Potentially Harmful Excipients in Neonatal Medications: An Observational and Cross-Regional Comparison of Japan and Europe
Received: 31-Oct-2018 / Accepted Date: 14-Nov-2018 / Published Date: 23-Nov-2018 DOI: 10.4172/2572-4983.1000172
Abstract
Objectives: We aimed to examine the administration of eight potentially harmful excipients of interest (EOI), including paraben, polysorbate 80, propylene glycol, benzoate, saccharin sodium, sorbitol, ethanol, and benzalkonium chloride, to hospitalized neonates in Japan and to compare the frequency of exposure to these excipients with previously reported European data.
Methods: Data on all medicines administered to neonates during hospitalization between May 2014 and March 2018 along with patients’ demographic data were extracted from the patients’ medical records. Excipients were identified from the Summaries of Product Characteristics.
Results: For parenteral medications, the records showed 178,858 prescriptions for 292 products administered to 1895 neonates. The EOI were found in 31,978 (17.9%) prescriptions for a relatively small number of products (n=29; 9.9%) and were administered to 1,454 (76.7%) neonates. In the parenteral prescriptions, benzyl alcohol, found in 20 (69.0%) products administered to 884 (46.6%) neonates, was the most common EOI. In enteral prescriptions, saccharin, found in nine (36.0%) products administered to 137 (13.7%) neonates, was the most common EOI. EOI administration was most frequent for the extremely preterm group of neonates. There was no difference in the number of EOI-containing prescription medications between Japan and Europe. However, a pan-European study reported a larger number of EOI-containing prescriptions and EOI-exposed neonates (OR: 2.5, 95%CI: 2.3 to 2.8, OR: 2.1, 95% CI: 1.8 to 2.5, respectively).
Conclusions: Neonates admitted to our center received several potentially harmful pharmaceutical excipients as in the previously studied European centers, but the frequency of exposure was lower at our center. Administration of pharmaceutical products in powder form may have contributed to lowering EOI exposure at our center.
Keywords: Excipient; Neonate; Cross-regional comparison; Enteral; Parenteral; Comparative study; Substitution
Introduction
Pharmaceutical excipients are necessary to maintain the quality and improve patient acceptability of medicines [1]. Excipients ideally have limited pharmacological activity; however, some are associated with toxicity in neonates [2]. The pharmacokinetics of excipients are different in neonates than in adults or older children and may additionally be affected by underlying medical conditions [1]. The extent of excipient use in neonatal medications in Japanese hospitals has never been reported. A few, European, single-center as well as pan- European studies suggested that neonates receive a significant amount of excipients, including potentially harmful ones [3-5]. Solvents and solubilizing agents, for example, are required for specific active pharmaceutical ingredients, and the extent of their use in medicines requiring suspension is unlikely to vary significantly between regions or institutions. Antimicrobials, in contrast, may be administered in solid or powder form (at least for single use formulations), which contain a relatively smaller amount of excipients than liquid formulations [6]. Sweeteners are needed only for enteral formulations. In Japan, in contrast to other countries, oral solid dosage forms (i.e., fine powder, dry-syrup, and granules) are the chief type of formulation used by medical institutes, and the frequency of excipient exposure may therefore be relatively lower. For example, ethanol, used to solubilize the liquid dosage form of medicines, is not essential for the oral solid dosage form. In this study, we aims were two-fold: first, to describe the extent of the administration of harmful excipients (or ‘excipients of interest’ (EOI), based on a previous study [2]) in the neonatal intensive care units at our center; and second, to compare the frequency of EOI exposure in our center with the findings of a previous European survey.
Methods
This single center study analyzed data on medications prescribed to neonates up to age 28 days who were admitted between May 1, 2014 and March 15, 2018. Details on sex, gestational age, weight at birth and route of administration were recorded. Data on demographics and all prescriptions except those for blood products, glucose and electrolyte solutions, and vaccines, were included. Each medicine was classified by its trade name (product), manufacturer, pharmaceutical dosage form, strength, and route of administration.
Identification of excipients
The excipient content of each medicine was identified from the summary of product characteristics (SmPC). If needed, a search was done to find the desired information from the manufacturers’ website.
Statistical analysis
The analysis was performed with SPSS software version 25 (IBM Japan, Ltd., Tokyo, Japan). Descriptive statistics were used to describe general excipient administration. The chi-square test was used to compare the frequency of EOI exposure. To estimate the influence of gestational age (GA) on the administration of each EOI, we compared the extent of EOI administration by GA category (<28 weeks, extremely preterm; 28~<32 weeks, very preterm; 32~<37 weeks, late preterm; and >37 weeks, term [7]).
Results
Of 3,927,194 live births in Japan during the study period, 8,544 (0.22%) were delivered at our center.
The extent and nature of EOI administration
In parenteral and enteral medicines, 1454 and 495 neonates received 31,987 and 1,534 prescriptions for 292 and 246 products containing 8 active ingredients, respectively. The demographic characteristics of the patients and their prescription data are shown in Table 1.
| (A) Parenteral prescription | |||||||
|---|---|---|---|---|---|---|---|
| GA category | Number of neonates | Mean BW (g, range) | Mean GA (weeks, range) | Number of prescriptions | Prescriptions per neonate | Total number of EOI per neonate | |
| Extremely preterm | <28 weeks | 53 | 676 (335-1817) | 25.3 (22.0-27.9) | 3680 | 69.4 | 85.4 |
| Very preterm | 28 to <32 weeks | 172 | 1347 (324-3148) | 30.6 (28.0-31.9) | 7634 | 44.4 | 54.9 |
| Late preterm | 32 to <37 weeks | 627 | 2250 (568-3928) | 35.7 (32.0-36.9) | 11736 | 18.7 | 22.6 |
| Term | >37 weeks | 602 | 2762 (408-4600) | 39.1 (37.0-42.0) | 8937 | 14.8 | 17.7 |
| (B) Enteral prescription | |||||||
| Extremely preterm | <28 weeks | 24 | 668 (335-1101) | 25.3 (22.9-27.7) | 120 | 5.0 | 11.8 |
| Very preterm | 28 to <32 weeks | 54 | 1311 (546-3148) | 30.5 (28.1-31.9) | 147 | 2.7 | 6.4 |
| Late preterm | 32 to <37 weeks | 141 | 2548 (980-3928) | 36.6 (32.0-36.9) | 357 | 2.5 | 3.1 |
| Term | >37 weeks | 276 | 2854 (408-4600) | 39.2 (37.0-42.0) | 910 | 3.3 | 4.3 |
GA: Gestational Age; BW: Birth Weight; EOI: Excipients of Interest
Table 1: Demographic characteristics and prescription data gestational age category of patients receiving at least one prescription.
Information on excipients was available for 538 (100%) products in 196,492 (100%) prescriptions. EOI were found in 33,589 (17.1%) prescriptions. Medications containing EOI were found with equal frequency in both parenteral and enteral formulations (OR 0.847, 95%CI 0.49 to 1.46).
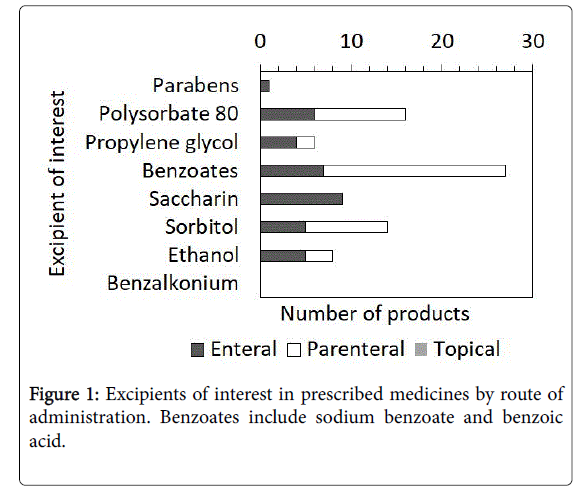
Figure 1 shows the proportion of EOI in the prescribed medicines classified by route of administration. In parenteral medicines, 9.9% of products contained EOI, and almost 76.7% (n=1454) of the neonates who received these medicines were exposed to at least one EOI. In parenteral prescriptions, benzyl alcohol, found in 18 (62.1%) products administered to 883 (60.7%) neonates, was the most common. In enteral medications, 10.2% of products contained EOI, and almost 49.8% (n=499) of the neonates who received these medicines were exposed to at least one EOI. In enteral prescriptions, saccharin, found in nine (36.0%) products administered to 137 (27.7%) neonates, was the most common.
Administration of excipients in this study
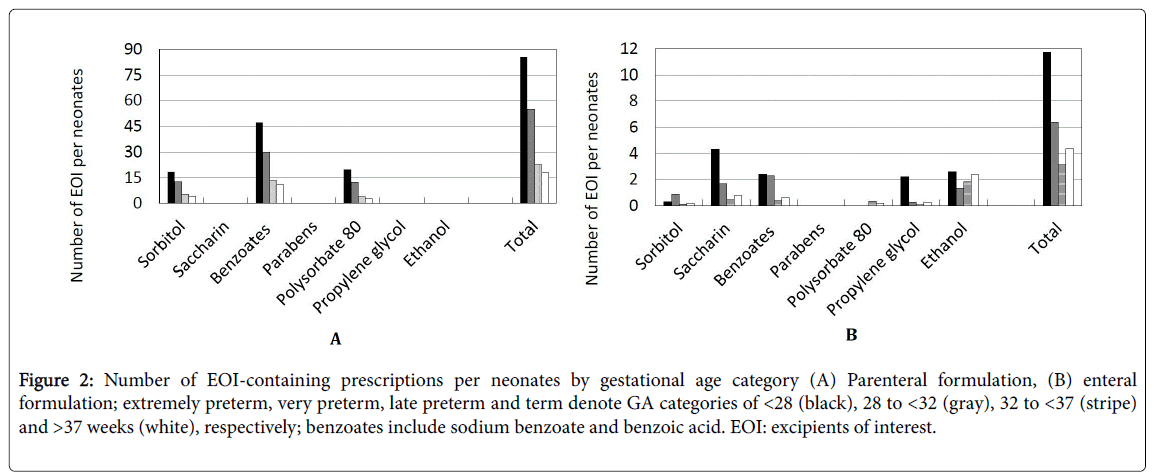
Figure 2 shows the administration of EOI classified by GA category. In parenteral formulations, the average number of EOI per neonate during hospitalization was 45.2 for all GA categories. Term neonates were less likely to receive sorbitol, benzyl alcohol, or polysorbate 80, and the average number of each excipient per neonate was 4.2, 10.7, and 2.9, respectively. In extremely preterm neonates, the average number of the same excipients per neonate was 18.3, 47.3, and 19.8, respectively. The number of prescriptions per neonate was inversely related to GA (Spearman correlation coefficient (ρ)=−0.99).
Figure 2: Number of EOI-containing prescriptions per neonates by gestational age category (A) Parenteral formulation, (B) enteral formulation; extremely preterm, very preterm, late preterm and term denote GA categories of <28 (black), 28 to <32 (gray), 32 to <37 (stripe) and >37 weeks (white), respectively; benzoates include sodium benzoate and benzoic acid. EOI: excipients of interest.
In enteral formulations, the average number of EOI per neonate during hospitalization was 6.4 for all GA categories. Term neonates were less likely to receive saccharin sodium, benzoates other than benzyl alcohol, or propylene glycol, and the average number of each excipient per neonate was 0.8, 0.6, and 0.3, respectively. In extremely preterm neonates, the average number of the same excipients per neonate was 4.3, 2.4, and 2.2, respectively. The number of prescriptions per neonate tended to be inversely related to GA (Spearman correlation coefficient (ρ)=−0.68).
Differences in administration of excipients between our center and European institutions
Our study showed no difference in the proportion of EOIcontaining products between our center and the European institutions (OR 0.9, 95% CI 0.7 to 1.1). Propylene glycol (OR 4.4, 95% CI 1.8 to 10.8), ethanol (OR 2.5, 95% CI 1.1 to 5.8), paraben (OR 72.4, 10.0 to 523.1), and saccharin sodium (OR 3.5, 95% CI 1.7 to 7.4) were more likely to be included in the products administered to neonates (Table 2A).
| (A) Number (%) of products | ||||
|---|---|---|---|---|
| Excipient | EU (n=530) | JPN (n=539) | OR | 95%CI |
| Polysorbate 80 | 18 (3) | 16 (3) | 1.1 | 0.6 to 2.3 |
| Propylene glycol | 26 (5) | 6 (1) | 4.4 | 1.8 to 10.8 |
| Ethanol | 20 (4) | 8 (1) | 2.5 | 1.1 to 5.8 |
| Paraben† | 71 (13) | 1 (0.2) | 72.4 | 10.0 to 523.1 |
| Benzoate‡ | 27 (5) | 27 (5) | 1.0 | 0.6 to 1.8 |
| Benzalkonium chloride | 10 (2) | 0 (0) | - | - |
| Saccharin sodium | 31 (6) | 9 (2) | 3.5 | 1.7 to 7.4 |
| Sorbitol | 24 (5) | 13 (2) | 1.9 | 0.9 to 3.7 |
| At least one EOI | 142 (27) | 164 (30) | 0.9 | 0.7 to 1.1 |
| (B) Number (%) of neonate | ||||
| Polysorbate 80 | 138 (19) | 674 (23) | 1.0 | 0.8 to 1.2 |
| Propylene glycol | 120 (17) | 53 (2) | 10.4 | 7.4 to 14.5 |
| Ethanol | 47 (6) | 435 (15) | 0.5 | 0.3 to 0.6 |
| Paraben† | 313 (43) | 1 (0.03) | 1958.3 | 274.2 to 13985.6 |
| Benzoate‡ | 82 (11) | 1008 (35) | 0.4 | 0.3 to 0.5 |
| Benzalkonium chloride | 26 (4) | 0 (0) | - | - |
| Saccharin sodium | 90 (12) | 138 (5) | 2.9 | 2.2 to 3.8 |
| Sorbitol | 57 (8) | 1464 (51) | 0.2 | 0.1 to 0.2 |
| At least one EOI | 456 (63) | 1952 (67) | 2.1 | 1.8 to 2.5 |
†Paraben includes propylparabens, ethylparabens and methylparabens, ‡Benzoate includes benzyl alcohol, benzoic acid and sodium benzoate, EU: European countries; JPN: Our Japanese Center; OR: Odds Ratio; CI: Confidence Intervals, The data from European countries were obtained from Nellis et al. [2].
Table 2: Number and proportion of products and prescriptions containing each excipient and the number of neonates exposed.
When we compared the number of neonates exposed to EOI, we found that 1952 neonates in 2897 were exposed to EOI at our center while 456 neonates in 726 neonates were exposed to EOI in the European study. The odds ratio between our center and the European centers was 1.2, and the 95% confidence intervals were ranged from 1.0 to 1.4. In particular, the frequency of exposure to propylene glycol (OR 10.4, 95% CI 7.4 to 14.5), paraben (OR 1958.3, 95% CI 274.2 to 13985.6), and saccharin sodium (OR 2.9, 95% CI 2.2 to 3.8) was higher in the European centers. In contrast, exposure to ethanol (OR 0.5, 95% CI 0.3 to 0.6), benzoate (OR 0.4, 95% CI 0.3 to 0.5), and sorbitol (OR 0.2, 95% CI 0.1 to 0.2) occurred more frequently at our center (Table 2B).
Proportion of drugs in each excipient by administration route
Table 3 shows the proportion of drugs in each excipient. The parenteral formulation was associated with a higher likelihood of benzyl alcohol use, and heparin sodium, used for anticoagulation in 877 neonates, accounted for 21757 (97.2%) of 22373 prescriptions containing benzyl alcohol as a preservative. Benzyl alcohol also used in 18 other products given to 885 neonates.
| (A) Parenteral formulation | |||
|---|---|---|---|
| Excipients | Brand name | Number of prescription (%) | Number of neonate (%) |
| Sorbitol | |||
| Multi-vitamine | Otsuka MV® injection | 6694 (74.1) | 644 (45.8) |
| Others | 2334 (25.9) | 1329 (94.6) | |
| Benzyl alcohol | |||
| Heparin sodium | Heparin sodium for injection | 21757 (97.2) | 877 (99.1) |
| Others | 616 (2.8) | 114 (12.9) | |
| Polysorbate 80 | |||
| Multi-vitamine | Otsuka MV® injection | 6694 (90.6) | 644 (98.9) |
| Others | 692 (9.4) | 176 (27.0) | |
| (B) Enteral formulation | |||
| Sorbitol | |||
| Soluble ferric pyrophosphate | Incremin® syrup | 97 (80.8) | 46 (78.0) |
| Others | 23 (19.2) | 16 (27.1) | |
| Saccharin | |||
| Multi-vitamin | Panvitan® powder | 165 (35.2) | 60 (43.5) |
| Phenobarbital | Phenobal® elixirs | 139 (29.6) | 42 (30.4) |
| Levocarnitine | L-Cartin FF® oral solution | 122 (26.0) | 29 (21.0) |
| Others | 43 (9.2) | 24 (17.4) | |
| Benzoates | |||
| Multi-vitamin | Panvitan® powder | 165 (41.4) | 60 (51.7) |
| Levocarnitine | L-Cartin FF® oral solution | 122 (30.6) | 29 (25.0) |
| Soluble ferric pyrophosphate | Incremin® syrup | 97 (24.3) | 46 (39.7) |
| Others | 23 (5.8) | 16 (13.8) | |
| Polysorbate 80 | |||
| Elemental diet for pediatrics | Elental® P | 47 (50.0) | 6 (26.1) |
| Others | 47 (50.0) | 18 (78.3) | |
| Propylene glycol | |||
| Phenobarbital | Phenobal® elixirs | 139 (94.6) | 42 (95.5) |
| Others | 8 (5.4) | 3 (6.8) | |
| Ethanol | |||
| Triclofos Sodium | Tricloryl® syrup | 793 (71.6) | 371 (82.1) |
| Phenobarbital | Phenobal® elixirs | 139 (12.6) | 42 (9.3) |
| Others | 175 (15.8) | 43 (9.5) | |
(A) Typical excipients used for parenteral formulation, (B) typical excipients used for enteral formulation. Drugs accounted for more than 10% of products were indicated.
Table 3: Proportions of drugs in each excipient by administration routes.
The frequency of ethanol was associated with the use of triclofos sodium for oral syrup formulations, which accounted for 793 (71.6%) of 1107 ethanol-containing enteral prescriptions. Phenobarbital elixir also contained ethanol and accounted for 139 (12.6%) of 1107 ethanolcontaining enteral prescriptions. Alfa-calcidol (Alfarol®) in its ethanolcontaining oral formulation was also able to substituted by an oral solid formulation in 76 prescriptions (28 neonates) to avoid neonatal exposure to ethanol (data not shown).
A multivitamin formulation for intravenous hyperalimentation prescribed in our center accounted for 6694 (74.1%) of 9028 sorbitolcontaining parenteral prescriptions. In enteral formulations, soluble ferric pyrophosphate accounted for 97 (80.8%) of 120 sorbitolcontaining prescriptions. Soluble ferric pyrophosphate is an oral iron preparation and can be administered as EOI-free sodium ferrous citrate granules (Ferromia® granules).
Saccharin was commonly used in enteral formulations as a sweetener (9 of 25 EOI-containing products), and 138 neonates were exposed to a saccharin-containing enteral formulation. Two hundred sixty-six prescriptions (56.7% of saccharin sodium-containing prescriptions) were liquid formulations. The oral formulation of phenobarbital elixir accounted for 29.6% of saccharin sodiumcontaining prescriptions although an EOI-free oral solid formulation of phenobarbital is available. The remaining 165 (35.2%) saccharincontaining prescriptions were for Panvitan® powder, an oral multivitamin formulation.
If the phenobarbital elixir had been administered in its oral powder dosage form, 42 (8.4%) neonates could have avoided exposure to ethanol, propylene glycol, and saccharin sodium.
Discussion
This is the first prospective study examining the administration of pharmaceutical excipients to neonates in Japan. The expansion of the generic drug market is reflected by the surprisingly broad range of medicines with a highly variable excipient content. We demonstrated that while about 10% of the prescribed medications contained at least one EOI, roughly 80% of treated neonates received at least one EOIcontaining drug. Especially in their parenteral formulation, daily-use heparin for anticoagulation and multivitamins for intravenous hyperalimentation accounted for a large number of the EOI-containing prescriptions. These findings suggest that a few, commonly used medicines were responsible for a large portion of EOI and that using the non-EOI containing formulation of this relatively small number of products may help a large number of neonates to avoid EOI exposure.
A study of Estonian neonatal units and a Brazilian neonatal unit reported that approximately 90% of neonates received at least one of the eight EOI [8,9]. In contrast, we observed a relatively low frequency of EOI exposure at our center. Furthermore, our center and the European centers differed in the administration of several EOI. Differences in the type of drug formulation used, as well as differences in hospital practices and protocols may account for some of these discrepancies. For example, ethanol is commonly used in liquid formulations as a solubilizing agent. In enteral formulations, ethanol was used in only five (20.0%) of the EOI-containing products in our study while it was used in 37 (78.7%) products in the European studies [2].
Generally, the powder dosage form was more frequently used than the liquid dosage form in Japan than in other countries. The liquid dosage form was generally used in single-dose formulations of sedatives, etc. Furthermore, four of five ethanol-containing products observed in our study were able to be substituted with an oral solid formulation in our center, suggesting that the frequency of ethanol exposure was reduced to the minimal level.
The administration of benzalkonium chloride was also lower in our study than in the European studies. A previous study indicated that 85% of prescriptions of topical formulations contained benzalkonium chloride. At our center, we did not observe the use of EOI-containing topical formulations.
Other factors associated with excipient use were route of administration and GA. Some of the variations in EOI administration between the GA groups in this study might have been influenced by the prescription periods and the large number of neonates enrolled.
Encouraging pharmaceutical companies to develop excipient-free products is a possible method of reducing neonatal exposure to EOI. Phenobarbital sodium injections usually contain benzyl alcohol as a preservative. In Japan, in response to a request from the Japan Pediatric Society, Nobelpharma Co. Ltd. launched Nobelbar®, an excipient-free injectable phenobarbital formulation for safe, intravenous, neonatal administration.
Pharmaceutical product and dosage form selection can also help to eliminate EOI-exposure. EOI-free versions of neonatal medications are available in Europe [2], and in Japan, a preservative-free heparin product is now available to replace benzyl alcohol-containing heparin sodium. Furthermore, there is an EOI-free oral solid dosage alternative to the phenobarbital elixir, alfacalcidol solution, and soluble ferric pyrophosphate syrup.
This study has some limitations. First, as a single-center study, its findings may not be generalizable to all Japanese institutions, especially given the fact that our neonatal unit is not very large (the eighteenth largest in Japan). As shown by a previous study, region may be a significant, independent determinant of EOI administration and might account for differences in the parameters examined [2]. To assess the level of neonatal exposure to harmful excipients in Japan, a multicenter study is needed. Second, the quantitative data on excipients in the SmPC was limited. Quantitative evaluation of neonatal EOI exposure requires accurate data on the content of EOI in each product. It is commonly known that excipients can reach a toxic level [10], and the need for toxicokinetic studies is being increasingly recognized [11].
Conclusion
The neonates in our center receive a number of excipients that are considered to be harmful. Comprehensive benefit and risk assessment of the use of excipients cannot be implemented until all the required quantitative data become available. Our study suggested that the neonates in our center were exposed to roughly equivalent levels of each EOI. However, exposure to benzyl alcohol and sorbitol contained in daily-use medicines was somewhat higher. The current EOI administration rates and known developmental risks in neonates warrant further toxicokinetic studies as well as the exploration of feasible alternatives.
Acknowledgments
This work was supported by the Research Program of the Japanese Agency for Medical Research and Development and was conducted as part of the “Regulatory science for better access to pediatric drugs in Japan” grant awarded to Hidefumi Nakamura (Division for Clinical Trials, Department of Clinical Research, National Center for Child Health and Development). We also thank James R. Valera (National Center for Child Health and Development, Department of Education for Clinical Research, Japan) for his English editorial support.
Conflict of Interest
All the authors declare no conflicts of interest.
Ethical Considerations
Ethics Committee approval was obtained in compliance with national guidelines. All data were anonymized before leaving the study sites.
Contributors
Study design: JS, HN and MA. Conduct of study: JS and HN. Identification of excipients: JS. Analysing data: JS and HN. Writing the manuscript: JS, HN, MA, YI and YA.
Funding
All the authors received no financial support for the research, writing, and/or publication of this article.
References
- Fabiano V, Mameli C, Zuccotti GV (2011) Paediatric pharmacology: remember the excipients. Pharmacol Res 63: 362-365.
- Nellis G, Metsvaht T, Varendi H, Lass J, Mesek I, et al. (2015) Potentially harmful excipients in neonatal medicines: a pan-European observational study. Arch Dis Child 100: 694-699.
- European Medicines Agency (2014) Questions and answers on propylene glycol and esters in the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’ (CPMP/463/00 Rev 1). London, United Kingdom.
- European Medicines Agency (2006) Reflection paper: formulations of choice for the paediatric population. London, UK.
- European Medicines Agency (2013) Reflection paper on the use of methyl-and propylparaben as excipients in human medicinal products for oral use. London, UK.
- Pawar S, Kumar A (2002) Issues in the formulation of drugs for oral use in children: role of excipients. Paediatr Drugs 4: 371-379.
- WHO (2013) Preterm birth. World Health Organization, Geneva, Switzerland.
- Lass J, Naelapää K, Shah U, Käär R, Varendi H, et al. (2012) Hospitalised neonates in Estonia commonly receive potentially harmful excipients. BMC Pediatr 12: 136.
- Souza A Jr, Santos D, Fonseca S, Medeiros M, Batista L, et al. (2014) Toxic excipients in medications for neonates in Brazil. Eur J Pediatr 173: 935-945.
- European Commission (2003) Excipients in the label and package leaflet of medicinal products for human use. Belgium.
- De Cock RFW, Knibbe CA, Kulo A, de Hoon J, Verbesselt R, et al. (2013) Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br J Clin Pharmacol 75: 162-171.
Citation: Saito J, Akabane M, Ishikawa Y, Nakamura H, Yamatani A (2018) Potentially Harmful Excipients in Neonatal Medications: An Observational and Cross-Regional Comparison of Japan and Europe. Neonat Pediatr Med 4: 172. DOI: 10.4172/2572-4983.1000172
Copyright: © 2018 Saito J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5107
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 4123
- PDF downloads: 984