Populus Tree Wood: A Noble Bioresource from Western Himalayas for the Development of Various Carbon Types for the Effective Application in Environment Protection i.e., Phenol Adsorption from Wastewater
Received: 22-Sep-2017 / Accepted Date: 26-Oct-2017 / Published Date: 30-Oct-2017 DOI: 10.4172/2155-6199.1000415
Abstract
Populus tree is one of the extensively available Bioresource in the foothills of Western Himalayas. Apart from its multifarious utilities, the activated carbon from the poplar wood has also been finding its enormous use in combating environmental contaminants. Waste aqueous effluents containing various hazardous chemicals, heavy metals and dyes etc. cause serious environmental problems which ultimately affect both flora and fauna adversely. Activated carbon (AC) is being widely used adsorbent for the removal of organic pollutants. The poplar wood carbon (PWC) when chemically activated with NaOH, HCl, HNO3, H3PO4, H2SO4, CH3COOH and ZnCl2 caused significant increase in surface area and pore size development. All the chemically activated carbons (CACs) were subjected to extensive physiochemical studies like SEM, XRD, FTIR, EDAX and surface area etc. Among all the carbons, the SEM and surface area analysis of H2SO4 activated carbon showed the maximum pore size of 7.1 μm and leading surface area of 1045 m2/g. These carbons are acidic with their pHzpc in the range of 3.6 to 4.1. The carbon is amorphous in nature as it is showing three typical broad peaks around 26.5, 44.4 and 80° in all the samples. Among the bulk density of these carbons, ZnCl2 and H2SO4 activated carbons have maximum of 428 and 427 kg/m3 respectively. The utilization of these carbons in environmental protection has been studied by carrying out adsorption studies for the removal of phenol. Direct proportion of phenol adsorption to the adsorbent concentration was well established. Effect of contact time on adsorption of phenol using 0.5 gm of adsorbent with 25 ml of 1000 ppm adsorbate showed 100% removal of phenol and the equilibrium was attained in almost an hour. Preliminary studies using poplar wood carbon for the elimination of highly hazardous contaminants has been carried out and the results have been very encouraging.
Keywords: Bioresource; Poplar wood carbon; Activated carbon; Chemically activated carbon (CAC); Environment protection
Introduction
India has been fascinating the entire world especially the scientific community due to her lush green and diverse flora. Himachal Pradesh a western Himalayan state of India, is one of the mega biodiversity centers of the world with all kinds of plants ranging from herbs to colossal trees. The altitude of this hilly state ranges from 350 meters to 6975 meters above mean sea level which make it the home for all kind of flora and fauna. Due to its mountainous geography, a variety of broad leafed species constitutes its forest. The Poplar is one such tree which finds its agricultural estate in abundance in Himachal. The tree is used not only as fodder, fuel and furniture etc., but has also been widely cultivated to combat soil erosion, deforestation and other environmental hazards. The global reported area of planted poplar was 6.7 million ha, of which 3.8 million ha (56%) were planted primarily for wood production and 2.9 million ha for environmental purposes [1]. Poplar agro forestry can now be found in many states in India. These include Uttar Pradesh, Punjab, Haryana, Jammu and Kashmir, Himachal Pradesh and Arunachal Pradesh [2]. Due to rapid Industrial growth and technological developments across the globe, there is nonstop quality degradation of both air and water. Along with it, even constant use of agricultural insecticides, pesticides and heavy metals is also proving to be a major contributor towards water pollution.
These pollutants along with Industrial effluents are persistently posing threat to whole biodiversity on the planet earth. As the problem is infuriating day by day, various techniques and technologies are being developed to combat the problem.
Among various techniques, adsorption is a very popular and widely used technique. This is due to its cost-effectiveness that this technique is finding its vast application in controlling the environmental hazards. Activated carbon is a tasteless, solid, microcrystalline, non-graphitic form of a black carbonaceous material with a porous structure [3-4]. Activated carbon has been regarded as only one of its kind, flexible and versatile adsorbent due to its salient and unique characteristics like large surface area, micro porous structure, high adsorption capacity and degree of reactivity [5-7]. There are many agricultural by- products which are considered as waste matter. These agricultural waste materials pose numerous disposal problems. But, an economic and green technology latent in these waste materials has been brought to the fore by the scientific world and this material is no more a waste material but is an eco-friendly as well as economic resource in controlling environmental pollutants. This requires only transformation of this agricultural waste material into activated carbon. Such agricultural waste material can be obtained from different agricultural and horticultural crops. For example, activated carbon can be obtained from various raw materials like Eucalyptus wood [8], bamboo [9], wood fiberboard waste [10], sugar cane bagasse [11], cotton stalks [12], Casuarina [13] and Pine tree [14] etc.
Phenol and phenolic compounds are among the highly toxic and hazardous pollutants. These compounds have not only poor biodegradability but also have extremely high toxicity. As a result, these pollutants pose a severe discharge problem. Based upon the intensity and gravity of health hazards associated with phenol, Environmental Protection Agency (EPA) has set a limit of 0.1 mg/L of it in waste water. The World Health Organization (WHO) has laid even stricter regulations for phenol and has laid 0.001 mg/L as the limit of phenol concentration in potable water [15].
Adsorption of such hazardous compounds on activated carbon has been found to be viable technology. Even USEPA has recommended activated carbon adsorption as one of the best available technologies (BAT) [16] in removal of organic compounds. Activated carbon possess perfect adsorption ability for relatively low molecular weight organic compounds such as phenols [17].
Poplar tree wood is also a good source for the preparation of activated carbon. The tree is abundantly available in Himachal Pradesh. Despite of its abundance in Himachal and even Northern India, the exploitation of poplar for its adsorption properties has not been much studied. The present study aims do deal with following issues i.e.,
• To develop carbon from this abundant resource i.e., poplar tree wood.
• To chemically activate the poplar tree wood carbon. The activating agents employed function as dehydrating agents that influence pyrolytic decomposition inhibiting the formation of tar and thereby enhancing the yield of carbon. The temperatures used in chemical activation are lower than that used in the physical activation process. As a result the development of a porous structure is better in the case of chemical activation method. Different chemicals (NaOH, HCl, HNO3, H3PO4, H2SO4 and ZnCl2) were used to have extensive properties like surface area, surface functional groups and composition etc. which are crucial for adsorption.
• Amongst these chemically activated carbons, utilizing the best carbon material for Environmental protection by using as an efficient adsorbent for the phenolic contaminants removal.
Materials and Methods
Adsorbent
Commercially available poplar activated carbon was procured from local supplier i.e., Anand Carbons, Jalandhar Road, Nasrala, Hoshiarpur Punjab, (INDIA)-146 022. This carbon was dried in an oven at 110C for 3 hours and was analyzed for various parameters like pH, ash content, and moisture content by using standardized methods. The surface area (BET) measurements of the adsorbents were obtained using BET method with nitrogen gas at 77K using Smartsorb surface area analyzer [Smart Instruments Ltd.]. The carbon was then activated using various chemicals using a standard method [18]. In the chemical activation, an important factor is the degree of impregnation [18] defined as
Impregnation ratio=(Weight of active agent added/weight of carbonizing material)
Chemically activated carbons i.e., Hydrochloric acid activated Poplar Wood Carbon (HCAPWC), Nitric acid activated Poplar Wood Carbon (NAAPWC), Sulphuric acid activated Poplar Wood Carbon (SAAPWC), Sodium Hydroxide activated Poplar Wood Carbon (SHAPWC), Phosphoric acid activated Poplar Wood Carbon (PAAPWC), Zinc chloride activated Poplar Wood Carbon (ZCAPWC) and Acetic acid activated Poplar Wood Carbon (AAAPWC) were prepared from poplar wood carbon at impregnation ratio of 0.25. The lower impregnation ratio was chosen only to see its effect on the characteristics of activated carbon even though almost all the published data only pertains to higher impregnation ratio of 0.5, 1, 2 and 3 etc. 30 gm each of poplar wood carbon was taken in seven different beakers. 50 ml of HCl, HNO3, H2SO4, NaOH, H3PO4, ZnCl2 and CH3COOH were added into these beakers respectively. These were then kept at 80°C for 12 hours. After taking them out, these were washed thoroughly with distilled water and followed by washing with 1% NaHCO3 solution. To ensure that no traces of acid and alkali are left out, the activated carbons were further washed extensively with hot distilled water until neutral pH.
In case of ZnCl2 activation, excess of it was leached out by immersing it in a 1 M HCl solution for about 24 hours in an oven at 80°C. It was repeatedly washed with hot distilled water until the chloride had disappeared from the wash water as ensured using AgNO3 solution. All activated carbon materials were then dried in an oven at 110°C. The chemicals used for the chemical activation of poplar wood carbon were analytical grade reagents. Acetic acid, Phosphoric acid and Hydrochloric acid were from Rankem with purity of 99.8%, 88% and not less than 35% respectively. Sodium Hydroxide, Phenol and Zinc chloride were from S.D. fine chemicals and CDH with purity of 85% and 99.5% and 97% respectively. Nitric acid, Sodium Bicarbonate and Sulphuric acid used were from Loba Chemicals and Qualigen with purity of 69-71%, 99.5% and 98% respectively.
Characterization techniques
pH, moisture, ash and bulk density: The pH and Bulk density of the Activated carbon sample was determined using following standard methods. The pH and ash were determined using standard reference methods i.e., ASTM D3838-80 [19] and ASTM D2866-94 [20] respectively. Whereas moisture was determined using ASTM D2867(09) 2014 [21]. Apparent or bulk density is a measure of the weight of material that can be contained in a given volume under specified conditions. To measure bulk density, a 10 mL cylinder was filled to a specified volume with dried adsorbent. The cylinder was weighed. The bulk density was then calculated as below by applying standard method i.e., ASTM D 2854-96., 2000b [22].
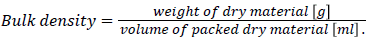
Porosity: Specific gravity (S) and bulk density (D) were used to determine the bulk density of the carbon. The following formula was used to calculate Porosity percentage.
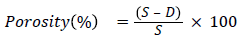
Scanning electron microscopy, XRD and surface area: Scanning electron microscopy (SEM) was used to study the surface morphology of adsorbent. The corresponding SEM micrographs being obtained using a Quanta 200 (FEI Company) Japan, at suitable magnification and resolution. The XRD analysis was carried out using the instrument with Model XRD X’pert Pro. The surface area (BET) measurements of the adsorbents were obtained using BET method with nitrogen gas at 77 K using Smartsorb surface area analyzer [Smart Instruments Ltd.].
Matter soluble in water and acid and volatile matter: Analysis of matter soluble in water and acid respectively is an indicator about the purity of the activated carbon. An estimation of purity in relation to water extractable substances can be had from the water soluble content of the activated carbon. Similar to ash content, even the acid soluble content gives a general indication of the purity of activated carbon. These were determined using standard methods. The standard method used for determining water soluble matter was ASTM D5029-98 (2014) [23]. Volatile matter was calculated using ASTM D 5832-98 (2014) [24]. It was calculated using the following method.
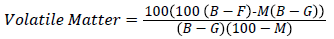
Where
G=Wt of empty crucible with lid (gm), B=Wt of empty crucible+lid +sample (before heating) in gm
F=Wt of empty crucible+lid+sample (after heating) in gm, M=Moisture content in %
Determination of pHZPC: The point of zero charge (pHZPC) is the pH at which the adsorbent is neutral to in aqueous suspension, and above which the total surface of the carbon is negatively charged [25]. Drift method [26] was used to measure it. The method used for the determination of pHZPC is as following.
Closed Erlenmeyer flasks were filled with 50 ml of 0.01M NaCl solutions. These were agitated at room temperature of about 25°C. The initial pH of each solution was fixed at value ranging from 2 to 12 by adding 0.1 M HCl or 0.1M NaOH solutions. After this, 0.5 gm of solid adsorbent was added to each flask. After an interval of 48 hours, the final pH was measured. pHZPC is the value that marks the point where the curve pHinitial versus the pHfinal intersects the first bisector.
Fourier transform infrared spectroscopy, CHNS analysis: The activated carbon has various functional groups on its surface. These surface functional groups also contribute towards adsorption capacity of the activated carbon. Fourier transform infrared spectroscopy (FTIR) is the technique which gives information about the functional groups. This technique was used to determine the vibrational frequency changes in the functional groups in the carbons. The spectra of carbons were measured by an FTIR spectrometer (Perkin Elmer) within the range of 400-4000 cm-1 wave number.
The CHNS analysis was done using vario MICRO Cube CHNS analyzer (Elementar GmbH ANALYSENSYS TEME). Iodine and methylene adsorption studies were done as per the standard procedures.
Adsorption studies: The adsorption studies were carried out with iodine and methylene blue. Iodine number is the elementary parameter used to characterize activated carbon performance. It is a measure of activity level. Methylene blue and iodine adsorption studies were done using standard methods i.e., ASTMD 2330-02 [27] and ASTM 4607-94 [28] respectively.
Phenol removal studies: The studies were carried out using aqueous solution of Phenol. Laboratory grade phenol was used for making standard solution of Phenol. The adsorption of phenol on active carbon was carried out using batch technique at room temperature. The concentration of phenol in aqueous solution was measured by UVvisible spectrophotometer (Double beam, Shimadzu UV-160A) at wavelength 269 nm, except where otherwise specified.
The difference of the initial and final phenol concentration was used to calculate the Phenol uptake as follows
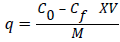
Where q is the uptake mg/g, C0 is the initial Phenol concentration, Cf is the final Phenol concentration (mg/ml). M is the amount of carbon and V is the volume of solution in ml.
Results and Discussion
The results of different physico-chemical properties of both the Activated as well as chemically activated carbons are as follows. The characterization of these adsorbents was done so as to obtain a better elucidation and interpretation of the mechanism involved during the adsorption process so as to highlight and differentiate the changes in physico-chemical properties of AC after chemical treatment and to provide complete comparison of CAC with precursor carbon AC.
Different activating agents have different effects on the carbon. These chemical activating agents have affirmative and favorable impacts on the carbon structure i.e., its pore structure and surface area etc. For example H3PO4 i.e., Phosphoric acid acts as a catalyst. This acid catalyst facilitates the process of bond cleavage, hydrolysis, dehydration and condensation, accompanied by cross-linking reaction between phosphoric acid biopolymers. Further, the volume occupied by Phosphoric acid is concurrent with the micropore volume of the activated carbon. As a result this acid may function as a template [29]. In case of activation with Zinc Chloride, the mechanism of action is different from that of Phosphoric acid. With zinc chloride, it is degradation of the material that takes place first followed by dehydration upon carbonization. Dehydration results in charring followed by aromatization of the carbon skeleton that ultimately leads to the formation of pore structure [30]. Acid treatment is generally used to oxidize the porous carbon surface. The oxidation has numerous effects on the activated carbon. Apart from enhancing the acidic property, it also results in the removal of the mineral elements also. In addition, oxidation also increases the hydrophilic nature of the surface [31].
Physical and physiochemical characterization
pH, moisture, ash, bulk density and porosity: The results obtained for pH, moisture, Ash, Bulk density and porosity of the AC and CAC as determined by the method prescribed above have been compiled in the Table 1.
| Type of PWC | pH | Moisture (%) |
Ash (%) | Bulk density (kg/m3) |
Porosity (%) |
|---|---|---|---|---|---|
| Untreated Poplar Wood Carbon | 6.58 | 15.50 | 3.19 | 377 | 79 |
| Hydrochloric acid activated Poplar Wood Carbon | 4.85 | 13.20 | 2.96 | 412 | 85 |
| Nitric acid activated Poplar Wood Carbon | 4.67 | 12.70 | 2.67 | 414 | 82 |
| Sulphuric acid activated Poplar Wood Carbon | 4.25 | 12.40 | 2.02 | 427 | 88 |
| Sodium Hydroxide activated Poplar Wood Carbon | 7.92 | 12.50 | 3.91 | 416 | 82 |
| Phosphoric acid activated Poplar Wood Carbon | 5.15 | 13.60 | 3.23 | 417 | 84 |
| Zinc chloride activated Poplar Wood Carbon | 6.35 | 12.80 | 1.67 | 428 | 86 |
| Acetic acid activated Poplar Wood Carbon | 5.60 | 16.20 | 2.87 | 418 | 82 |
Table 1: pH, moisture, ash, bulk density, porosity activated as well as chemically activated poplar wood carbon.
The crude activated carbon is acidic in nature with its pH as 6.58. It has been reported by Ahmedna and Okieimen [32,33] that for most application carbon pH 6-8 is acceptable. Even in literature, various studies for the removal of heavy metals has shown that maximum adsorption of Cr (III), Cu (II), Zn(II), Cd (II) and Pb (II) was found to occur at pH 4.5–6.5 [34]. Though the moisture content of the carbon does not have any effect on its adsorptive power, but it definitely contributes in diluting the carbon. Consequently, an additional weight of carbon is required during treatment process. Any porous material will have the tendency to absorb moisture. The moisture content in this work was found to be 12.4 to 16.2%. This has been found close to activated carbons from Thevetia peruviana [35]. Moisture content, according to Aziza et al. [36] has a relationship with porosity (α) of a given carbon. Adsorbent with high moisture content is expected to swell less. This would hinder the pore size expansion for adsorbate uptake. Another highly important factor for better and efficient adsorption is Porosity. It is also interrelated to the bulk density and specific gravity of activated carbon. The porosity of these activated carbons find similarity to the one obtained from coir pith by physical and chemical activation method [18]. Activated carbon may not be pure carbon as it may contain some impurities depending on the type of starting material. For a good activated carbon, it is desirable that it must have low ash content. Higher ash content negatively influences the adsorptive properties of activated carbon. The ash content influences the adsorption of gases too [37]. Faust and Aly [38] suggests that the typical ash content of activated carbons is around 5-6%. However, all the activated carbons under study had lower ash content than the typical activated carbons in the range of 1.67-3.91% by mass.
pHzpc, volatile matter and matter soluble in acid and water: Table 2 evidently sums up the results of pHzpc, Volatile matter, Matter soluble in acid and water. pHzpc is an important characteristic for any activated carbon. It puts in the picture diverse characteristics of the activated carbon. It not only affirms the acidic/basic nature of the adsorbent but also points towards the type and kind of activated carbon. Even the net surface charge of the carbon in solution is also known by the PHzpc value. It is the collective influence of all the functional groups of activated carbon determines pHzpc, i.e., the pH at which the net surface charge on carbon was zero. It is known that the net charge on carbon surface is positive at a solution pH lower than that corresponding to the point of zero charge (pHzpc) of the surface and is negative at a solution pH higher than pHzpc. The presence of strong acidic groups is suggested by the value of the pHzpc obtained for these activated carbon samples. The acidic value for pHzpc also resemble to the activated carbon obtained from Balsamodendron caudatum wood waste [39].
| Type of Carbon | pHzpc | Water soluble matter(%) | Acid soluble matter(%) | Volatile Matter(%) |
|---|---|---|---|---|
| UPWC | 3.60 | 1.30 | 3.08 | 19.60 |
| HCAPWC | 3.80 | 0.94 | 2.34 | 21.53 |
| NAAPWC | 3.80 | 0.82 | 2.21 | 22.04 |
| SAAPWC | 3.90 | 0.71 | 2.20 | 21.64 |
| SHAPWC | 4.10 | 0.82 | 2.08 | 20.96 |
| PAAPWC | 3.70 | 1.01 | 2.11 | 20.76 |
| ZCAPWC | 4.00 | 0.96 | 2.13 | 21.73 |
| AAAPWC | 3.70 | 0.88 | 2.11 | 18.82 |
Table 2: pHzpc, water and acid soluble matter and volatile matter of activated as well as chemically activated poplar wood carbon.
The presence of organic compounds present in the raw material gives way to volatile matter. Microporous structure is developed when the volatiles are released as an outcome of decomposition of the organic part [40]. Actually, the volatile matter remains clogging in the carbon pores. Low volatile content is desirable as it leads to higher porous carbon. There are reports showing that low volatile matter content implies the high porosity of the adsorbent [41].
Solubility studies of carbon in acid and water were also performed to evaluate and infer the amount of impurities present in the carbon prepared by different carbonization process. These impurities in the carbon may adversely affect the expected quality of the treated water during treatment. The acid leachable matter is found to be slightly higher than the water soluble matter. The matter soluble in water and acid has been found almost similar to the one found in case of activated carbon from Ceiba pentradenta wood waste [42]. Among all, activation with sulphuric acid proves to be the best activation.
Morphological characterization
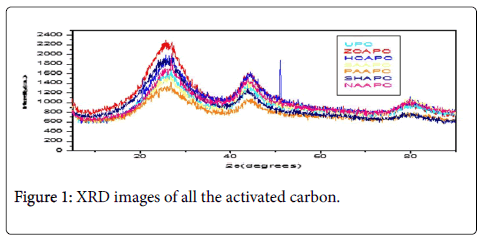
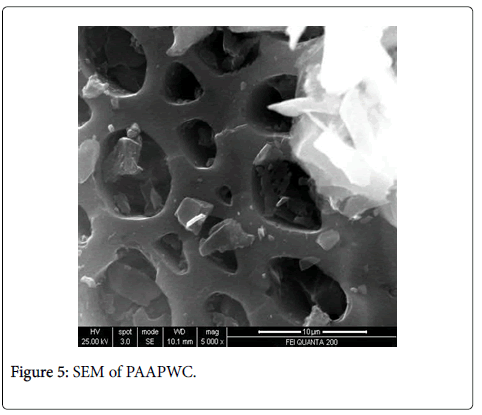
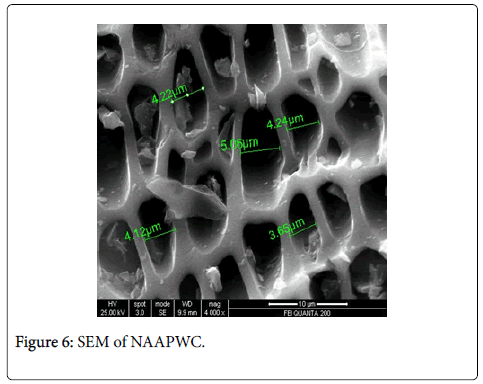
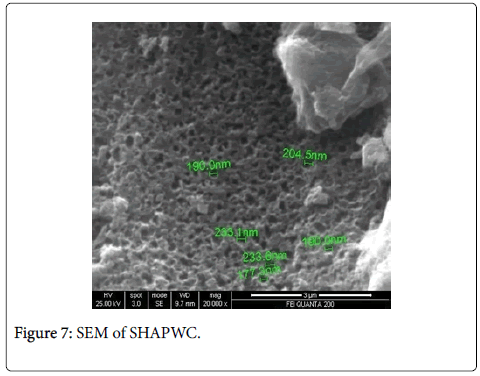
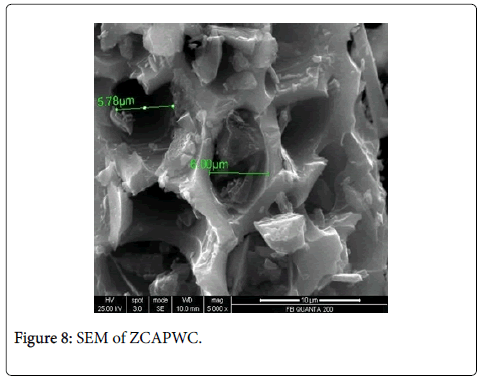
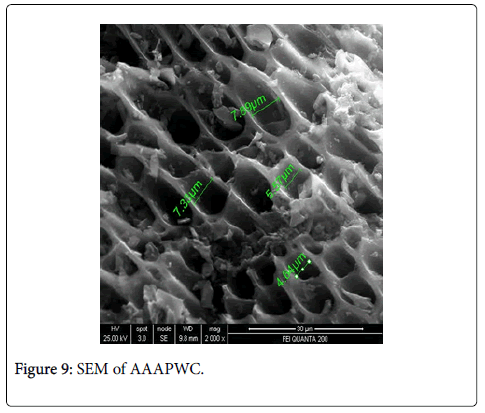
Scanning electron microscopy, XRD and fourier transform infrared spectroscopy: The effect of chemical treatment was definitely visible as there was increase in the surface area. The increase in surface area was more prominent in case of treatment with sulphuric acid (1045 m2/g) which was followed by Zinc chloride (1025 m2/g) and nitric acid (1017 m2/g) activation respectively. The SEM enables the direct observation of the changes in the surface microstructures of the carbons due to the modifications. XRD pattern of these poplar carbons are shown in Figure 1.
Three broad peaks around 26.5, 44.4 and 80° are observed in all the samples. These peaks confirm the presence of amorphous carbon in the presently studied samples. All the activated carbons have peaks situated at approximate 26° (2ɵ) corresponding to the mineralogical phase of graphite. The highest intensity peaks have been observed for the Zinc chloride activated carbon indicating that the development of atomic order and crystallite size were accelerated with zinc chloride. Two extra peaks with the amorphous carbon peaks in HCAAPWC sample can be indexed to HCl.
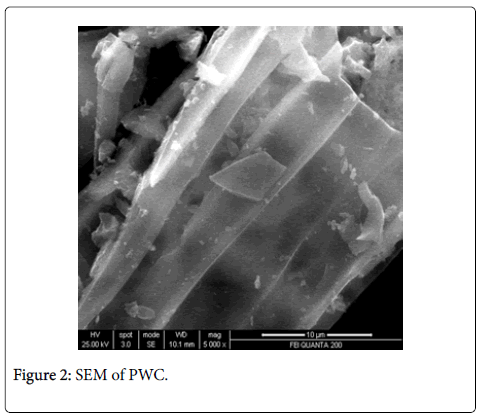
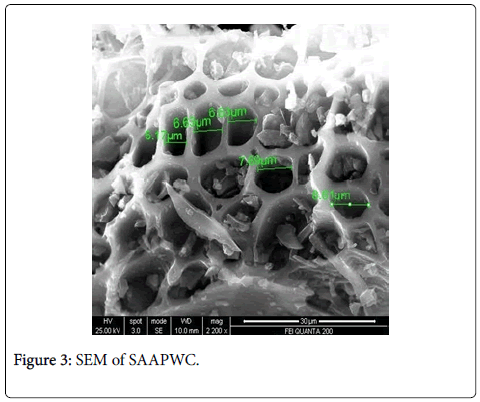
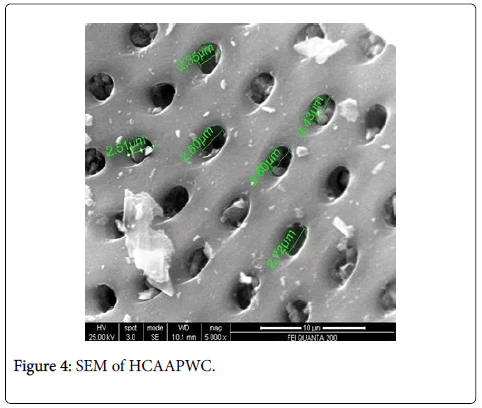
The scanning electron microscope images of all the carbons show the definite development of pores upon chemical treatment. The SEM images from Figures 2-9 indicate the porous nature of these carbons. Pores of different sizes have been formed and developed as a result of different chemical treatment. Among all the chemically activated carbons, it’s the sulphuric acid activation which resulted in the largest pore size of 7.1 μm whereas the treatment with sodium hydroxide resulted in a pore size of 189.7 nm. Zinc chloride and acetic acid activation also increased the pore size of the UPC. Zinc chloride permits the development of the porosity of materials, so the more it’s there in the precursor, the more the pores of the activated carbon are opened [43]. The results for surface area and pore size have been summarized in Table 3. Moreover, the surface area and the porosity obtained with such a low impregnation ratio have resulted into a far superior activated carbon as compared to some of already published literature [44,45]. The uniqueness of this carbon from poplar has been compared in Table 4.
| Type of Carbon | Surface area (m2/g) | Avg. Pore size |
|---|---|---|
| Untreated Poplar Wood Carbon | 961 | - |
| Hydrochloric acid activated Poplar Wood Carbon | 1005 | 2.60 µm |
| Nitric acid activated Poplar Wood Carbon | 1017 | 4.30 µm |
| Sulphuric acid activated Poplar Wood Carbon | 1045 | 7.10 µm |
| Sodium Hydroxide activated Poplar Wood Carbon | 985 | 189 nm |
| Phosphoric acid activated Poplar Wood Carbon | 998 | 5.80 µm |
| Zinc chloride activated Poplar Wood Carbon | 1025 | 5.90 µm |
| Acetic acid activated Poplar Wood Carbon | 980 | 6.40 µm |
Table 3: Surface area and pore size of untreated and treated poplar wood carbon.
| Source of activated carbon | Activating agent |
Impregnation ratio | Surface Area (kg/m3) | Porosity (%) | Reference |
|---|---|---|---|---|---|
| Balsamodendron caudatum wood waste (BAC1) | H2SO4 Process + Thermal Activation under N2 flow | - | 505 | 49.19 | B. Sivakumar et. al. [41] |
| Balsamodendron caudatum wood waste (BAC2) | H3PO4 Process + Thermal Activation under N2 flow | - | 458 | 43.64 | |
| The poplar wood barks | Steam activation | - | 555.92 | - | Jiahui Zhang et al. [46] |
| Iggesund Pine tree | H3PO4 (50%) | 1 | 256 | Daniella Birbas [47] | |
| Cuban Pine tree | H3PO4 (50%) | 1 | 180 | ||
| Iggesund Pine tree | H3PO4 (40%) | 1 | 172 | ||
| Cuban Pine tree | H3PO4 (40%) | 2 | 54 | ||
| Poplar Tree Wood SAAPWC Tree wood | H2SO4 | 0.15 | 1045 | 88 | Present study |
| Poplar Tree Wood carbon PWC |
- | - | 961 | 1045 | Present study |
Table 4: Comparison of Current activated carbon with few references from the published literature.
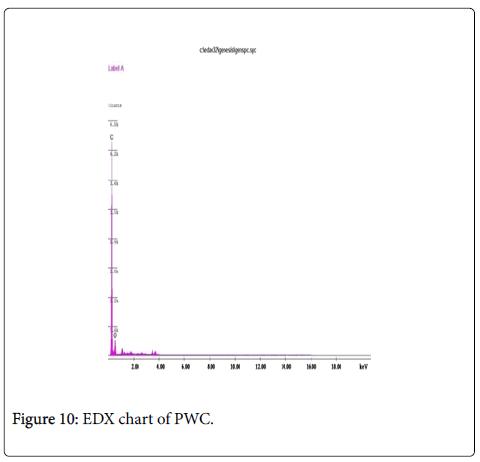
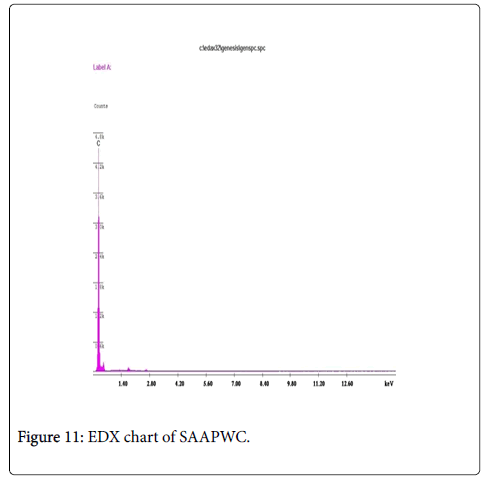
The EDX results (Figures 10 and 11) also confirm that these materials primarily consist of carbon and oxygen to very varied proportions. Even in case of samples activated with other activating agents the results do not show the presence of other elements. As EDX analysis of the samples practically does not show the presence of Zinc; neither does it show chlorine which could explain the rather good adsorbent properties particularly for this activated carbon [46].
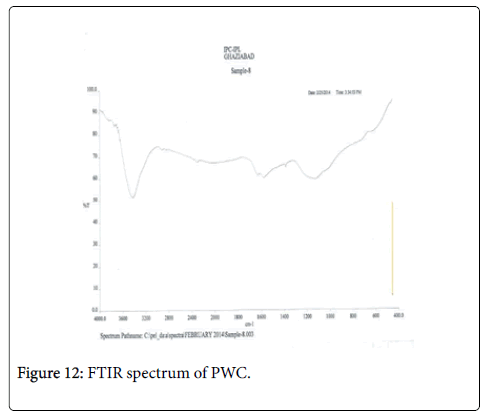
The IR spectrum is as follows (Figures 12 and 13). The spectra consist of major peaks in the areas 1000-1200 cm-1, 1500 -1600 cm-1 and 3300-3500 cm-1 apart from some minor peaks around 2800-3000 cm-1. The presence of peaks around 1600-1700 cm-1 advocates the presence of carbonyl group coming from the stretching of C=C bond [47,48]. There are two bands characteristic of aliphatic groups -CH3 and -CH2- around 2800-2900 cm-1 and (2900-3000 cm-1) [49,50]. Around 3200-3500 cm-1 vibration of OH appears in all the samples, suggesting the presence of the hydroxyls groups and chemically absorbed water on the surface. The lines observed around 1450-1500 cm-1 could match up the vibrations in the plane of the aromatic rings [51]. Table 5 compiles the major IR peaks of these carbons from poplar tree wood.
| Sl. No | Type of functional group | Wave numbers |
|---|---|---|
| 1 | OH/-NH group | 3400-3500 cm-1 |
| 2 | Aliphatic –CH3 | 2800-3000 cm-1 |
| 3 | -C=O group | 1600-1700 cm-1 |
| 5 | -C-O stretching | 1000-1200 cm-1 |
| 6 | In plane skeletal vibration in aromatic ring | 1450-1550 cm-1 |
Table 5: Type of functional groups present in untreated and treated poplar wood carbon.
Chemical composition
CHNSO Analysis and Fixed carbon: The samples of AC as well as CACs were analyzed for the carbon, nitrogen, hydrogen, sulphur and oxygen percentage. The results as compiled in Table 6 show that the content of nitrogen and sulphur is less than 1%. The carbon content of these activated carbons is quite high. It is maximum in case of Phosphoric acid treated activated carbon followed by acetic acid and hydrochloric acid treated poplar wood carbons. The carbon content is better than the activated carbons obtained from eucalyptus wood [52]. The presence of hydrogen and oxygen influence the adsorptive properties of activated carbon. The functional groups of these elements offer sites where molecules of water and other polar substances or easily polarizable gases and vapours are adsorbed [52,53].
| Sl. No | Type of PWC | N(%) | C(%) | H(%) | S(%) | O(%) | Fixed Carbon (%) |
|---|---|---|---|---|---|---|---|
| 1 | UPWC | 0.38 | 78.10 | 1.62 | 0.30 | 19.60 | 63.19 |
| 2 | HCAPWC | 0.44 | 80.10 | 1.58 | 0.13 | 17.74 | 62.31 |
| 3 | NAAPWC | 0.74 | 75.90 | 1.88 | 0.10 | 21.40 | 62.39 |
| 4 | SAAPWC | 0.39 | 75.50 | 1.86 | 0.32 | 21.97 | 59.74 |
| 5 | SHAPWC | 0.39 | 77.60 | 1.45 | 0.11 | 20.45 | 64.83 |
| 6 | PAAPWC | 0.39 | 80.80 | 1.65 | 0.17 | 17.01 | 64.41 |
| 7 | ZCAPWC | 0.44 | 51.60 | 1.51 | 0.11 | 46.36 | 63.80 |
| 8 | AAAPWC | 0.40 | 80.50 | 1.45 | 0.12 | 17.48 | 61.51 |
Table 6: Elemental and Fixed carbon content of the activated poplar carbon and its untreated precursor.
The fixed carbon percentage calculated from the values of moisture, ash and volatile matter. The amount of fixed carbon is almost similar as to the one obtained from wattle wood [54].
Adsorption studies
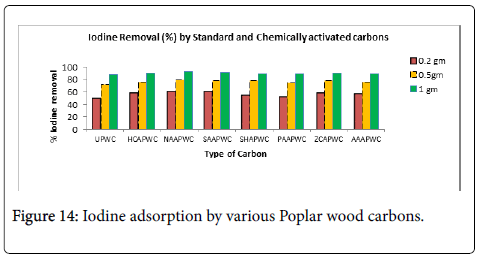
Iodine adsorption: The results of iodine absorption studies have been plotted in the following graph (Figure 14). Iodine number is a measure of activity level. That means activity level is directly proportional to iodine number. Higher the iodine number implies higher degree of activation. The typical range of iodine value stands 500–1200 mg/g. Iodine value indicates activated carbon’s ability to adsorb impurities of low molecular weight. It is also a measure of the micropore content of the activated carbon (0 to 20 Å, or up to 2 nm). It is equivalent to surface area of carbon between 900 m2/g and 1100 m2/g. The iodine number in mg/g gives a ballpark figure of the surface area in m2/g [55] and measure the porosity of pores of dimensions between 1-1.5 nm [56].
Methylene value: Methylene blue value gives an indication of the adsorption capacity of an activated carbon for molecules with high molecular weights. Some carbons have mesopore structure which adsorb medium size molecules such as dye Methylene blue. The methylene blue value is highest in case of Suphuric acid activated carbon. Enhanced adsorption of methylene blue that benefited from the addition of mesopores has also been reported [57,58]. Table 7 compiles the data of methylene blue value and iodine adsorption. Methylene blue is adsorbed on the acidic sites of the activated carbon and accessible to pores with diameters (20-50 A° or 2-5 nm) structure which adsorb medium size and large size substances. Activated carbon is known to be amphoteric i.e., its surface can be positively or negatively charged due to proton association or dissociation of surface functional groups (carboxylic and phenolic groups in particular) depending on the solution pH. The surface is negatively charged at pH>pHpzc, favoring adsorption of cationic species.
| Type of Carbon | Methylene Value (mg/gm) | Iodine value (mg/gm) |
|---|---|---|
| Untreated Poplar Wood Carbon | 320 | 1100 |
| Hydrochloric acid activated Poplar Wood Carbon | 331 | 1120 |
| Nitric acid activated Poplar Wood Carbon | 330 | 1140 |
| Sulphuric acid activated Poplar Wood Carbon | 340 | 1140 |
| Sodium Hydroxide activated Poplar Wood Carbon | 328 | 1130 |
| Phosphoric acid activated Poplar Wood Carbon | 329 | 1115 |
| Zinc chloride activated Poplar Wood Carbon | 336 | 1120 |
| Acetic acid activated Poplar Wood Carbon | 328 | 1115 |
Table 7: Methylene value and Iodine number of various activated carbons.
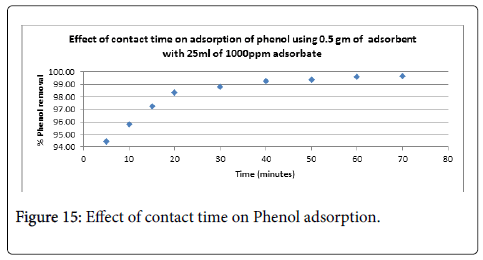
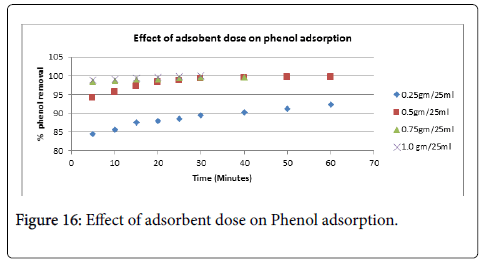
Phenol removal: The relationship between contact time and phenol removal by activated carbon obtained by different chemical activation of PWC for three different initial concentrations is presented in Figures 15 and 16 at natural pH of the solution. It can be seen from the Figure that an increase in initial phenol concentration results in decrease in the phenol removal. It can be concluded that the rate of phenol binding with activated carbon is more at initial stages, which then gradually decreases and becomes almost constant after 50 minutes. This indicates that these carbons would require less residence time for the complete removal of phenol. Similar types of observations have already been reported by several authors [59,60]. The phenol removal vs time curves are single, smooth and continuous. This definitely indicate about the possible monolayer coverage of phenol on the surface of the adsorbent.
Conclusion
From the results of the present investigation,
• A novel activated carbon can be conveniently and economically obtained from poplar tree.
• The Iodine removal efficiency is more for HNO3 and H2SO4 activated carbon and followed by Sodium Hydroxide activated carbon whereas Methylene blue value is highest for H2SO4 and ZnCl2 activated carbon.
• The study reflects that the standard activated carbon has a surface area of 961 m2/g. The Chemical activation with H2SO4, ZnCl2 and HNO3 has shown an increase in the surface area which is 1045 m2/g, 1025 m2/g and 1017 m2/g respectively.
• The pH of these carbons is different and ranges from 4.25 to 7.92. It is 7.92 in case of 1 M NaOH treated carbon and 4.25 in case of 1 M H2SO4 treated carbon.
• The data clearly indicate that all carbons have a good percentage of fixed carbon. The data pertaining to the matter soluble in water and acid indicates that all carbons contain very less amount of impurities. The ash content also shows variation as it is maximum for NaOH treated carbon i.e., 3.91% and minimum in case of 1% ZnCl2 treated carbon.
• The pore size shows a significant difference as it is minimum in case of NaOH activated carbon (189.7 nm) and maximum in case of H2SO4 activated carbon (7.1 μm).
• The preliminary studies using 0.5 gm of this chemically activated poplar carbon per 25 ml of 3000 ppm phenol solution have shown 98% removal of phenol. Similar encouraging results have been also obtained with heavy metals like Zinc and Lead.
• Among all the treatments, chemical activation with Sulphuric acid has been found to be most encouraging and promising followed by Zinc chloride activation. As all these chemical pollutants cause severe Environmental problems, these results definitely foreshow that these carbons are effective in removal of pollutants and thus proving to be useful in environmental protection.
• The overall physiochemical characterization of chemically activated precursors of poplar carbon definitely indicates that this carbon could prove to be a very good, economic and efficient adsorbent for adsorption and removal of various environmental contaminants.
• The activated carbon from poplar wood is superior to that which has been prepared from the poplar wood bark in terms of Specific area, Iodine adsorption value
Acknowledgement
The authors thank Director, DRDO-BU centre for providing access to all equipment placed at their disposals which were necessary for the realization of this work. They also thank Dr. Usha Rani, HOD, Dept. of Env. Sciences, Bharathiar University, Mr. Allen, S.R.F, DRDO-BU centre and Sh. Dinesh Kumar Sharma, Food Analyst, H.P. for their continuous and valuable support.
References
- FAO (2005) Report of the 22nd Session of the Commission and of the 42nd Session of its Executive Committee. Food and Agriculture Organization of the United Nations, Santiago, Chile.
- Newman SM (1997) Poplar agroforestry in India. Forest Ecology and Management 90: 13-17.
- Smisek M, Cemey S (1970) Active carbon manufacture, properties and applications. Elsevier pub, Comp, New York, USA, pp: 562-563.
- Budavari S, O’Neil MJ, Smith A, Heckelman PE, Kinneary JF (1996) The Merck Index, Merck Research Laboratories Division of Merck & Co. Inc., Whitehouse Station, NJ, USA pp: 137-138.
- Bansal R, Donnet J, Stoeckli F (1998) Active Carbon. Marcel Dekker Inc, New York, USA pp: 1-163.
- Nakagawa K, Mukai SR, Suzuki T, Tamon H (2003) Gas adsorption on activated carbons from PET mixture with a metal salt. Carbon 41: 823-831.
- Williams PT, Reed AR (2006) Development of activated carbon pore structure via physical and chemical activation of biomass fiber waste. Biomass and bioenergy 30: 144-152.
- Amaya A, Corengia M, Cuna A, Vivo DJ, Sarachik A, et al. (2015) Preparation of harcoal pellets from Eucalyptus wood with different binders. J Ener Nat Res 4: 34-39.
- Ma X, Yang H, Yu L, Chen Y, Li Y (2014) Preparation, Surface and Pore Structure of High Surface Area Activated Carbon Fibers from Bamboo by Steam Activation. Materials 7: 4431-4441.
- Juan Jin X, Mei Zhu Y (2014) Absorption of Phenol on Nitrogen-Enriched Activated Carbon from Wood Fiberboard Waste with Chemical Activation by Potassium Carbonate. J Chem Eng Process Technol 5: 4.
- Erlich C, Bjornbom E, Bolado D, Giner M, Fransson TH (2006) Pyrolysis and gasification of pellets from sugar cane bagasse and wood. Fuel 85: 1535-1540.
- Putun AE, Ozbay N, Onal EP, Putun E (2005) Fixed bed catalytic pyrolysis of cotton stalk for liquid and solid products. Fuel processing technology 86: 1207-1219.
- Swamy AVVS, Devi KS (2012) Biosorption Of Phenols. Int J Eng Res Appl 2: 262-265.
- Mukosha L, Onyango MS, Ochieng A, Kasaini H (2013) Development of Better Quality Low-Cost Activated Carbon from South African Pine Tree (Pinus patula) Sawdust: Characterization and Comparative Phenol Adsorption. International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering 7: 228-238.
- WHO (1963) International Standards for Drinking Water. Geneva, Switzerland, pp: 40-42.
- Adams CD, Watson TL (1996) Treatability of s-altrazine herbicide metabolites using powdered activated carbon. J Environ Eng 122: 327-330.
- Dabrowski A, Podkoscielny P, Hubicki Z, Barczak M (2005) Adsorption of phenolic compounds by activated carbon-a critical review. Chemosphere 58: 1049-1070.
- Namasivayam C, Kadirvelu K (1997) Activated carbons prepared from coir pith by physical and chemical activation methods. Bioresource technology 62: 123-127.
- Standard ASTM (1999) Standard test method for pH of activated carbon. Designation, ASTM D pp: 3838-3880.
- ASTM DESIGNATION: D 2866-83 (1991) Standard Test Method for Total Ash Content of Activated Carbon. Annual Book of ASTM Standards, Section 15, p: 343.
- ASTM D2867 (2009) Standard test methods for moisture in activated carbon. ASTM International, West Conshohocken, PA, 10.1520/D2867-09.
- American Society for Testing and Materials (2009) Standard Test Method for Apparent Density of Activated Carbon: Designation: D2854-09 ASTM.
- ASTM D 5029-98 (2014) Standard test method for water soluble in active carbon. S.1. American society for testing and materials (ASTM), USA.
- ASTM (2014) Standard-Standard Test Method for Volatile Matter Content of Activated Carbon Samples. S.I.American society for testing and materials (ASTM) D pp: 5832-5898.
- Y Leon CL, Solar JM, Calemma V, Radovic LR (1992) Evidence for the protonation of basal plane sites on carbon. Carbon 30: 797-811.
- Newcombe G, Hayes R, Drikas M (1993) Granular activated carbon: importance of surface properties in the adsorption of naturally occurring organics. Colloids and Surfaces A: Physicochemical and Engineering Aspects 78: 65-71.
- ASTM D 2330-02, ASTM Standards (2000) Standard test method for determination of methylene blue number of activated carbon.
- ASTM D4607-94, ASTM Standards (2006) Standard Test Method for Determination of Iodine Number of Activated Carbon. ASTM, USA, pp: 1-5.
- Zuo S, Yang J, Liu J, Cai X (2009) Significance of the carbonization of volatile pyrolytic products on the properties of activated carbons from phosphoric acid activation of lignocellulosic material. Fuel Processing Technology 90: 994-1001.
- Caturla F, Molina-Sabio M, Rodriguez-Reynoso F (1991) Preparation of activated carbon by Chemical activation with ZnCl2. Carbon 29: 999-1007.
- BazuÃ…?a PA, Lu AH, Nitz JJ, Schuth F (2008) Surface and pore structure modification of ordered mesoporous carbons via a chemical oxidation approach. Microporous Mesoporous Mater 108: 266-275.
- Ahmedna M (2000) Granular activated carbons from agricultural by-products: Preparation, properties, and application in cane sugar refining. Bulletin of Louisana State University Agriculture Centre 54.
- Okieimen FE, Okieimen CO, Wuana RA (2007) Preparation and characterization of activated carbon from rice husk. J Chem Soc 32: 126-136.
- Abdel-Ghani NEDT, El-Chaghaby GA (2008) The use of low cost and environment friendly materials for the removal of heavy metals from aqueous solutions. Current World Environment 3: 31-38.
- Raffiea Baseri J, Palanisamy PN, Sivakumar P (2012) Preparation and characterization of activated carbon from Thevetia peruviana for the removal of dyes from textile waste water. Advances in Applied Science Research 3: 377-383
- Aziza A, Odiakosa A, Nwajei G, Orodu V (2008) Modification and characterization of activated carbon derived from Bumper sawdust and disk sawdust to remove lead (II) and cadmium (II) effluent water. In CSN Conference proceeding. Chemical Society of Nigeria. Delta chem pp: 235-243.
- Faust SD, Aly OM (1983) Chemistry of Water Treatment, Butterwort Pub. Woburn Food and Fertilizer Technology Center for the Asian and Pacific Regions (FFTCAPR). Processing of coconut shell into activated carbon/charcoal.
- Sivakumar B, Kannan C, Karthikeyan S (2012) Preparation and characterization of activated carbon prepared from Balsamodendron Caudatum wood waste through various activation process. Rasayan J Chem 5: 321-327.
- Malik R, Ramteke D, Water S (2006) Physico-chemical and surface characterization of adsorbent prepared from groundnut shell by ZnCl2 activation and its ability to adsorb colour. Indian journal of chemical Technology 13: 329-333.
- Olowoyo DN, Orere EE (2012) Preparation and characterization of activated carbon made from palm-kernel shell, coconut shell, groundnut shell and obeche wood (Investigation of apparent density, total ash content, moisture content, particle size distribution parameters). International Journal of Research in Chemistry and Environment 2: 32-35.
- Geeta k, Velmani N, Karthikeyan S, Shabudeen PSS (2014) Ceiba Pentradenta wood waste activated carbon for waste water treatment. Carbon-Sci tech 6: 395-406.
- Ahmadpour A, Do DD (1997) The preparation of activated carbon from Macademia nut shells by chemical activation. Carbon 35: 1723-1732.
- Zhang J, Zhang W (2014) Preparation and Characteristics of Activated Carbon from Wood Bark and Its Use for Adsorption of Cu (II). Materials Science 20: 474-478.
- Birbas D (2011) Preparation of Activated Carbon: Forest residues activated with Phosphoric Acid and Zinc Sulfate.
- Dina DJD, Ntieche AR, Ndi JN, Ketcha JM (2012) Adsorption of acetic acid onto activated carbons obtained from maize cobs by chemical activation with zinc chloride (ZnCl2). Res J Chem Sci 2: 42-49.
- Akhter MS, Chughtai J, Smith DM (1985) The structure of hexane soot I: Spectroscopic studies. Appl Spectro. Carbon 39: 143-153.
- Tuah AK, Orskop ER (1987) The degradation of untreated and treated maize cobs and cocoa pod husk in the rumen. In Proceedings of the fourth annual workshop held at the institute of animal research, Mankon Station, Bamenda, Cameroun pp: 363-378.
- Tang MM, Bacon R (1964) Carbonization of cellulose fibers–I Low temperature pyrolysis. Carbon 2: 11-20.
- Vinke P, Van der Eijk M, Verbree M, Voskamp AF, Bekkum H (1994) Modification of the surfaces of a gas-activated carbon and a chemically activated carbon with nitric acid, hypochlorite, and ammonia. Carbon 32: 675-686.
- Khalil BL (1999) Surface area and pore structure of hardened Portland cement/silica fume pastes. Adsorp Sci Tec 9: 729-740.
- Tancredi N, Medero N, Möller F, PÃriz J, Plada C, et al. (2004) Phenol adsorption onto powdered and granular activated carbon, prepared from Eucalyptus wood. Journal of Colloid and Interface Science 279: 357-363.
- Hassler JW (1974)Â Purification with activated carbon. Chemical Publishing Company, New York, USA.
- Ngernyen Y, Tangsathitkulchai C, Tangsathitkulchai M (2006) Porous properties of activated carbon produced from Eucalyptus and wattle wood by Carbon dioxde activation. Korean J Chem Eng 23: 1046-1054.
- Gergova K, Petrov N, Eser S (1994) Adsorption properties and microstructures of activated carbons from agricultural by-products by steam pyrolysis. Carbon 32: 693-702.
- Collin GJ, Fauziah HAA, Hasnul FMZ, Siti FD (2006) Treatment of landfill leachate in Kayu Madang. Sabah: Porosity and Adsorption Studies (Part 2), Asian Chemistry Letters 10: 89-94.
- Xiaojun M, Guangjie Z (2011) Variations in the microstructure of carbon fibers prepared from Liquefied wood during carbonization. J Appl Polym Sci 121: 3525-3530.
- Pelekani C, Snoeyink VL (2000) Competitive adsorption between atrazine and methylene blue on activated carbon: The importance of pore size distribution. Carbon 38: 1423-1436.
- Banat FA, Al-Asheh S, Al-Makhadmeh L (2004) Utilization of raw and activated date pits for the removal of phenol from aquoeus solutions. Chem Eng Tech 27: 80-86.
- Goud VV, Mohanty K, Rao MS, Jayakumar NS (2005) Phenol removal from aqueous solutions using Tamarind nut shell activated carbon: Batch and column study. Chem Eng Tech 28: 814-821.
- Namasivayam C, Ranganathan K (1995) Removal of Cd(II) from wastewater by adsorption on waste Fe(III)/Cr(III) hydroxide. Water Research 29: 1737-1744.
Citation: Kumar RD, Kannan GK, Kadirvelu K (2017) Populus Tree Wood: A Noble Bioresource from Western Himalayas for the Development of Various Carbon Types for the Effective Application in Environment Protection i.e., Phenol Adsorption from Wastewater. J Bioremediat Biodegrad 8: 415. DOI: 10.4172/2155-6199.1000415
Copyright: © 2017 Kadirvelu K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6635
- [From(publication date): 0-2017 - Nov 25, 2025]
- Breakdown by view type
- HTML page views: 5594
- PDF downloads: 1041