technique is similar to that of a lateral flow assay, but a vertical flow pattern is utilized instead for a faster diagnosis time. Both assays use the principles of ELISA and require the formation of an antibodyantigen complex, immobilization of the capture antibody onto a readout layer, and immobilization of the labelled detection antibody to produce a color signal. The main advantages of Vertical Flow Assays (VFA) include the use of gravitational forces and capillary forces and the ability to easily multiplex the assay. Additionally, the VFA sensor response is faster than that of a Lateral Flow Assay (LFA), and the test results can be evaluated by untrained users [9,12]. The pore size of the membrane and the optimized flow rate determine the sensitivity of the test. VFA has a faster detection time than LFA because of the immediate reaction between the specific antigen and the conjugated detection antibody. The signal can be detected with the naked eye, and it can be quantified using imaging techniques, such as those used in smartphone reader [12]. According to Chen, et al. a vertical flow pattern can increase the flow rate and decrease the required pore size of a membrane. As a result, vertical flow methods can be up to 5 times more sensitive than lateral flow methods. In this article, we report the development of a paper-based, point-of-care vertical flow immunoassay platform for SARS-CoV-2/COVID-19 diagnosis that is user-friendly, low-cost, flexible, and rapid. The platform can detect the SARS-CoV-2 spike and nucleocapsid proteins in addition to human antibodies (IgG and IgM) in a singleplex manner. Additionally, multiplex detection can be used to detect both viral proteins and human antibodies with one biosensor platform in a single device. Therefore, our novel and flexible paper-based platform can detect various types of biomarkers related to COVID-19. We utilized the principles of colloidal gold nanoparticle-based sandwich ELISA in combination with vertical flow microfluidics. The singleplex platform was challenged in the presence of other types of coronaviruses and pathogenic viruses to evaluate the specificity capability of our assay for SARS-CoV-2 detection. In addition, the detection capability of our platform was evaluated in various specimen types (saliva, nasal fluid, blood, and urine). This diagnostic platform demonstrated high sensitivity in the 100 pg/mL limit of detection range and high specificity to the SARS-CoV-2 SP. In addition, the platform demonstrated its potential capability to differentiate between coronaviruses, which is important as new coronavirus variants emerge and for avoiding false positive results. The platform detected the SARS-CoV-2 SP in all specimens without the need to pre-treat the specimens prior to testing. Saliva provided the highest signal intensity (sensitivity) among the specimen types tested. The multiplexing capability of the platform for rapid detection of the SARS-CoV-2 spike protein and IgG and IgM antibodies was also evaluated. The results demonstrated the ability of this platform to provide rapid multiplexing within 10 minutes. Due to the platform’s high specificity, flexibility to be adapted for testing with multiple specimen types, and multiplexing capabilities, we believe this platform will be useful for meeting the specific testing requirements of various testing locations and accelerating the detection of COVID-19 at various stages of the disease infection.
Materials and Methods
Materials
Recombinant SARS-CoV-2 S1 (His), anti-SARS-CoV-2 Spike MAb (detection), anti-SARSCoV-2 Spike MAb (capture), anti-SARSCoV- 2 Nucleocapsid MAb (detection), and anti-SARSCoV-2 Nucleoprotein MAb (capture) were purchased from Creative Diagnostics (Shirley, NY). Rabbit IgG and Goat anti-Rabbit IgG were purchased from EMD Millipore-Sigma (Burlington, MA). Anti- Human IgG capture and anti-Human IgM capture Ab were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Pooled human nasal fluid was obtained from Innovative Research, Inc. (Novi, MI). Saliva and normal urine were obtained from Lee Biosolutions, Inc (Maryland Heights, MO). Whole human blood from healthy donors was acquired from Research Blood Components, LLC (Watertown, MA). Human Immunodeficiency Virus 1 (HIV 1) Antigen, Influenza A Virus Antigen, MERS-CoV Spike Protein S1, and SARS-CoV Spike Protein S1 were obtained from East Coast Biologics, Inc (North Berwick, ME). Bilirubin, cholesterol, human albumin, and human gamma globulin were obtained from MP Biomedicals, LLC (Solon, OH). Sucrose, uric acid, and Dulbecco's Phosphate Buffer Saline (DPBS) were acquired from Thermo Fisher Scientific, Inc. (Waltham, MA). Tween 20 was purchased from Sigma Aldrich (St. Louis, MO). Double-sided adhesive was provided by FLEXcon (Spencer, MA). The nitrocellulose membrane, Whatman chromatography paper, and blotting paper were bought from GE Healthcare Life Sciences (Pittsburgh, PA).
Sample preparation
The samples tested in the diagnostic platform were prepared with fresh human blood, saliva, nasal fluid, and urine spiked with SARSCoV- 2 spike and nucleocapsid proteins or antibodies against SARSCoV- 2 (IgM or IgG). SARS-CoV-2 positive blood samples in the presence of endogenous substances such as bilirubin, cholesterol, uric acid, albumin, and gamma globulin were prepared. Pretreatment of the specimens used in this study was not required prior to testing.
Synthesis of colloidal gold-anti-SARS-CoV-2 proteins
The Point of Care Testing (POCT) platform presented in this study was designed for singleplex detection of the spike or nucleocapsid antigenic proteins of SARS-CoV-2. In addition, multiplex detection of IgG/IgM antibodies against SARS-CoV-2-SP and spike antigenic protein was also performed. Therefore, the SARS-CoV-2 S-protein antigen, anti-spike protein mAb, and antiNucleocapsid protein mAb were conjugated to colloidal gold nanoparticles according to the manufacturer’s protocol (DCN Diagnostics, Carlsbad, CA). Briefly, all the proteins mentioned above were dialyzed in a 10 mM sodium phosphate buffer solution using 10K MWCO dialysis devices. Then, conjugation was performed by mixing the colloidal gold solution (AuNP) titrated at the suggested pH values (7.0-9.0) with 0.004-0.012 mg/mL of each protein for 15 minutes at Room Temperature (RT). This was followed by blocking each solution for 30 minutes using the blocking solution in the conjugation kit. The resulting solutions were centrifuged at 14,000 × g for 10 minutes. The supernatant was removed, and the resulting conjugated anti-spike protein mAb, anti- Nucleocapsid protein mAb, and S-protein antigen pellets were resuspended in the buffer provided by the kit. The resulting conjugated solutions were stored at 4°C until use.
Fabrication of the paper-based diagnostic platform for singleplex and multiplex detection of SARS-CoV-2/ COVID-19
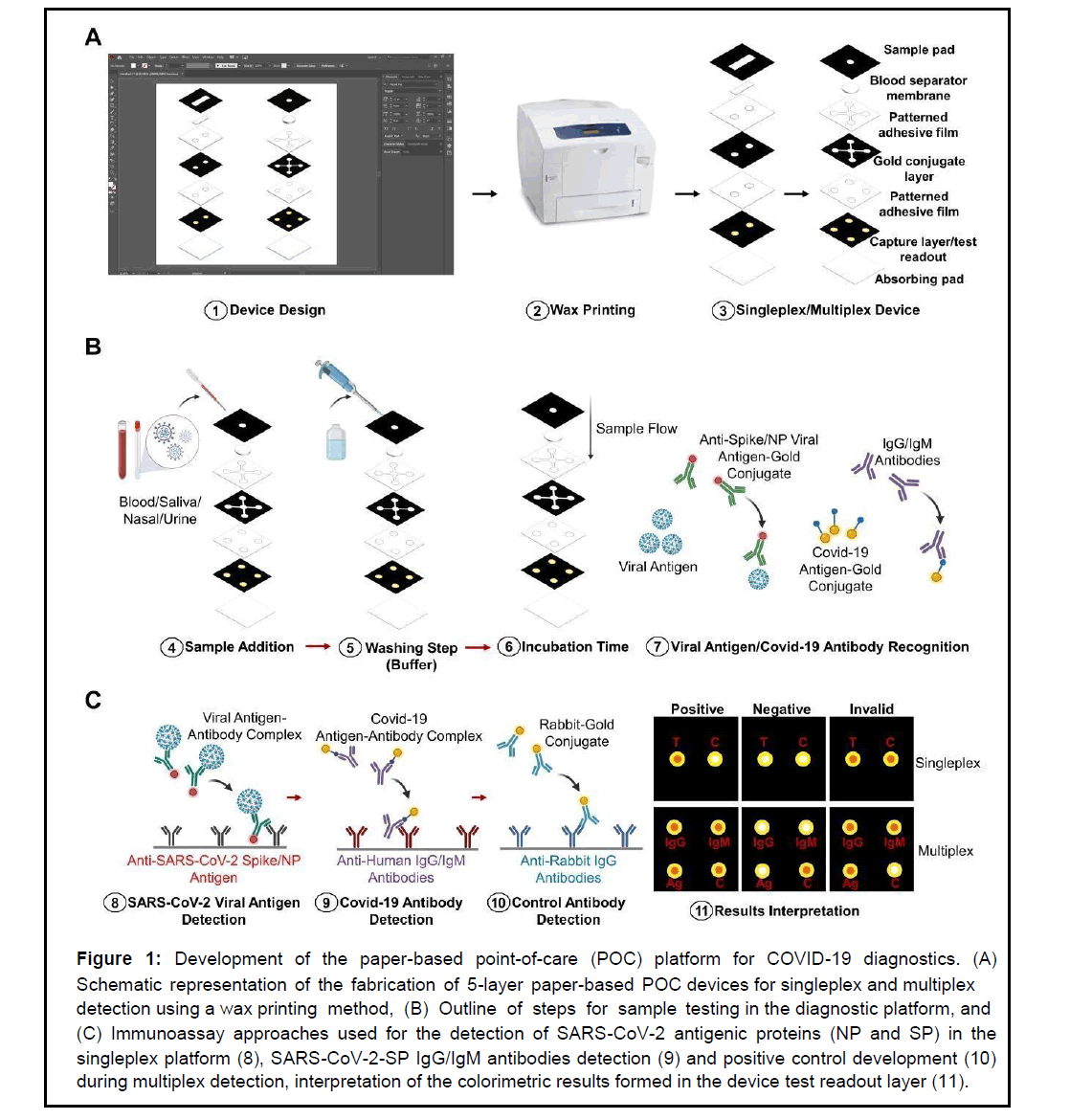
The paper-based POCT platform proposed in this study was fabricated using the principles of sandwich enzyme-linked immunosorbent assay (ELISA) and vertical flow microfluidics for rapid detection of COVID-19 biomarkers. The 5-layer platform used in this study for singleplex or multiplex detection was designed using Adobe Illustrator (Figure 1). The singleplex platform was designed with only two testing zones on the device test readout layer. The testing zones enabled colorimetric detection of a single analyte and a control. Similarly, the multiplex platform was designed with 4 testing zones for simultaneous detection of 3 analytes and a control. Wax printing using a Xerox ColorQube 8580 printer was used to create the hydrophobic areas that surround the active hydrophilic test zones on the proposed devices. This was done on every layer except the blotting and separator layers. The sample pad and conjugate layers of these devices were printed on Whatman No 1 chromatography sheets. The test readout layer was printed on the nitrocellulose membrane. To induce the melting of the wax, which created hydrophobic boundaries to define the sample zones, the paper layers were baked in an oven at 130°C for 30 seconds. Ultimately, the devices were cut using a guillotine-type paper cutter, and the layers of the devices were assembled. Adhesive films patterned with open holes and channels were created with a laser cutter (GuangZhou Amonstar Trade CO., Ltd, KH-3020). The adhesive films were placed on the backside of each layer prior to assembling. Lastly, the device was created by stacking all the layers one after the other, starting with the sample pad as the first layer and the blotting paper as the final bottom layer (Figure 1A). The fully assembled device dimensions were the same (1.75 cm by 1.75 cm) for the singleplex and multiplex platforms.
Figure 1: Development of the paper-based point-of-care (POC) platform for COVID-19 diagnostics. (A) Schematic representation of the fabrication of 5-layer paper-based POC devices for singleplex and multiplex detection using a wax printing method, (B) Outline of steps for sam ple testing in the diagnostic platform, an d (C) Immunoassay approaches used for the detection of SARS-CoV-2 antigenic proteins (NP and SP) in the singleplex platform (8), SARS-CoV-2-SP IgG/IgM antibodies detection (9) and positive control development (10) during multiplex detection, interpretation of the colorimetric results formed in the device test readout layer (11).
Immunoassay implementation of singleplex and multiplex testing platform
Preparation of the immunoassay platform for singleplex detection (SARS-CoV-2-NP or SP) required the treatment of the conjugate layer with a conjugate treatment solution (0.05% Tween 20 and 1% BSA). The conjugate layer was then treated with a colloidal gold-labeled anti-SARS-CoV-2-NP or SP antibody solution. The solution was allowed to air dry at room temperature. This was followed by treating the test readout layer with the anti-SARS-CoV-2-NP or SP capture antibodies at 3 and 4 mg/mL concentrations, respectively. The test layer was then treated with a blocking solution (0.05% Tween 20 and 1% BSA). Next, the layers of these devices were assembled as explained in section 2.4. Testing was then initiated by adding 15 μL of samples positive to the SARS-CoV-2-NP or SP (100 μg/mL, 100 ng/mL, or 100 pg/ mL) to the device sample zone. This was followed by the immediate addition of 30 μL of washing buffer (DPBS). The results were interpreted by peeling the devices’ layers apart to reveal the colorimetric results in test readout layer. Color interpretation was possible using the naked eye and quantified on images taken by an Android phone using the NIH ImageJ software. The grey intensity was quantified, and statistical analysis of the results was carried out. The detection time was within 5 minutes, and triplicate tests were performed for each concentration tested. Similarly, the same procedure was followed for the multiplex immunoassay platform preparation. Briefly, the conjugate layer was treated with colloidal gold-labeled anti-SARS-CoV-2-SP antibody, SARS-CoV-2 S-protein antigen (IgG/IgM), and Rabbit IgG (positive control) at the corresponding test zones. In addition, the test readout layer was treated with a 2 mg/mL antibody solution of antiSARS-CoV-2 S-protein, anti-Human IgG/ IgM, or anti-Rabbit IgG. Devices were assembled, and testing was performed in triplicates at 100 μg/mL in unmodified human blood. Testing took 10 minutes.
Analytical specificity for pathogenic viruses and endogenous interferents
The cross-reactivity and possible interference of the singleplex platform for SARS-CoV-2 SP detection was also assessed by testing antigens of different pathogenic viruses (SARS-CoV, MERS-CoV, HIV, and Influenza A) and endogenous substances that can potentially interfere with the detection efficiency of this testing platform. Each pathogenic virus was analyzed at a concentration of 100 ng/mL in samples negative for SARS-CoV-2. The diagnostic platform was challenged with individual blood samples positive for each virus at the concentration specified above. The specificity of the platform was evaluated by quantifying the red color signal observed in the test zone of each device as a result of the detection of each pathogenic virus. Statistical analysis was performed, and the results were compared to a negative control and the positive signal for SARSCoV- 2 detection. Interference for SARS-CoV-2 detection in the presence of endogenous substances was also evaluated in the diagnostic platform. A cocktail composed of 100 ng/mL SARS-CoV-2 SP viral antigen, 0.2 mg/mL uric acid, 120 mg/mL albumin, 120 mg/mL gamma globulin, 0.6 mg/mL bilirubin, and 5 mg/mL cholesterol in unmodified human blood was tested. Samples were tested in replicates of three, and colorimetric results were evaluated by quantification using NIH ImageJ software. Statistical analysis was performed to determine if the presence of potentially interferent substances altered the ability of the platform to detect SARS-CoV-2.
SARS-CoV-2 detection performance in the presence of different bodily fluids
To examine the detection profile of the proposed POCT platform in different bodily fluids, specimens such as saliva, unmodified human blood, nasal fluid, and urine were spiked with SARS-CoV-2 antigenic S-protein at a concentration of 100 ng/mL. The specimens were then individually tested in the singleplex POCT platform by adding 15 L of the specimen to the device sample zone. Colorimetric results were observed within 5 minutes, and the results were quantified using NIH ImageJ software. Statistical analysis was used to evaluate differences in the detection sensitivity of the assay as a result of changes in the sample media.
Statistical analysis
The statistical analyses were performed using GraphPad Prism (La Jolla, CA, U.S.A.). The statistical data was evaluated by one-way ANOVA in combination with Bonferroni tests. In this work, the data was represented as an average ± standard deviation (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001).
Results and Discussion
In this work, a rapid and tunable platform for SARS-CoV-2/ COVID-19 diagnostics was developed. Our simple, flexible, low-cost, portable, and immunoassay-based vertical flow platform can perform rapid point-of-care diagnosis of COVID-19 at all stages of the disease development. Singleplex testing of the SARS-CoV-2 virus Spike Protein (SP) and Nucleocapsid Protein (NP) were evaluated. In addition, multiplex detection of SARS-CoV antibodies (IgG and IgM) and SARS-CoV-2 antigens in a single device was examined. Furthermore, the specificity of our platform for the detection of the SARS-CoV-2-SP in the presence of other cross-reactant viruses and endogenous substances was characterized. The detection of the SARSCoV- 2-SP in different bodily fluids was also studied.
Platform development for SARS-CoV-2/COVID-19 detection
Indication of a COVID-19 infection is extremely nonspecific since the symptoms of this disease are similar to those of other illnesses such as viral pneumonia, cough, or fever. Therefore, user-friendly, rapid, and low-cost point-of-care diagnostic platforms with high specificity are critically needed for confirming suspected COVID-19 cases and limiting the spread of the virus among communities [13]. Currently, COVID-19 diagnostics are limited to a specific type of test, sample type, or stage of the disease. These key factors affect the test outcomes of current COVID-19 diagnostic tools potentially leading to misdiagnosis or false results [14,15].
In this work, a flexible paper-based platform for singleplex and multiplex colorimetric detection of SARS-CoV-2/COVID-19 was developed by integrating the principles of sandwich Enzyme-linked Immunosorbent Assay (ELISA) with microfluidics in a vertical flow arrangement (Figure 1). The singleplex platform was engineered for rapid individual testing of single antigenic proteins of the SARSCoV- 2 virus (SP or NP) in one diagnostic device (Figure 1A). Detection of multiple analytes (SARS-CoV-2 (SP) and IgG/IgM antibodies) was also accomplished in a single diagnostic device with the multiplex detection platform (Figure 1B). A vertical flow microfluidics approach was utilized during the development of the 5-layer single-plex and multiplex platforms because it was ideal for the rapid diagnosis of SARS-CoV-2/COVID-19 within sample volumes and time frames appropriate for minimally invasive POC diagnostics (e.g. <50 μL and <10 minutes). This approach allowed for rapid detection performance and flexibility in the multiplex diagnostic capability of the platform. Additionally, it eliminated hook effect problems, which are limiting factors in current diagnostic devices where lateral flow microfluidics is utilized as a proposed method for sample wicking to the device test zone [5,16,17]. In addition, literature studies have shown vertical flow microfluids to be up to 5 times more sensitive than lateral flow approaches [5]. Therefore, a vertical flow approach was the selected method for increasing the sensitivity and limiting the possibility of false negative results, especially in the later stages of COVID-19 disease development where the viral load may be reduced.
Throughout the development of this platform, key factors such as ease of use in operating the test and evaluating the final results, portability, and specimen type were considered. This diagnostic platform was developed for users with no medical experience. As such, strict procedures are not required to be followed, and samples, such as saliva, blood from finger pricking, or urine, will not need any pretreatment before testing. Literature studies have demonstrated that saliva is a reliable specimen for SARS-CoV-2 detection because it causes less patient discomfort and is a useful non-invasive sample for patient self-collection [18-21]. In addition, whole blood specimens obtained by finger pricking are desired for SARS-CoV-2 antibody testing because they eliminate major pre-analytical obstacles such as pre-treatment of blood specimens (centrifugation). Furthermore, venous blood sampling prevents patients from self-testing at point of care [22]. Therefore, the flexibility, simplicity, and ability of this platform to use different specimen types without pre-treatment procedures were important parts in the successful development of our immunoassay platform. Additionally, the ability of the platform to be easily operated by non-trained medical personnel and its light weight and small size (1.75 cm by 1.75 cm) for easy portability were important factors also considered and optimized. Simple operation and portability of POCT devices are key requirements for the effective utility of POCT devices in various testing settings [23]. By simply adding the required specimen type followed by a washing buffer and then peeling off the device readout layer, patients can interpret the colorimetric results of the test and determine if they infected by the virus using the naked eye. The possibility of patients being able to self-test from home further reduces the risk of spreading the virus.
The sensitivity, specificity, and colorimetric detection efficacy of this immunoassay platform were other key factors considered during the development process of this platform. To elaborate, optimal colorimetric results and higher sensitivities during analyte testing were obtained by using 20 nm gold nanoparticles during the gold nanoparticle-conjugation process of monoclonal antibodies (mAbs) against SARS-CoV-2 antigenic proteins (SP and NP) and anti-SARSCoV- 2-SP antibodies in the singleplex and multiplex platforms, respectively. It is well established in literature studies that gold nanoparticles of approximately 20 nm in size are preferred for fabrication of rapid immunoassays because they provide optimal colorimetric results at high intensities visible to the naked eye and enhance the detection sensitivity of the assay platform [24,25]. In addition, literature examples have also specified the need for specific antibodies against SARS-CoV-2 to increase the specificity of current immunoassays and eliminate cross reactivity problems that may result from considerable homology between SARS-CoV-2 and other coronaviruses, such as SARS-CoV [26]. Thus, commercially available monoclonal antibodies specific for SARS-CoV-2 detection were utilized in this platform.
Overall, the engineering approach used to develop this paper-based diagnostic platform allowed for singleplex detection of the antigenic proteins (SP and NP) for the SARS-CoV-2 virus in various specimen types without pre-treatment prior to testing. Only 15 μL of the testing analyte were required, and results were obtained in 5 minutes. In addition, the flexibility of this platform for multiplexing also allowed for rapid detection of multiple SARS-CoV-2/COVID-19 analytes (SARS-CoV-2-S(P) and IgG/IgM antibodies) in 10 minutes using only 30 μL of unmodified human blood in a single device. Altogether, our novel platform’s ability to be tailored to a specific detection pattern and sample type, its portability, and its low cost, as a result of inexpensive paper-based materials used for its fabrication, demonstrate the ability of this platform to be a potential alternative for rapid testing of SARS-CoV-2/COVID-19 in various testing sites.
SARS-CoV-2/COVID-19 platform detection capability
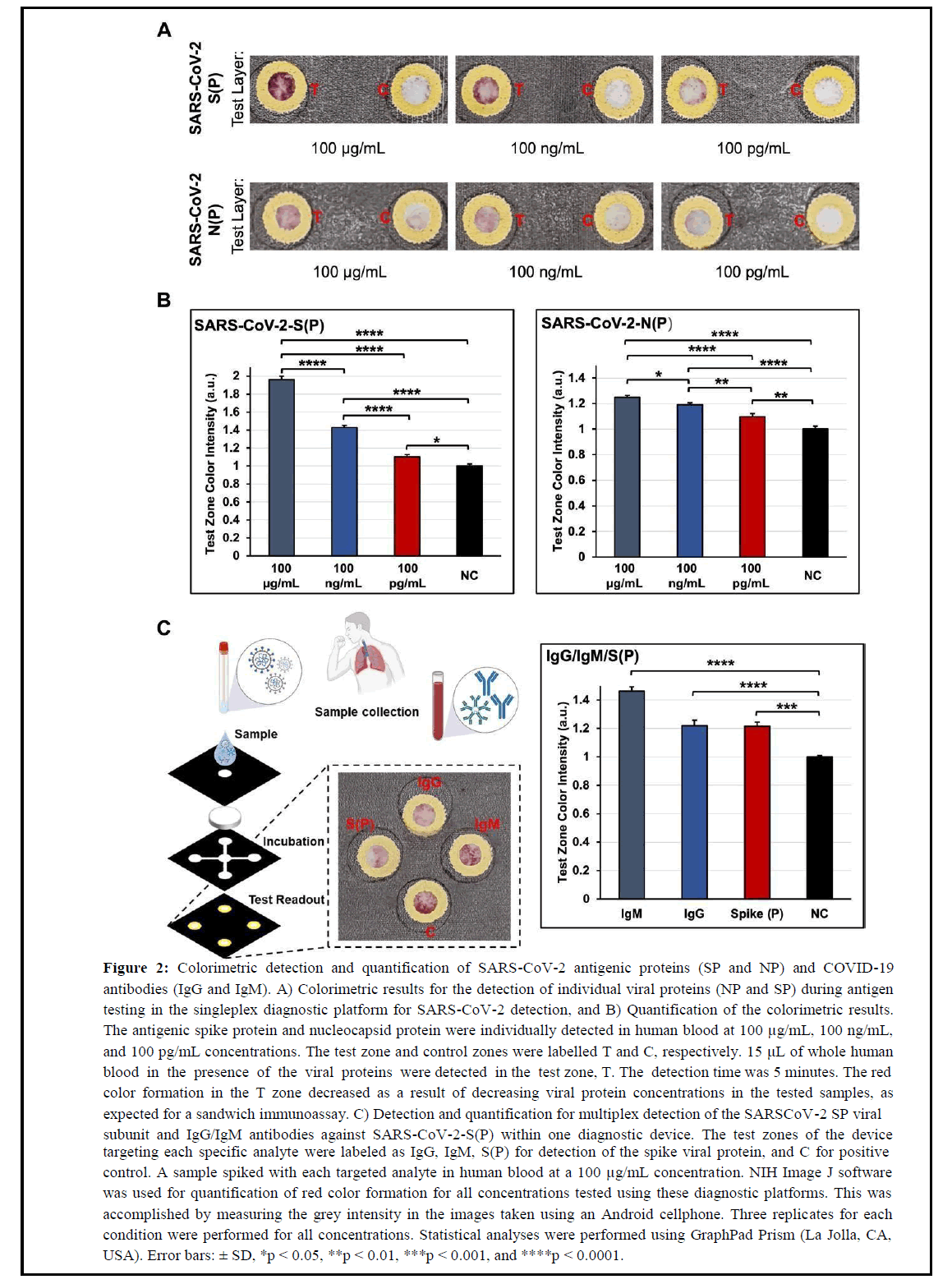
The detection capability of our flexible POCT platform for SARSCoV- 2/COVID-19 diagnosis was confirmed by performing testing of SARS-CoV-2 antigenic species (SP and NP) and antibodies (IgG/IgM) against the SARS-CoV-2-S(P) virus in unmodified fresh human blood. To demonstrate the detection affinity of the singleplex platform to SARS-CoV-2 SP and NP viral antigens alone, diluted samples at concentrations ranging from 100 μg/mL to 100 pg/mL were added to the sample zones on the devices. Colorimetric results were evaluated within 5 minutes after the sample addition by quantifying the color intensity formed in the device’s test zone using images taken with an Android cellphone. NIH ImageJ software was used for quantification of the colorimetric results, and significant differences among detected concentrations were assessed through statistical analysis. In Figure 2A, the colorimetric results for the detection of the SARS-CoV-2 SP and NP antigenic species showed a decreasing trend in color intensity as the concentration of the SP and NP viral antigens in the tested samples decreased, as expected. However, the detection affinity of the mAbs used in this sandwich immunoassay against the SARS-CoV-2 SP viral antigen proved to be higher compared to the results shown for the detection of the SARS-CoV-2 NP antigen. These results are in accordance with literature studies showing higher sensitivities for singleplex assays targeting both of these antigenic species individually in single devices [27]. The results for the detection sensitivity of the SARS-CoV-2 SP antigen is superior, and Limits of Detection (LOD) for each detected viral analyte (SP and NP) are within similar concentration ranges as tested in this assay (100 pg/mL) [27]. However, given the ability of our diagnostic platform to be flexible and meet specific testing requirements, this platform can potentially be arranged for multiplex detection of both antigenic species in a single device and contribute to more valid testing results if necessary [28]. In Figure 2B, the quantification results for the detection of both SARSCoV- 2 antigenic proteins confirm the colorimetric observations presented in this assay. In addition, significant statistical differences between each concentration (100 μg/mL, 100 ng/mL, and 100 pg/mL) tested for each antigenic protein were observed. Similarly, a significant statistical difference was also observed between each concentration and the negative control test zone in the assay.
Figure 2: Colorimetric detection and quantification of SARS-CoV-2 antigenic proteins (SP and NP) and COVID-19 antibodies (IgG and IgM). A) Colorimetric results for the detection of individual viral proteins (NP and SP) during antigen testing in the singleplex diagnostic platform for SARS-CoV-2 detection, and B) Quantification of the colorimetric results. The antigenic spike protein and nucleocapsid protein were individually detected in human blood at 100 μg/mL, 100 ng/mL, and 100 pg/mL concentrations. The test zone and control zones were labelled T and C, respectively. 15 μL of whole human blood in the presence of the viral proteins were detected in the test zone, T. The detection time was 5 minutes. The red color formation in the T zone decreased as a result of decreasing viral protein concentrations in the tested samples, as expected for a sandwich immunoassay. C) Detection and quantification for multiplex detection of the SARSCoV-2 SP viral subunit and IgG/IgM antibodies against SARS-CoV-2-S(P) within one diagnostic device. The test zones of the device targeting each specific analyte were labeled as IgG, IgM, S(P) for detection of the spike viral protein, and C for positive control. A sample spiked with each targeted analyte in human blood at a 100 μg/mL concentration. NIH Image J software was used for quantification of red color formation for all concentrations tested using these diagnostic platforms. This was accomplished by measuring the grey intensity in the images taken using an Android cellphone. Three replicates for each condition were performed for all concentrations. Statistical analyses were performed using GraphPad Prism (La Jolla, CA, USA). Error bars: ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
The multiplexing capability of this diagnostic platform was also assessed through preliminary studies where the SARS-CoV-2 SP viral subunit and IgG/IgM antibodies against SARS-CoV-2-S(P) were targeted simultaneously within one diagnostic device (Figure 2C). This study was performed as a proof-of-principle to demonstrate the flexibility of this diagnostic platform and its ability to be easily customized for multiplexed testing for COVID-19.
Multiplexing diagnostic tests for COVID-19 has the potential to accelerate the detection of COVID-19 at different stages of the disease or infection progress. In addition, such diagnostic tools could be valuable in sites (e.g. school) where information regarding immunity and infection could be beneficial for proper quarantine measures [1]. To perform this experiment, a sample spiked with each targeted analyte in unmodified human blood at a 100 μg/mL concentration was used. As shown in the results in Figure 2C, a colorimetric signal for the analytes tested in this platform was observed in all the individual test zones specific for each analyte (IgG, IgM, SP viral antigen, and positive control labeled as C). To confirm the absence of non-specific binding due to false-positive results, previous experiments using solutions negative to each analyte were also performed, and colorimetric results were only observed in the positive control test zone, as expected.
These results suggest no interference between the detection capability of each detection and capture mAb utilized for sandwich ELISA in this platform. Additionally, the quantified results in Figure 2C demonstrated statistically significant differences when colorimetric results from each targeted analyte were compared to a negative control.
The overall results of this study validated the ability of our diagnostic platform to perform rapid detection of SARS-CoV-2 viral subunits at concentrations as low as 100 pg/mL and serological testing against SARS-CoV-2 antibodies. The colorimetric results can be qualitatively assessed by the naked eye. Three replicates were performed for each test, and no false positive results were observed during this work.
Analytical specificity of COVID-19 platform for SARSCoV- 2 antigen detection in the presence of other viruses and potential interferents
In this work, the cross-reactivity and possible interference of the singlex platform for SARS-CoV-2 SP detection was also assessed by testing different pathogenic viruses (SARS-CoV, MERS-CoV, HIV, and Influenza A) and endogenous substances (Sec. 2.6) that can potentially interfere with the detection efficiency of the platform. The spike antigens of each pathogenic virus were analyzed at a 100 ng/mL concentration in samples negative to SARS-CoV-2.
Interferent endogenous substances were tested in the presence of the SARS-CoV-2 SP (100 ng/mL) in unmodified human blood. Samples were tested in replicates of three, and colorimetric results were evaluated using NIH ImageJ software to quantify color intensity (Figure 3). The tests performed in this study were conducted following the current recommendations issued by the FDA for data submission requests to support the pre-EUA/EUA of a diagnostic POCT for SARS-CoV-2 antigen testing [29].
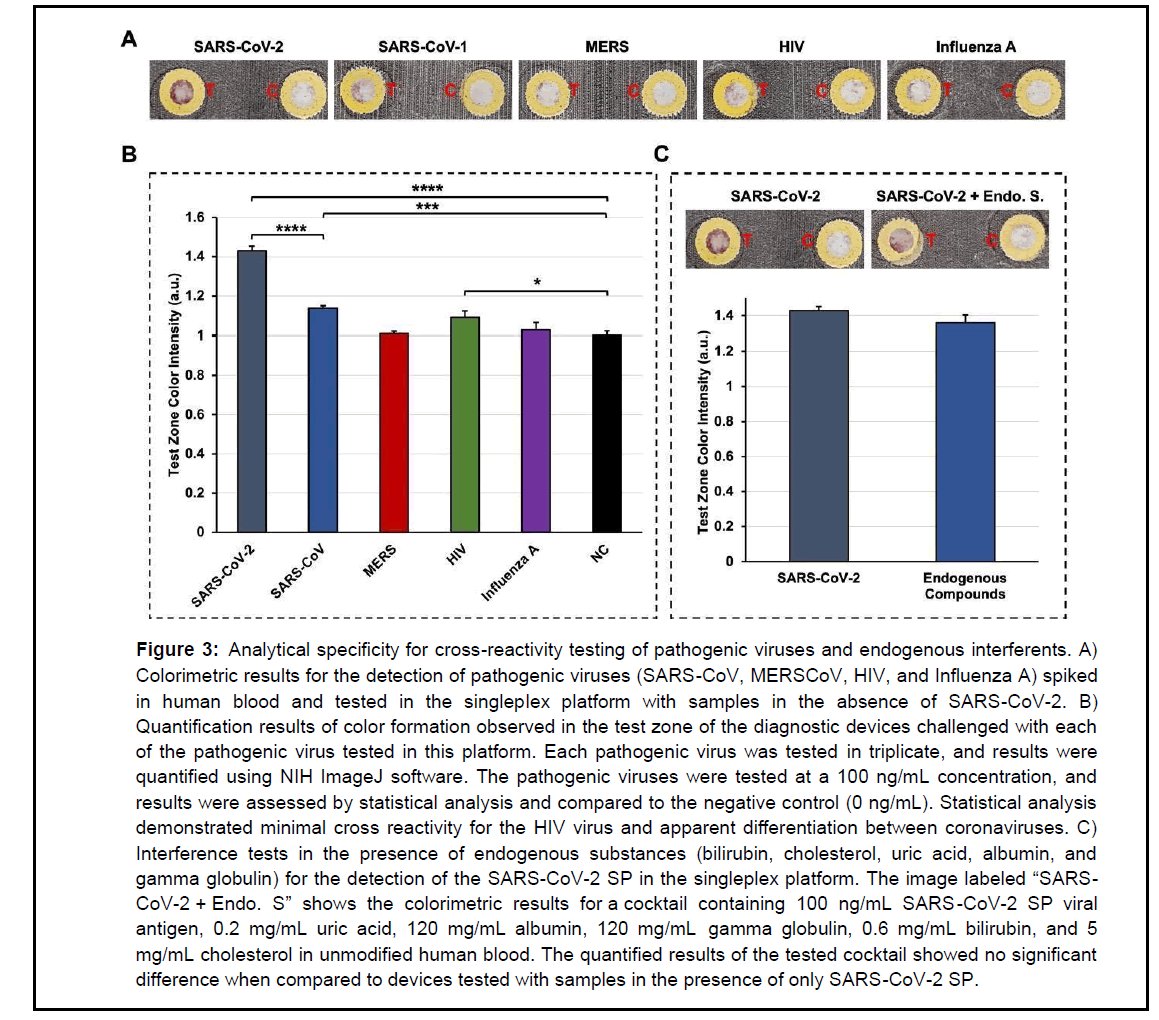
Figure 3: Analytical specificity for cross-reactivity testing of pathogenic viruses and endogenous interferents. A) Colorimetric results for the detection of pathogenic viruses (SARS-CoV, MERSCoV, HIV, and Influenza A) spiked in human blood and tested in the singleplex platform with samples in the absence of SARS-CoV-2. B) Quantification results of color formation observed in the test zone of the diagnostic devices challenged with each of the pathogenic virus tested in this platform. Each pathogenic virus was tested in triplicate, and results were quantified using NIH ImageJ software. The pathogenic viruses were tested at a 100 ng/mL concentration, and results were assessed by statistical analysis and compared to the negative control (0 ng/mL). Statistical analysis demonstrated minimal cross reactivity for the HIV virus and apparent differentiation between coronaviruses. C) Interference tests in the presence of endogenous substances (bilirubin, cholesterol, uric acid, albumin, and gamma globulin) for the detection of the SARS-CoV-2 SP in the singleplex platform. The image labeled “SARSCoV- 2 + Endo. S” shows the colorimetric results for a cocktail containing 100 ng/mL SARS -CoV-2 SP viral antigen, 0.2 mg/mL uric acid, 120 mg/mL albumin, 120 mg/mL gamma globulin, 0.6 mg/mL bilirubin, and 5 mg/mL cholesterol in unmodified human blood. The quantified results of the tested cocktail showed no significant difference when compared to devices tested with samples in the presence of only SARS-CoV-2 SP.
In Figure 3A, colorimetric results for the detection of coronaviruses, such as SARS-CoV and MERS, showed only a slight red color signal in the testing zone of the POCT platform when challenged with a blood sample positive for SARS-CoV (100 ng/mL). The detection of MERS showed no red color formation when tested at the same 100 ng/mL concentration. This may be due to SARS-CoV sharing similar homology with SARS-CoV-2. However, Figure 3B demonstrates a significant difference in the signal intensity for the quantification result for SARS-CoV-2 when detected at the same concentration (100 ng/mL) and compared to the results obtained for SARS-CoV. The colorimetric detection signal of SARS-CoV-2 is evidently in the higher end of the LOD of this diagnostic platform. The signal intensity resulting from the quantification result for MERS is confirmed to be not statistically significant because it is in the same quantification range as the negative control (NC, 0 ng/mL). Therefore, these results suggest the possibility of this diagnostic platform to distinguish among different coronaviruses since these results can only be expected from POCT tools with enhanced specificity against SARS-CoV-2, which seems to be the case for our detection platform. A similar testing trend has also been observed in other literature examples, but colorimetric differentiation with the naked eye was less evident in these examples than for our platform [30]. Furthermore, the quantification results for Influenza A showed no cross reactivity as its quantification results were similar to the negative control (NC). The quantification results for the HIV virus, however, seemed to show a minimal cross-reaction during detection. Lastly, in Figure 3C, the results for SARS -CoV-2 detection (100 ng/mL) in the presence of various endogenous compounds (0.6 mg/mL bilirubin, 5 mg/mL cholesterol, 0.2 mg/mL uric acid, 120 mg/mL albumin, and 120 mg/mL gamma globulin) showed no significant statistical difference when compared to the device tested with a solution in the presence of SARS-CoV-2 only. Overall, the COVID-19 platform presented in this study demonstrates enhanced sensitivity in detecting the SARS-CoV-2 SP antigen with minimum cross reactivity problems as a result of interference with other organisms. In addition, this platform proved to be potentially suitable to differentiate among coronaviruses.
SARS-CoV-2 detection performance in the presence of different bodily fluids
Appropriate specimen type and sample collection are key factors for determining the successful performance of SARS-CoV-2 diagnostic tools. However, depending on the application and method for diagnosis, a suitable sample type must be used. Among them, nasal fluid, as well as saliva, urine, and blood, have been used to diagnose patients infected by the COVID-19 virus [31-33]. To further investigate the detection profile of our singleplex immunoassay platform, various specimen types (saliva, nasal fluid, blood, and urine) were tested. Each specimen was spiked with the SARS-CoV-2 SP at 100 ng/ mL, and statistical analysis was performed to evaluate the results. Triplicate experiments were done for each specimen type (Figure 4).
In Figure 4A, colorimetric results can be observed in the test readout layer for each diagnostic device where a different specimen type was tested. Non-false positive results were observed, and color intensity on the sample test zone could be differentiated from the NC zone in the tested devices by the naked eye. These results suggest the potential capability of this diagnostic tool to be adapted to work with multiple specimen types if they are appropriate for the detection method of a targeted SARS-CoV-2 analyte. Interestingly, the quantification results in Figure 4B demonstrate that the saliva sample containing the SARS-CoV-2 SP analyte provided the highest signal intensity (sensitivity) among all tested specimens. Blood samples showed the second highest sensitivity followed by nasal fluid. A statistical difference was observed between the detection signal of all specimen types, except no significant difference was
Figure 4: SARS-CoV-2 detection performance in different bodily fluids using our POC diagnostic platform. A) Colorimetric results for the detection of the SARS-CoV-2 antigenic SP in samples such as saliva, nasal fluid, human blood, and urine. The SARS-CoV-2 SP viral protein was spiked in each specimen at a concentration of 100 ng/mL and tested in single devices as triplicates. B) Quantification of the colorimetric results obtained from the images taken with an Android cellphone camera. The colorimetric results were quantified using NIH ImageJ software, and results were evaluated through statistical analysis using GraphPad Prism (La Jolla, CA, USA). Note: Error bars: ± SD, *p<0.05, **p<0.01, and ****p<0.0001. Our paper-based microfluidic platform successfully detected the antigenic proteins of SARS-CoV-2 in blood, saliva, nasal fluid, and urine. The saliva specimen provided the highest signal intensity (sensitivity) among all tested specimens. A statistical difference was observed in the detection signal among all specimen types, except between blood and nasal fluid, where no significance difference was observed.
observed between blood and nasal fluid. The urine specimen showed the lowest sensitivity among all samples. These results are expected as literature studies have not shown a lot of evidence supporting the presence of viral ribonucleic acid (RNA) in urine [32]. However, several literature studies have suggested that SARS-CoV-2 detection in saliva samples provides results with higher sensitivity than results obtained using nasopharyngeal specimens [18,21,33,34]. Since saliva is a reliable and non-invasive sample type for patient self-collection, the ability of this platform to provide the highest sensitivity results within this specimen type serves as steppingstone for advancing this research closer to a platform for SARS-CoV-2 self-testing at home.
Overall, the ability of this platform to provide successful results by allowing for the testing of various specimen types without pretreatment in the same platform may be a valuable solution for establishing a better prognosis for patients infected by the SARSCoV- 2 virus.
Conclusion
In summary, a flexible paper-based platform for singleplex and multiplex colorimetric detection of SARS-CoV-2/COVID-19 was developed in this study. The combination of vertical flow microfluidics and sandwich immunoassay was the chosen approach for the development of this POCT platform. This approach allowed for high assay sensitivity and rapid results within time frames and specimen volumes closer to minimally invasive diagnostics (e.g., <50 μL and <10 minutes). The test results can be interpreted using the naked eye by untrained users similar to home-based pregnancy tests. Multiple specimen types can be used to detect SARS-CoV-2 related analytes in our testing platform. The singleplex design of the platform allowed for individual testing of the spike and nucleocapsid antigenic proteins of the SARS-CoV-2 virus at concentrations within the 100 pg/mL detection range. Additionally, the multiplex detection design of our POC device demonstrated simultaneous detection of IgG and IgM antibodies and the SARS-CoV-2 SP in a single platform using human blood. The assay platform provided non-significant interference against other pathogenic viruses and demonstrated the potential to differentiate between other coronaviruses such as MERS-CoV and SARS-CoV. To further asses the detection profile of this diagnostic platform, singleplex testing was performed using various specimen types. While our platform achieved SARS-CoV-2 detection in blood, nasal fluid, saliva, and urine, saliva provided the most sensitive results. Our technology does not only offer a potential rapid and lowcost alternative to current immunoassay-based techniques for SARSCoV- 2 diagnostics, but it can also provide flexibility by being able to adapt to the specific testing requirements needed by various testing locations.
Author Contributions
G.C.U. and D.L. conceived of the study; D.L. conducted the experiments; D.L., C.M., and G.C.-U. wrote, revised, and edited the manuscript. All authors have read and approved the manuscript.
Acknowledgements
This work was supported by the University of Massachusetts Lowell faculty start-up funds. The patent application for this work has been filed but it is not published yet.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Masterson AN, Muhoberac BB, Gopinadhan A, Wilde DJ, Deiss FT, et al. (2021) Multiplexed and high-throughput label-free detection of rna/spike protein/igg/igm biomarkers of sars-cov-2 infection utilizing nanoplasmonic biosensors. Anal Chem 93:8754-8763.
[Crossref] [Google scholar] [PubMed]
- Soler M, Estevez MC, Cardenosa-Rubio M, Astua A, Lechuga LM (2020) How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sens 5:2663-2678.
[Crossref] [Google scholar] [PubMed]
- Moitra P, Alafeef M, Dighe K, Frieman MB, Pan D (2020) Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14:7617-7627.
- Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, et al. (2020) Diagnosing COVID-19: The diseaseand tools for detection. ACS Nano 14:3822-3835.
[Crossref] [Google scholar] [PubMed]
- Nguyen NN, McCarthy C, Lantigua D, Camci-Unal G (2020) Development of diagnostic tests for detection of SARS-CoV-2. Diagnostics 10:905.
[Crossref] [Google scholar] [PubMed]
- Bastos ML, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, et al. (2020) Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ 370:m2516.
[Crossref] [Google scholar] [PubMed]
- Posthuma GAT, Korf J, van Amerongen A (2009) Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem 393:569-582.
[Crossref] [Google scholar] [PubMed]
- Chen P, Gates-Hollingsworth M, Pandit S, Park A, Montgomery D, et al. (2019) Paper-based Vertical Flow Immunoassay (VFI) for detection of bio-threat pathogens. Talanta 191:81-88.
[Crossref] [Google scholar] [PubMed]
- Singh AT, Lantigua D, Meka A, Taing S, Pandher M, et al. (2018) Paper-based sensors: Emerging themes and applications. Sensors 18:2838.
- Reboud J, Xu G, Garrett A, Adriko M, Yang Z, et al. (2019) Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities. Proc Natl Acad Sci USA 116:4834-4842.
[Crossref] [Google scholar] [PubMed]
- Clarke O, Goodall B, Hui H, Vats N, Brosseau CL (2017) Development of a SERS-based rapid vertical flow assay for point-of-care diagnostics. Anal Chem 89:1405-1410.
- Jiang N, Ahmed R, Damayantharan M, Ünal B, Butt H, et al. (2019) Lateral and vertical flow assays for point‐of‐care diagnostics. Adv Healthc Mater 8:e1900244.
[Crossref] [Google scholar] [PubMed]
- Nguyen T, Duong Bang D, Wolff A (2020) 2019 novel coronavirus disease (COVID-19): Paving the road for rapid detection and point-of-care diagnostics. Micromachines 11:306.
[Crossref] [Google scholar] [PubMed]
- Toptan T, Eckermann L, Pfeiffer AE, Hoehl S, Ciesek S, et al. (2021) Evaluation of a SARS-CoV-2 rapid antigen test: Potential to help reduce community spread? J Clin Virol 135:104713.
[Crossref] [Google scholar] [PubMed]
- Hu E (2020) COVID-19 testing: Challenges, limitations and suggestions for improvement. Prepr. 2020040155.
[Crossref] [Google scholar] [PubMed]
- Park J, Park JK (2017) Pressed region integrated 3D paper-based microfluidic device thatenables vertical flow multistep assays for the detection of C-reactive protein based on programmed reagent loading. Sens Actuators B Chem 246:1049-1055.
- Moumita M, Shankar K, Abhiman P, Shamasundar BA (2019) Development of a sandwich vertical flow immunogold assay for rapid detection of oxytetracycline residue in fish tissues. Food Chem 270:585-592.
[Crossref] [Google scholar] [PubMed]
- Williams E, Bond K, Zhang B, Putland M, Williamson DA (2020) Saliva as a noninvasive specimen for detection of SARS-CoV-2. J Clin Microbiol 58:e00776-00720.
[Crossref] [Google scholar] [PubMed]
- Azzi L, Carcano G, Gianfagna F, Grossi P, Dalla Gasperina D, et al. (2020) Saliva is a reliable tool to detect SARS-CoV-2. J Infect 81:e45-e50.
[Crossref] [Google scholar] [PubMed]
- Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, et al. (2020) Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med 383:1283-1286.
[Crossref] [Google scholar] [PubMed]
- To KKW, Tsang OTY, Leung WS, Tam AR, Wu TC, et al. (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARSCoV-2: An observational cohort study. Lancet Infect Dis 20:565-574.
- Andrey DO, Cohen P, Meyer B, Torriani G, Yerly S, et al. (2020) Diagnostic accuracy of Augurix COVID‐19 IgG serology rapid test. Europ J Clin Invest 50:e13357.
[Crossref] [Google scholar] [PubMed]
- Tu J, Torrente‐Rodríguez RM, Wang M, Gao W (2020) The era of digital health: A review of portable and wearable affinity biosensors. Adv Funct Mater 30:1906713.
- Omidfar K, Khorsand F, Azizi MD (2013) New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens Bioelectron 43:336-347.
[Crossref] [Google scholar] [PubMed]
- Lou S, Ye JY, Li KQ, Wu A (2012) A gold nanoparticle-based immunochromatographic assay: The influence of nanoparticulate size. Analyst 137:1174-1181.
[Crossref] [Google scholar] [PubMed]
- Terry JS, Anderson LB, Scherman MS, McAlister CE, Perera R, et al. (2021) Development of a SARS-CoV-2 nucleocapsid specific monoclonal antibody. Virol 558:28-37.
[Crossref] [Google scholar] [PubMed]
- Cai Q, Mu J, Lei Y, Ge J, Aryee AA, et al. (2021) Simultaneous detection of the spike and nucleocapsid proteins from SARS-CoV-2 based on ultrasensitive single molecule assays. Anal Bioanal Chem 413:4645-4654.
[Crossref] [Google scholar] [PubMed]
- Barlev-Gross M, Weiss S, Ben-Shmuel A, Sittner A, Eden K, et al. (2021) Spike vs. nucleocapsid SARS-CoV-2 antigen detection:application in nasopharyngeal swab specimens. Anal Bioanal Chem 413:3501-3510.
[Crossref] [Google scholar] [PubMed]
- FDA. Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices.
- Lee JH, Choi M, Jung Y, Lee SK, Lee CS, et al. (2021) A novel rapid detection for SARS-CoV-2 spike 1 antigens using human angiotensin converting enzyme 2 (ACE2). Biosens Bioelectron 17:112715.
[Crossref] [Google scholar] [PubMed]
- Yan Y, Chang L,Wang L (2020) Laboratory testing of SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2 (2019‐nCoV): Current status, challenges, and countermeasures. Rev Med Virol 30:e2106.
[Crossref] [Google scholar] [PubMed]
- Peng L, Liu J, Xu W, Luo Q, Chen D, et al. (2020) SARS‐CoV‐2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol 92:1676-1680.
[Crossref] [Google scholar] [PubMed]
- Kashiwagi K, Ishii Y, Aoki K, Yagi S, Maeda T, et al. (2021) Immunochromatographic test for the detection of SARS-CoV-2 in saliva. J Infect Chemother 27:384-386.
[Crossref] [Google scholar] [PubMed]
- Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, et al. (2020) Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. MedRxiv.
[Crossref] [Google scholar] [PubMed]
Citation: Lantigua D, McCarthy C, Una GC (2022) Point of Care Platform for Detection of SARS-CoV-2/COVID-19. J Infect Dis Ther 10:486. DOI: 10.4172/2332-0877.1000486
Copyright: © 2022 Lantigua D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3747
- [From(publication date): 0-2022 - Jul 14, 2025]
- Breakdown by view type
- HTML page views: 3119
- PDF downloads: 628