Prediction of Multiple Sclerosis after Childhood Isolated Optic Neuritis
Received: 18-Nov-2015 / Accepted Date: 13-Jan-2016 / Published Date: 20-Jan-2016 DOI: 10.4172/2572-4983.1000S1003
Abstract
Isolated optic neuritis in adults (ON) is the most common initial manifestation of multiple sclerosis (MS). Conversion to MS after childhood ON is not well determined. We aimed to identify risk factors predicting MS following ON and to develop risk profiles with adjusted clinical follow-up based on current diagnostic tools. Medical records of 42 cases with isolated ON between 1970 and 2005 were analysed. In 2006 and 2007 all patients received a clinical follow-up investigation including ophthalmological and neurological examination, visual evoked potentials (VEPs), somatosensory evoked potentials (SEPs) and cerebral magnetic resonance imaging (cMRI). Investigation was performed to a mean follow-up of 18 years (3-38 years). 14% of all patients showed MS-like lesions in cMRI. Additional neurologic symptoms or abnormal cMRI at initial presentation indicating dissemination in space significantly altered the risk of MS (OR 16.0, 95% CI [1.5; 176.5], p = 0.020), (OR 4.6, 95% CI [0.7; 31.0], respectively). Severe visual loss and funduscopic affection reduced the likelihood for progression to MS (OR 0.2, 95% CI [0.0; 1.5]). Children presenting with isolated ON, neurological impairment at onset or especially coordinative dysfunction at follow-up and demyelinating lesions in cMRI at disease onset were at high risk for the development of MS.
Keywords: Multiple sclerosis; Childhood optic neuritis; Magnetic Resonance Imaging; Risk factors of multiple sclerosis; Corticosteroid treatment; Funduscopic pathology
Introduction
Optic neuritis (ON) is an acute or subacute inflammation of the optic nerve with sudden loss of visual acuity, visual field deficits (VFD), retrobulbar pain commonly without funduscopic pathology.
ON is rare in childhood, incidence is estimated with 4/100.000/year. Despite other causes ON occurs as an isolated episode, but it is more frequently associated with multiple sclerosis (MS) [1-4]. Acute, unilateral ON in adults is associated with an increased risk of development of MS and further alterations with additional findings (lesions indicative of MS on magnetic resonance imaging (MRI)). Conversion rate to MS in children with ON has been described with 4-56% [5,6] However, a recent study of a Korean cohort of children by Kim and co-workers reported a conversion rate of 7.7% [7]. MRI seems to be of prognostic value as recent studies predict a higher conversion to MS after pediatric ON [8-10]. Oligoclonal bands (OCB) might as well be a positive predictor of MS, but could not be statistically proven in a recently published study by Heussinger et al. [9]. Despite abnormal MRI Waldman et al. [10] proposed that development of MS after ON in childhood is age-depended. Visual evoked potentials (VEP) and other parameters (age, sex, neurological deficit) in children presenting with ON are not well determined [5,6,11,12] and studies with sufficient long-term clinical follow-up and suitable diagnostic methods are difficult to conduct. Former studies are mainly based on retrospective data of medical records and none of them include clinical examination at follow-up [5].
It is well-accepted that early treatment, already in the state of clinical isolated syndrome (CIS) significantly postpones the conversion to definite MS and influences the course of the disease [1]. Thus, identification of children with ON at high risk for the development of MS is essential, because they are supposed to benefit from early MS therapy.
We aimed to identify risk factors predicting the development of MS following ON during childhood. Therefore, we systematically reviewed medical records of 42 patients and performed clinical follow-up investigations including ophthalmological and neurological examination, VEP, somatosensory evoked potentials (SEP) and MRI after a mean surveillance period of 18 years (3-38 years). Using a standardized study protocol we aimed to reach high comparability.
Materials and Methods
Study population
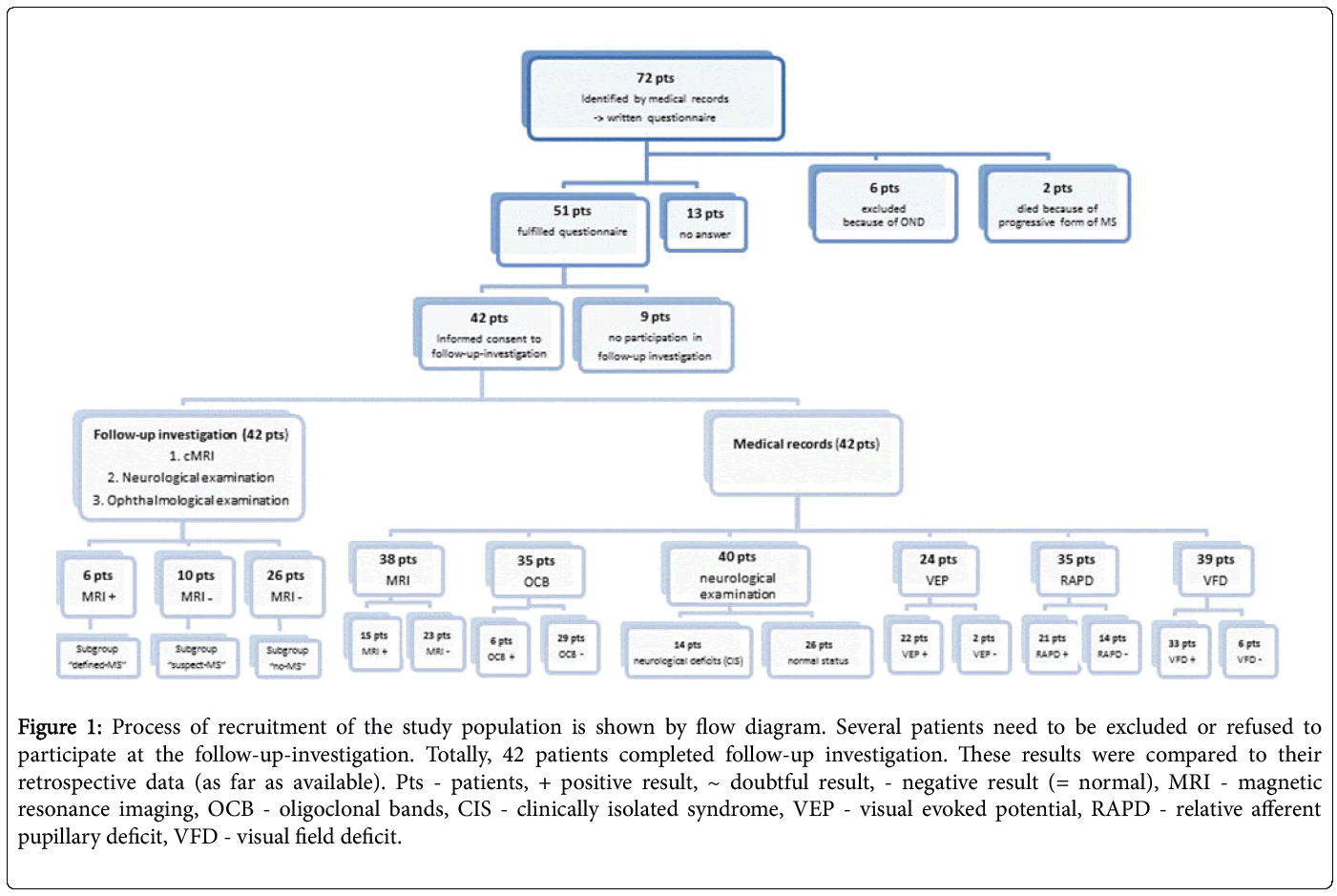
72 patients with uni- or bilateral ON under 16 years at presentation with ON during 1970-2005 in the University Children’s Hospital Muenster, Department of General Pediatrics-Neuropediatrics were identified by medical records. All of them were asked to complete a written questionnaire concerning profession, family history, sports, nutrition and activity of daily life. 13 individuals refused study participation or did not respond to the invitation. 6 patients had to be excluded afterwards because not fulfilling the inclusion criteria. 9 patients answered the written questions but did not agree to take part in the study. 2 patients died during follow up period at ages of 25 and 27 years because of a rapid progression of MS. A total of 42 patients answered the questionnaire and participated in the follow-up investigation (Figure 1). The drop-out rate of these patients did not influence the results and conclusion of the study.
Figure 1: Process of recruitment of the study population is shown by flow diagram. Several patients need to be excluded or refused to participate at the follow-up-investigation. Totally, 42 patients completed follow-up investigation. These results were compared to their retrospective data (as far as available). Pts - patients, + positive result, ~ doubtful result, - negative result (= normal), MRI - magnetic resonance imaging, OCB - oligoclonal bands, CIS - clinically isolated syndrome, VEP - visual evoked potential, RAPD - relative afferent pupillary deficit, VFD - visual field deficit.
Definition of ON
Uni- or bilateral ON was defined as acute or subacute loss of acuity with at least one of the following symptoms: intra- and/or periocular pain on eye movement, relative afferent pupillary deficit (RAPD), impairment of colour vision, VFD, funduscopic disc appearances like disc pallor, papillitis, swelling of the optic nerve, prominence of the optic nerve, oedema and hyperaemia or abnormal VEP. Patients were excluded from the study if evidence of toxic, metabolic, vascular, hereditary or compressive aetiology or retinal lesions was present. ON was defined as bilateral if both eyes were affected simultaneously or within two weeks, and classified sequentially if both eyes were affected two weeks to three months after unilateral ON.
Retrospective data
Analysis of medical data of each patient was based on medical records of the neuropediatric and ophthalmologic department of the University of Muenster. Neurologic and ophthalmologic data at ON onset was available for all patients, radiological data including computer tomography (CT) or MRI were missing in patients with ON in early years when these techniques were not applied frequently (9.5%). Disease duration was defined as period from first symptom to visual acuity of at least 0.8 Infection and headache during ON were recorded if they occurred two weeks before or within two weeks after ON onset. Therapeutic treatment was divided in three groups related to the Optic-Neuritis-Treatment-Trial (ONTT) by Beck et al. [13]. Patients who received intravenous corticosteroids for at least three days and oral corticosteroids for at least eleven days were included in group
1. Patients who received only oral corticosteroids for at least 14 days were included in group 2. Patients without medical treatment were included in group 3. Comparable to the placebo-group in the ONTT. Cerebrospinal fluid (CSF) findings of initial lumbar puncture were regarded as positive if either mild CSF pleocytosis between 5-50/μl leukocyte counts, immunoglobulin increase, or OCB could be detected; related to the recommendation of Pohl et al. [14]. Electroencephalogram (EEG) was considered pathological if compatible with ON (e.g., presence of an occipital focus or pathological waves above the region of the visual cortex). MRI-data was evaluated as positive if typical evidence of demyelination apart from the optic nerve was described (Referring to published data of Hahn C et al. [15]). Additionally laboratory investigations including blood parameters as well as auto-immunological studies were considered and divided in four groups associated with either signs of special infection or unspecific increase of infection parameters. Recurrence of ON in the same or other eye was noticed as second episode if ON occurred after three months of the initial event or one month after the end of therapy. Additionally the results of the neurologic examination during ON, the VEP if examined, the ophthalmologic findings apart from visual acuity and the period between single relapses were recorded.
Ophthalmologic examination
Fundoscopy was performed in all 42 participants by a single experienced ophthalmologist to reduce the interobserver bias with indirect ophthalmoscopic investigation with 15 dioptre (D) lenses and direct ophthalmoscopy with superfield lenses. Colour vision was tested with red items, RAPD was detected by the swinging-flashlight test, pain on eye movement was proved palpatoric, intraocular pressure was measured with a Topcon®CT-80 tonometer after local anaesthesia with oxybuprocain, visual acuity was measured with an ophthalmic optic device. Optic field data were collected with Humphrey® Field Analyser/HFA II.
Neurologic examination
Neurologic examination was performed by the author (EC). Neurologic deficits were recorded according to the functional systems of expanded disability status scale (EDSS). It should be mentioned that EDSS is not a suitable tool in pediatric MS. But as standardized systems in children are lacking, EDSS was used as the investigators endeavoured to ensure a high level of comparability within the study population [16,17]. A detailed history with special regard to autoimmune diseases, relapses, recent and past infections, heredity of MS and family diseases was taken.
Neurological examination and cMRI of one study-participant was accomplished in Klinikum Innenstadt Children’s hospital in the neuropediatric department of Prof. Dr. med. Heinen; Munich, Bavaria. Ophthalmologic examination of this patient was performed in the medical office “Augenärzte am Stacchus” by an experienced ophthalmologist with long clinical practice in Munich, Bavaria.
Electrophysiological examination
Measurements of all 42 patients were performed by two medical technical assistants with long-time clinical experience and practical knowledge. Evoked potentials were registered with NIHON KOHDEN MEB 71026-Neuropack 2®, for VEP a HITACHI monitor was used.
VEP stimulus was a black and white checkerboard pattern. For analysis two sweeps were documented. Amplitude (peak to peak) and latency of P100, P25 and P40 were recorded. Normal limits for P100, P25 and P40 latency and amplitude were set according to the criteria of Tekavcic-Pompe et al. [18] and the averaged values of healthy children in the University Children’s Hospital Muenster, Department of General Pediatrics-Neuropediatrics. Upper limit for P100 latency was defined as 110 ms after half field stimulation for both eyes.
MRI
All MRIs included FLAIR, T1 and T2 weighted TSE-sequences performed on a 1.5 Tesla. MRI results were analysed according to the Poser Criteria [19] as MRI at follow-up (2006, 2007) as well as MRI at ON onset, which dated back to 1970 were considered for analysis. It is obvious, that meanwhile newly diagnostic criteria were defined [20] but subsequent change of initial analysis were not performed to avoid inaccuracy with clinical data.
Statistical analysis
Statistical analyses were performed using SAS® software, Version 9.2 of the SAS System for Windows and IBM SPSS® Statistics 20 for Windows (IBM Corporation, Somers, NY, USA). For group comparison of two groups, the Fisher’s exact test was used and the odds ratios (OR) with 95% confidence intervals (CI) were carried out. Inferential statistics are intended to be exploratory (hypotheses generating), not confirmatory, and are interpreted accordingly. The local significance level was set to 0.05. No adjustment for multiple testing was performed.
The study was approved by the local ethic committee of University Hospital Muenster. Written informed consent was signed by all subjects or their parents if they were minors at time of the study. (Reference Number 2007-382-f-S).
Results
First, retrospective data of 42 patients (Figure 1) are presented. Secondly, the results of the follow-up investigation are shown. All 42 patients received a follow-up investigation. The mean follow-up period was 18 years (3-38 years, Median 14 years); the mean age at follow-up investigation was 28 years (11-50 years). The results of each examination a) ophthalmological b) neurological c) electrophysiological and d) cMRI are listed below in detail.
In Figure 1 Process of recruitment of the study population is shown by flow diagram. Several patients need to be excluded or refused to participate at the follow-up-investigation. Totally, 42 patients completed follow-up investigation. These results were compared to their retrospective data (as far as available). Pts-patients, + positive result, ~ doubtful result,-negative result (= normal), MRI-magnetic resonance imaging, OCB-oligoclonal bands, CIS-clinically isolated syndrome, VEP-visual evoked potential, RAPD-relative afferent pupillary deficit, VFD-visual field deficit.
Retrospective data
ON occurred unilateral in 24 patients (57%) (12 left/12 right); bilateral in 18 patients (43%). 12 patients (67%) showed a bilateral ON at first presentation, 6 patients (33%) had bilateral sequential ON. Mean duration of the disease was 34.3 days. Therapy defined as group 1 (IV steroid plus oral tapering) or group 2 (only oral steroid) was started in 30 patients (71%; 15 patients in each group). 12 patients (29%) received no therapy (group 3). Average time to therapy was 5.6 days after onset. At presentation of ON or within two weeks before or after disease onset 2 patients (5%) additionally suffered from an infection (of the upper airways), 40 patients (95%) showed no signs of infection. 16 patients (38%) reported headache at onset or in course of ON. Apart from ON 14 patients (33%) showed dissemination in space with additional neurological symptoms at ON onset. Subsequently these patients need to be classified as CIS patients and are labeled (Table 1-3). In 26 patients (62%) no further neurological dysfunction apart from the optic pathway could be found. In two patients (5%) no data of neurological status was available. CSF analysis was performed in 35 patients (83%), 6 patients (17%; 14%) (First number refers to the particular subgroup, second number expresses the proportion of the total study population [n = 42]) showed OCB. In 29 patients (83%; 69%) CSF was normal (Figure 1).
| Retrospective data | Number of pts | % of pts |
|---|---|---|
| Mean age at onset | 8.3 years | |
| Sex (female : male) | 3:03 | |
| ON (bilateral : unilateral) | 3:03 | |
| Relapse | 1 | 16.70% |
| Treatment (Cortisone) | 5 | 83.30% |
| Fundoscopy (pathological) | 4 | 66.70% |
| Fundoscopy (normal) | 2 | 33.30% |
| VFD | 6 | 100% |
| OCR positive | 1 | 16.70% |
| Neurological deficits (= CIS) | 4 | 66.70% |
| Demyelinating lesions in MRI | 4 | 66.70% |
| Follow-up data | ||
| Mean age at follow-up | 24.8 years | |
| Mean follow-up | 16.5 years | |
| Optic pallor | 5 | 83.30% |
| Optic pallor + VFD | 3 | 50.00% |
| Fundoscopy (normal) | 1 | 16.70% |
| Coordination deficits | 5 | 83.30% |
| Coordination deficits + >1 FS | 2 | 33.30% |
| Normal neurological status | 1 | 16.70% |
| VEP-Latency (>110ms) | 3 | 50.00% |
Subgroup, defined-MS" (n = 6)
Table 1: Characteristics of subgroup “defined-MS”.
| Retrospective data | Number of pts | % of pts |
|---|---|---|
| Mean age at onset | 9.8years | |
| Sex (female : male) | 6:04 | |
| ON (bilateral : unilateral) | 5:05 | |
| Relapse | 0 | 0.00% |
| Treatment (Cortisone) | 5 | 50.00% |
| Fundoscopy (pathological) | 7 | 70.00% |
| Fundoscopy (normal) | 3 | 30.00% |
| VFD | 8 | 80.00% |
| OCR positive | 2 | 20.00% |
| Neurological deficits (= CIS) | 5 | 50.00% |
| Demyelinating lesions in MRI | 4 | 40.00% |
| Follow-up data | ||
| Mean age at follow-up | 35.2 years | |
| Mean follow-up | 25.4 years | |
| Optic pallor | 8 | 80.00% |
| Optic pallor + VFD | 5 | 50.00% |
| Fundoscopy (normal) | 0 | 0.0% |
| Coordination deficits | 6 | 60.00% |
| Coordination deficits + ≥ 1 FS | 3 | 30.00% |
| Normal neurological status | 4 | 40.00% |
| VEP-Latency (>110ms) | 6 | 60.00% |
Subgroup, suspect-MS" (ri = 10)
Table 2: Characteristics of subgroup “suspect-MS”.
| Retrospective data | Number of pts | % of pts |
|---|---|---|
| Mean age at onset | 10.4 years | |
| Sex (female : male) | 18:08 | |
| ON (bilateral : unilateral) | 10:16 | |
| Relapse | 5 | 19.% |
| Treatment (Cortisone) | 20 | 77.% |
| Fundoscopy (pathological) | 24 | 92.% |
| Fundoscopy (normal) | 2 | 8.% |
| VFD | 22 | 85.% |
| OCB positive | 3 | 12.% |
| Neurological deficits (= CIS) | 5 | 19.% |
| Demyelinating lesions in MRI | 7 | 27.% |
| Follow-up data | ||
| Mean age at follow-up | 26.2 years | |
| Mean follow-up | 15.7 years | |
| Optic pallor | 21 | 81.% |
| Optic pallor + VFD | 14 | 54.% |
| Fundoscopy (normal) | 3 | 12.% |
| Coordination deficits | 8 | 31.% |
| Coordination deficits volFS | 5 | 19.% |
| Normal neurological status | 12 | 46.% |
| VEP-Latency (>110ms) | 14 | 54.% |
Subgroup, no-MS" (n = 26)
Table 3: Characteristics of subgroup “no-MS”.
Laboratory parameters offered non-specific signs of infection in terms of an acute inflammation in eight patients (19%). 33 patients (79%) revealed normal infection parameter; in 1 patient (2%) information was lacking. EEG-recordings were performed in 31 patients (74%) at onset of ON and showed an occipital focus in the area of the visual cortex in 11 patients (36%; 26%). 7 patients (23%; 17%) demonstrated non-specific changes, in 13 patients (42%; 31%) EEG was normal. 22 (92%; 52%) of 24 patients showed pathological VEP in the affected eye. MRI at disease onset was abnormal in 15 patients (40%; 36%); 1 patient showed defined MS-lesions.
Normal MRI scans were found in a total of 23 patients (61%; 55%). Visual acuity below 0.4 in the affected eye was evident in 38 patients (91%), 33 patients (87%; 79%) showed only perception of hand movements or even no light perception. 4 patients (10%) demonstrated an visual acuity between 0.4-0.8 in the affected eye at ON. RAPD was obvious in 21 out of 35 patients (60%; 50%), 17 of 36 examined patients (47%; 41%) reported pain associated with eye motility at ON onset. Fundoscopy was pathologic in 35 patients (83%) (Figures 2a and 2b).
In Tables 1-3 the selected parameters of each subgroup are presented. In the upper part of the table retrospective data are listed and results of follow-up investigation are shown below. ON-optic neuritis, VFD-visual field deficit, OCB-oligoclonal bands, CISclinically isolated syndrome, VEP-visual evoked potential, FSfunctional system (= deficit in 1 or more functional system, according to the EDSS).
Optic pallor occurred in 10 patients (29%; 24%), oedema in 24 patients (69%; 57%) and 1 patient (3%; 2%) presented with optic atrophy. 7 patients (17%) showed a normal fundoscopy. 33 of 39 patients (85%; 79%) suffered from a VFD at ON onset. In 3 individuals (7%) perimetry could not be performed according to a severe loss of visual acuity and/or the young patients age at presentation of ON. A total of 6 patients (14%) developed a relapse of ON, in 5 patients (83%; 12%) in the formerly affected eye.
Follow-up investigation
Follow-up investigation of the patients was performed after an average period of 18 years (3-38 years, Median 14 years) and only once in each patient.
Ophthalmological data
9 patients (21%) showed RAPD, among them 8 patients (89%) unilateral (3 left; 5 right) and 1 patient (11%) bilaterally. All patients with RAPD additionally demonstrated an optic pallor as well as a VFD. 12 patients (29%) had a colour vision deficit, 9 patients (75%) unilateral (2 left, 7 right). Colour vision deficit was bilateral in 3 patients (25%). 2 patients (5%) were bilaterally colour-blind at followup. 3 patients (7%) declared pain of eye motility, pain of repulsion existed in 2 patients (5%). All 42 patients showed a normal internal pressure of the eyes (internal pressure of eyes was measured at 15.5 +/- 5.5 mmHg) and none of them showed diplopia. 32 patients (76%) offered optic pallor, in 20 patients (48%) in one eye, in 12 patients (29%) pallor was diagnosed bilaterally. Atrophy of the optic nerve in addition to the optic pallor was detected in 1 patient. 8 of 20 patients (40%) with optic pallor also showed a VFD, 7 patients (35%) had an isolated optic pallor without any additional pathologic finding. A pathologic perimetry was documented in all 12 patients with bilateral optic pallor. 6 of these patients (50%) showed a reduced optic field together with optic pallor. No optic pallor was diagnosed in 8 patients (19%) thereof four patients (50%) showed a completely normal ophthalmological assessment. In 3 of these patients (38%) an isolated colour vision deficit in the former affected eye was diagnosed whereat 1 of these 3 patients showed additional pathological findings in perimetry. 5 patients (12%) had a pathologic perimetry with reduced determination of external borders of the optic field (3 bilateral; 2 unilateral). A total of 21 patients (50%) showed pathology in the 30°- threshold, in 12 patients (5 left, 7 right) (57%) unilaterally located, 9 patients (43%) demonstrated bilateral pathological results. A total of 19 patients (45%) showed normal perimetry. All 42 patients participated in the ophthalmological investigation (Table 1-3). OCT was not performed within the follow-up investigation.
Neurological data
Neurological deficits at follow-up were divided according to the functional systems of EDSS. It should be mentioned that EDSS is not a suitable tool in pediatric MS. But in the absence of an appropriate system in children, EDSS was used as prior studies in pediatric population did before [16,17]. None of the patients show clinical signs suggestive of a previous demyelination (e.g., Lhermitte sign). 10 patients showed pyramidal tract signs, 5 patients with mild hemi- or paraparesis. 4 individuals (80%) showed unilateral disability, in 1 case (20%) motor impairment occurred bilaterally. 5 patients (12%) demonstrated only pathologic reflex status without disability (3 bilateral, 2 unilateral). In 24 patients (57%) cerebellar impairment was found. 3 patients (13%) showed unilateral coordination deficits, in 21 patients (88%) coordinative dysfunction was detected bilateral. 11 patients (26%) demonstrated failure in coordination together with deficits in further functional systems. 5 patients (12%) demonstrated brainstem involvement. 3 patients (60%) suffered from trigeminal hypaesthesia, 1 patient (20%) showed hypoglossal paresis. The sensory system was affected in 1 patient (2%) together with decreased perception of temperature. 1 patient (2%) showed urinary and fecal incontinence. This patient additionally demonstrated sensory deficits, brainstem impairment, pyramidal tract involvement and severe coordinative deficits. All patients participated in neurological investigation (Table 1-3). Cognitive assessment was not included in the follow-up investigation.
Electrophysiological data
19 patients (45%) showed VEP-latency over 110 ms (normal values of latency 100 +/- 10 ms), in 7 patients (37%) in the left, in 6 patients (32%) in the right eye and in 6 patients (32%) bilaterally. VEPdifferences were detectable in 10 patients (24%). VEP was performed in all patients, except of one patient with bilateral blindness, and was only performed in 1 patient VEP in one eye because of unilateral blindness and lack of fixation (Tables 1-3). SEP was normal in all individuals. Measurement of SEP of upper and lower limb was performed in 42 patients except of one patient due to a severe spasticity of the right leg. Therefore only SEPs of the left tibial nerve were recorded.
Statistical data
Results of the univariate analysis are shown in Table 4.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Sex (male) | 2.3 | (0.370; 13.667) | 0.39 |
| Treatment (Cortisone) | 1.5 | (0.146; 15.461) | 1 |
| ON unilateral | 0.6 | (0.105; 3.7237) | 0.666 |
| Infection | 5.0 | (0.266;93.958) | 0.345 |
| Neurological deficits | 16 | (1.451; 176.451) | 0.02 |
| OCB | 1.3 | (0.107; 14.949) | 1 |
| VEP | 1.1 | (0.041; 27.300) | 1 |
| MRI | 4.6 | (0.673; 31.048) | 0.164 |
| Visual acuity | 0.2 | (0.011; 3.758) | 0.345 |
| RAPD | 2.8 | (0.264; 29.047) | 0.621 |
| Motility pain | 2.9 | (0.433; 19.281) | 0.372 |
| Fundoscopy (pathological) | 0.2 | (0.018; 1.546) | 0.15 |
| VFD | 2.4 | (0.112; 51.995) | 1 |
| Relapse | 0.8 | (0.0795; 8.880) | 1 |
| FU - Coordinative deficit | 5.0 | (0.511; 48.909) | 0.196 |
| FU - VEP- latency | 0.5 | (0.084; 3.514) | 0.664 |
| FU - Fundoscopy (pathological) | 1.2 | (0.113; 12.585) | 1 |
Table 4: Results of univariate analysis are shown. Odds Ratios and corresponding p-value for MS according to risk factors are presented. Risk factors referring to discussion section are highlighted in bolt print. ON-optic neuritis, OCB-oligoclonal bands, VEP-visual evoked potentials, MRI-magnetic resonance imaging, RAPD-relative afferent pupillary deficit, VFD-visual field deficit, FU-follow-up.
MRI data
A total of 6 patients (14%) were identified with indicative lesions of MS. 10 patients (24%) showed suspected lesions in MRI at follow-up. The majority of 26 patients (62%) showed normal MRI scans. Longitudinal spinal lesions suspicious of neuromyelitis optica (NMO) were detected in none of the patients [21]. According to the MRI results, study population was divided into 3 subgroups (“defined-MS”, “suspect-MS”, and “no-MS”). To focus on the differences and to simplify comparison, characteristics of each subgroup were presented in Tables 1-3.
Discussion
After a mean follow-up interval of 18 years (3-38 years) MS following childhood ON was established in only 14%, which is low compared to adults [2]. Kim et al. [7]recently published a low conversion rate of 7.7% to MS after childhood ON in a cohort of Korean children which is consistent with our presented data. Based on our results several potential predictors for the development of MS following childhood ON could be identified.
Neurological deficits at presentation of ON or in clinical follow-up showed a positive prediction for development of MS, reflecting dissemination in space as defined in the McDonald criteria [20]. In neurological examination at clinical follow-up 83% of the subgroup “defined-MS” presents neurological deficits in coordination, compared to 31% in subgroup “no-MS”. In this subgroup 46% demonstrates a completely normal neurological status-in comparison to only 17% in the subgroup “defined-MS”. An OR of 5.0 (95% CI 0.5; 48.9, Table 4) shows a five times higher risk for development of MS in individuals with impairment of coordination at clinical follow-up. Neurologic symptoms at onset of ON are present in 67% of the patients in subgroup “defined-MS”, in contrast to only 19% of the patients in subgroup “no-MS”. This parameter reached statistical significance (OR = 16, 95%CI 1.5; 176.5, p = 0.020). Already 2006 Wiljeto et al. [22] reported an association of future MS and occurrence of neurologic symptoms (mostly involving pyramidal tract, brainstem and cerebellar function) at ON onset, confirmed by Riikonen et al. [5], who correlated a lack of neurologic impairment at ON onset with a reduced risk for progression of MS. Subsequently these patients with neurological deficits at ON have to be defined as CIS according to the consensus definitions by Krupp et al. [23]; especially if they show demyelinating lesions indicative for MS on MRI. These patients were pursued in statistical analysis in our study to avoid further division of an already limited study population. But CIS patients are specially indicated in each subgroup (Table 1-3). Based on our results CIS patients seem to preferentially develop MS than children with isolated ON (66.7 vs. 19.2%). Further comparison of CIS and ON patients in our study is lacking as both groups were not directly opposed to in detailed analysis. But studies concerning the possible different risk profiles are mandatory [24]. The study cannot contribute to the presence or amount of cognitive deficits in MS as there were not tested in follow-up investigation. At time of study conduct neurocognitive changes were not in focus of MS. To address this important topic a clinical investigation is already planned.
In follow-up investigation, optic pallor was established over all subgroups in at least 80% (Ø81%). Consistent to the literature, optic pallor could be considered as residual symptom of ON without relationship to development of MS. Further parameters (VFD, RAPD, and VEP) in ophthalmological investigation did not differ between subgroups. Hence, according to our data VEP-recordings had no prognostic prediction for the development of MS (Table 4).
67% of the subgroup “defined-MS” presented MS-lesions in initial MRI in contrast to only 27% in subgroup “no-MS”. Recent studies [9,22] confirmed a strong correlation between conversion to MS and detection of lesions on MRI at ON onset. No lesions in MRI significantly reduced the risk of progression to MS [8,25,26]. In the published 10-years follow-up of the ONTT [3] already one single lesion in MRI doubled the risk of MS (22% vs. 56%). This data is corroborated by our findings showing that 67% in subgroup “defined- MS” presented with lesions in initial MRI in our cohort. In Fisher’s exact test the p-value for this parameter was 0.07 (data not shown) predicting development of MS. However, because of the small sample size, statistical significance could not be reached. Our results contribute to recent findings confirming that neurologic symptoms in combination with MRI-lesions significantly altered the risk of MS [6,22]. Furthermore our data underline the important predictive role of MRI as already stated by Heussinger et al. [9] at ON onset and MRI should therefore be performed in any child with ON.
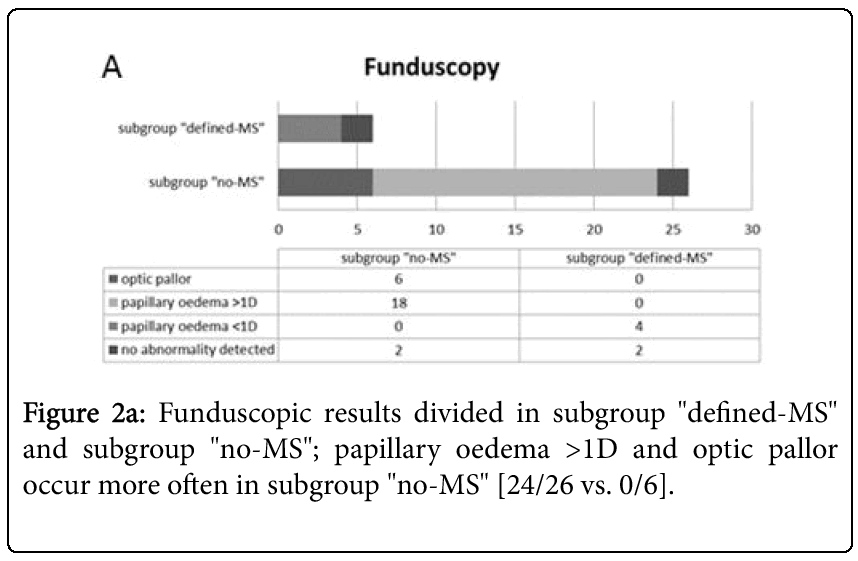
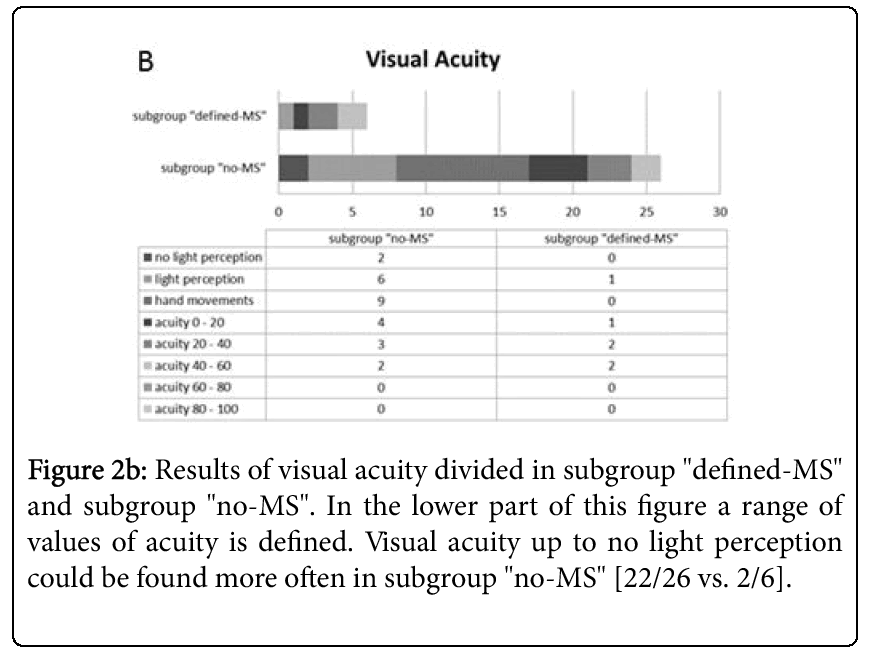
67% of subgroup „defined-MS” showed a pathological fundoscopy at onset of ON compared to 92% in the subgroup “no-MS”. In subgroup “no-MS” investigation revealed a severe optic pallor or distinct papilla oedema with prominence of the papilla up to 2 D or additional funduscopic findings. Subgroup “defined-MS” showed only mild pathologies in funduscopic examination without additional changes (Figure 2a). 33% of the patients in subgroup “defined-MS” showed no ophthalmological pathology at ON, only 7% of the subgroup “no-MS” had normal fundoscopy. Also severe loss of visual acuity with transient complete loss of vision at onset of ON (Figure 2a) was associated with reduced risk of MS (Table 4). 21 of 26 patients in subgroup “no-MS” showed visual acuity below 20% whereas 4 of 6 patients in subgroup “defined-MS” had a visual acuity of 20% or higher (Table 1,3). Recently published studies [18,26] predict good recovery of ON, which includes severe visual deficit [8,12]. Probably the high capability for remyelination in children is responsible for good recovery despite the high affection of the optic nerve. According to this, the authors of the ONTT pointed out that severe swelling of the optic nerve, total loss of vision and additional, funduscopic lesions at onset of ON reduce the risk of MS development and halved the risk of later MS in female patients [3]. Our study as well showed a protective effect of abnormal fundoscopy which can also be statistically confirmed with an OR of 0.2 (95% CI 0.0; 1.5). In addition loss of visual acuity seems to be a negative predictor as univariate analysis revealed an OR of 0.2 (95% CI 0.0; 3.8).
Only 6 patients showed OCB at ON. Thus, no prognostic value of OCB can be estimated based on our data. Probably early lumbar puncture (within 2 days of ON) was responsible for the high number of negative OCB cases as discussed elsewhere [14].
The predictive value of uni- or bilateral ON was variable [6,22]. Unilateral ON was presented with 62% in subgroup “no-MS” which supports a low conversion rate of isolated juvenile ON to MS. This is contradictory to recently published data where unilateral ON was associated with MS comparable to adults [9]. Therefore childhood ON has probably to be distinguished from ON with adult onset. Our data are consistent with the results of a meta-analysis by Waldman et al. [10] and supports an age-dependent characteristic and risk profile of childhood ON. Because of our small sample size the prognostic role of sex, age at onset and relapse rate remained unanswered.
Regarding the equal distribution of intravenous versus oral treatment in each subgroup, type of steroid application played no major role (see supplementary data). Studies concerning intravenous versus oral application are not available in children, studies in adults cannot emphasize for any application [27]. ONTT in the 1-, 2-, and 5- year analysis [4,13,28] denies a difference between the treatment and placebo group. In patients treated with intravenous corticosteroids in the first month a significant recovery of vision was proven. ONTT mentions a lower conversion rate to MS after childhood ON of 8% in the group treated with intravenous corticosteroids compared to 13% of the patients which received oral corticosteroid therapy. Further studies confirm the protective effect of corticosteroid treatment [6]. Our study fails to establish special predictors in childhood ON which recommends early beginning of immunmodulatory therapy. Based on the good tolerability and safety profile of interferon-therapy in children [29,30] accompanied by the evidence that treatment of CIS patients significantly delays progression to MS early therapy initiation seems to be favourable. Each patient was examined by the same physician following standard protocols to avoid interobserver bias.
Conclusion
To our knowledge the presented study is the first single center investigation of patients with childhood ON based on a long-term clinical follow-up with standardized examination protocols in ophthalmology, neurology, electrophysiology and cMRI after a mean surveillance period of 18 years combined with retrospective analysis. Conversion rate to MS after childhood ON is low compared to ON in adults (14% vs. 40% [2]). Neurologic impairment at onset of ON significantly alter the risk for the development of MS. Lesion load on initial MRI indicating early dissemination in space was identified as potential predictor for the conversion to MS. Severe visual loss and funduscopic pathologies showed a negative correlation concerning the development of definite MS. Our data highlights the importance of a detailed clinical examination and regular MRI in childhood ON to estimate the risk for the development of MS. Further prospective studies with a large, well-defined study population and long-term follow-up are needed to corroborate the presented findings.
Acknowledgement
We thank all patients of the study and their families for the participation, patience and information; the medical technical assistants of the neuropediatric department Mrs Böcker and Mrs Stroscio together with the radiological technical assistants of the Institute of Radiology in particular Mrs Eink for their support.
References
- Kappos L (2007) Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 370: 389-397.
- Nilsson P, Larsson EM, Maly-Sundgren P, Perfekt R, Sandberg-Wollheim M (2005) Predicting the outcome of optic neuritis: evaluation of risk factors after 30 years of follow-up. J Neurol 252: 396-402.
- Beck RW, Trobe JD, Moke PS, Gal RL, Xing D, et al. (2003) High- and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trial.Arch Ophthalmol 121: 944-949.
- Beck RW, Trobe JD (1995) What we have learned from the Optic Neuritis Treatment Trial.Ophthalmology 102: 1504-1508.
- Riikonen R, Donner M, Erkkilä H (1988) Optic neuritis in children and its relationship to multiple sclerosis: a clinical study of 21 children.Dev Med Child Neurol 30: 349-359.
- Lucchinetti CF, Kiers L, O'Duffy A, Gomez MR, Cross S, et al. (1997) Risk factors for developing multiple sclerosis after childhood optic neuritis.Neurology 49: 1413-1418.
- Kim YM, Kim HY, Cho MJ, Kwak MJ, Park KH, et al. (2015) Optic Neuritis in Korean Children: Low Risk of Subsequent Multiple Sclerosis.Pediatr Neurol 53: 221-225.
- Bonhomme GR, Waldman AT, Balcer LJ, Daniels AB, Tennekoon GI, et al. (2009) Pediatric optic neuritis: brain MRI abnormalities and risk of multiple sclerosis.Neurology 72: 881-885.
- Heussinger N, Kontopantelis E, Gburek-Augustat J, Jenke A, Vollrath G, et al. (2015) Oligoclonal bands predict multiple sclerosis in children with optic neuritis.Ann Neurol 77: 1076-1082.
- Waldman AT, Stull LB, Galetta SL, Balcer LJ, Liu GT (2011) Pediatric optic neuritis and risk of multiple sclerosis: meta-analysis of observational studies.J AAPOS 15: 441-446.
- KENNEDY C, CARTER S (1961) Relation of optic neuritis to multiple sclerosis in children.Pediatrics 28: 377-387.
- Visudhiphan P, Chiemchanya S, Santadusit S (1995) Optic neuritis in children: recurrence and subsequent development of multiple sclerosis.Pediatr Neurol 13: 293-295.
- Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, et al. (1992) A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group.N Engl J Med 326: 581-588.
- Pohl D, Rostasy K, Reiber H, Hanefeld F (2004) CSF characteristics in early-onset multiple sclerosis.Neurology 63: 1966-1967.
- Hahn CD, Shroff MM, Blaser SI, Banwell BL (2004) MRI criteria for multiple sclerosis: Evaluation in a pediatric cohort.Neurology 62: 806-808.
- Charvet LE, Beekman R, Amadiume N, Belman AL, Krupp LB (2014) The Symbol Digit Modalities Test is an effective cognitive screen in pediatric onset multiple sclerosis (MS).J Neurol Sci 341: 79-84.
- Ghezzi A, Pozzilli C, Grimaldi LM, Moiola L, Brescia-Morra V, et al. (2013) Natalizumab in pediatric multiple sclerosis: results of a cohort of 55 cases.Mult Scler 19: 1106-1112.
- Tekavcic-Pompe M, Stirn-Kranjc B, Brecelj J (2003) Optic neuritis in children--clinical and electrophysiological follow-up.Doc Ophthalmol 107: 261-270.
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, et al. (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols.Ann Neurol 13: 227-231.
- Polman CH (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292-302.
- Argyriou AA, Makris N (2008) Neuromyelitis optica: a distinct demyelinating disease of the central nervous system.Acta Neurol Scand 118: 209-217.
- Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, et al. (2006) The clinical features, MRI findings, and outcome of optic neuritis in children.Neurology 67: 258-262.
- Krupp LB (2013) International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 19: 1261-7.
- Lee CG, Lee B, Lee J, Lee M (2015) The natural course of clinically isolated syndrome in pediatric patients.Brain Dev 37: 432-438.
- Tintore M, Sastre-Garriga J (2009) Role of MRI criteria and OB for diagnosing multiple sclerosis in patients presenting with clinically isolated syndromes.Mult Scler 15: 407-408.
- Absoud M (2010) Childhood optic neuritis clinical features and outcome. Arch Dis Child, 2010.
- Burton JM (2009) Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev 3: CD006921.
- Beck RW (1993) The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. The Optic Neuritis Study Group. The New England journal of medicine 329: 1764-1769.
- Banwell B, Reder AT, Krupp L, Tenembaum S, Eraksoy M, et al. (2006) Safety and tolerability of interferon beta-1b in pediatric multiple sclerosis.Neurology 66: 472-476.
- Tenembaum SN, Segura MJ (2006) Interferon beta-1a treatment in childhood and juvenile-onset multiple sclerosis.Neurology 67: 511-513.
Citation: Elpers C, Amler S, Grenzebach U, Allkemper T, Fiedler B, et al. (2015) Prediction of Multiple Sclerosis after Childhood Isolated Optic Neuritis. Neonat Pediatr Med 1: S1003. DOI: 10.4172/2572-4983.1000S1003
Copyright: © 2015 Elpers C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4983
- [From(publication date): 0-2015 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 4056
- PDF downloads: 927