Predictive Value of the TSH/FT4 Ratio in Women with Suspected PE or GH
Received: 19-Oct-2017 / Accepted Date: 02-Nov-2017 / Published Date: 09-Nov-2017 DOI: 10.4172/2376-127X.1000357
Abstract
Objective: In the present study, we aimed to examine whether the TSH/FT4 ratio after the second trimester can predict the prevalence of preeclampsia (PE) or gestational hypertension (GH).
Study design: Retrospective case-control study.
Methods: We collected TSH and FT4 serum levels after the second trimester in 133 pregnant women with suspected PE or GH. Participants were divided into 2 groups, the PE+GH group and the non-PE+GH group and conducted the retrospective study for the two groups to evaluate the background and the prevalence of PE or GH were retrospectively evaluated.
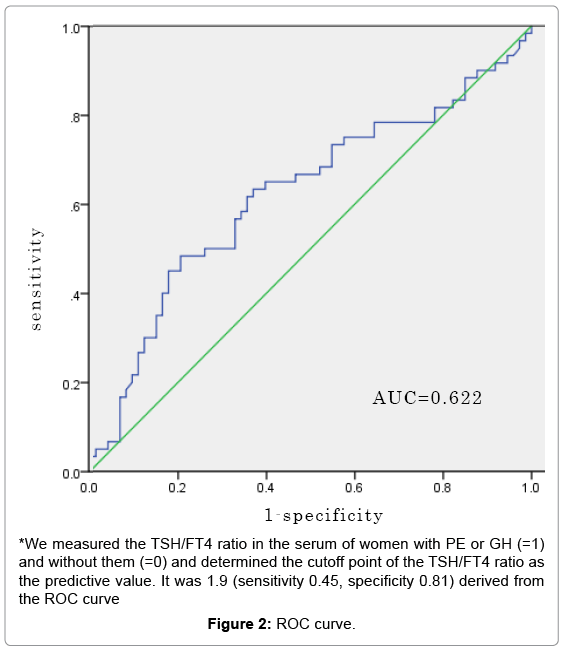
Results: Among the participants in the PE+GH group, mean age, body mass index (BMI) at no pregnancy and BMI at delivery were 34.5 ± 6.7 years, 22.3 ± 3.9 kg/m2 and 26.0 ± 4.0 kg/m2, respectively. Among the participants in the non-PE+GH group, mean age, BMI at no pregnancy and BMI at delivery were 32.9 ± 5.5 years, 22.5 ± 4.8 kg/m2 and 26.1 ± 4.4 kg/m2, respectively. There were no significant differences observed between the two groups. The cutoff point of the TSH/FT4 ratio was 1.9 (sensitivity 0.45, specificity 0.81), which was derived from the receiver operating characteristic curve. The adjusted odds ratio of PE or GH prevalence was 3.60 (95% CI: 1.62-8.02).
Conclusion: The TSH/FT4 ratio after the second trimester may aid in the prediction of PE or GH prevalence.
Keywords: Preeclampsia; Gestational hypertension; TSH; FT4
Introduction
Preeclampsia (PE) is considered to be caused by a vascular endothelial cell disorder and has recently been associated with soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF).
The disorder results from abnormal maternal spiral artery remodeling [1].
In normal pregnancy, trophoblastic cells penetrate the decidua as well as the alternate vascular endothelial cells or vascular muscles of the maternal spiral arteries, resulting in maternal spiral artery remodelling [2]. In PE, abnormal maternal spiral artery remodeling causes persistent hypoxia in fetal-placental circulation.
Hypoxia in the placenta stimulates sFlt-1 production and suppresses PlGF production [3,4].
sFlt-1 overproduction and low PlGF levels reduce circulating vascular endothelial growth factor (VEGF) and suppress angiogenesis in the placenta.
Thus, hypoxia results in a vicious cycle in the placenta with PE. sFlt-1 and PlGF can pass through the placenta and enter the maternal circulation, resulting in high levels of sFlt-1 and low levels of PlGF [5,6].
In glomerular epithelial cells, overexpression of VEGF causes the functional disorder of glomerular cells and proteinuria presents when the balance between sFlt-1 and VEGF is lost [7,8].
Primary hypothyroidism increases the risk of prevalence of PE and superimposed PE [9].
A sFlt-1/PlGF ratio of ≤ 38 can predict the absence of PE within 4 weeks [10].
In umbilical cord serum, sFlt-1 shows a positive linear association with TSH and PlGF shows a positive linear association with FT4 [11].
We hypothesized that the TSH/FT4 ratio, as with the sFlt-1/PlGF ratio, may be associated with the prevalence of PE or gestational hypertension (GH) and examined whether TSH/FT4 ratio could be measured more easily than the sFlt-1/PlGF ratio.
Methods
This matched case-control study was conducted between April 2014 and March 2016 at the Department of Obstetrics and Gynecology, Osaka City General Hospital, Osaka City, Japan.
Our hospital is located in the Miyakojima Ward of Osaka City. The population of Miyakojima Ward is approximately 104,000, with the birth of approximately 870 infants each year. Our hospital has 1063 beds and fulfills the role of a perinatal medical center. The maternal-fetal intensive care unit (MFICU) consists of 6 beds, the neonatal intensive care unit (NICU) 12 beds and the growing care unit (GCU) 18 beds.
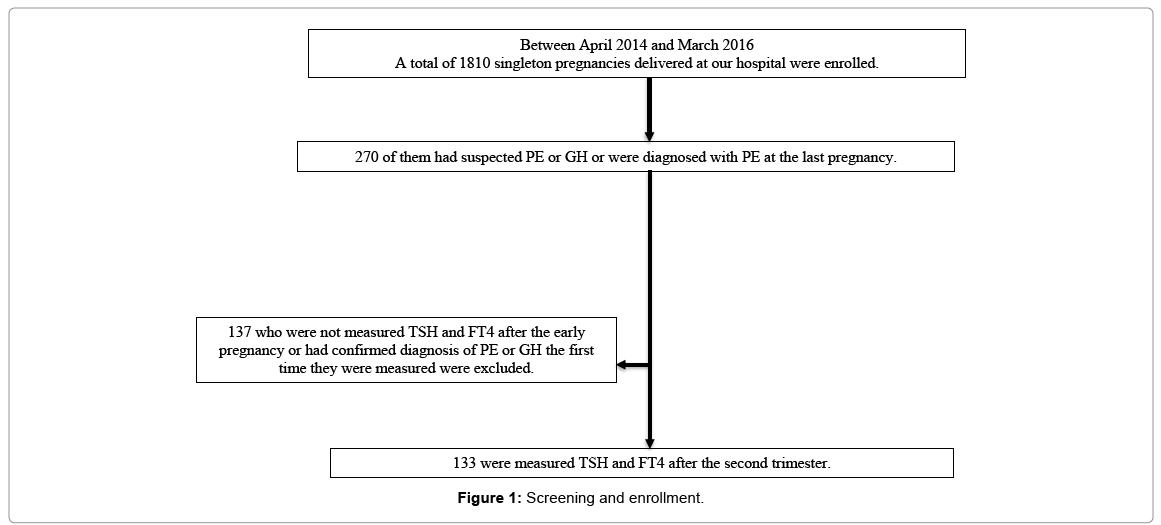
A total of 1810 singleton pregnancies delivered at our hospital were enrolled: 270 of them had suspected PE or GH or were diagnosed with PE at the last pregnancy. The analysis included 133 eligible pregnancies in which TSH and FT4 could be measured after the second trimester (Figure 1).
We divided the participants into 2 groups: 1 group with PE or GH (PE+GH group) and 1 group without PE or GH (non-PE+GH group).
We compared background and PE or GH prevalence between the groups.
Data were analyzed using SPSS Statistics version 18.0 and statistical analysis was performed using t test, chi-square test, ROC analysis and multiple logistic regression analysis.
A p value
Results
Among the PE+GH group, mean age, BMI at no pregnancy and BMI at delivery were 34.5 ± 6.7 years, 22.3 ± 3.9 kg/m2 and 26.0 ± 4.0 kg/m2, respectively. Among the non-PE+GH group, mean age, BMI at no pregnancy and BMI at delivery were 32.9 ± 5.5 years, 22.5 ± 4.8 kg/m2 and 26.1 ± 4.4 kg/m2, respectively. There were no significant differences observed between the 2 groups (Table 1).
| PE or GH group | Non PE or GH group | P value | |
|---|---|---|---|
| Age (years) | 34.5 ± 6.7 | 32.9 ± 5.5 | 0.125 |
| BMI before pregnancy (kg/m2) | 22.3 ± 3.9 | 22.5 ± 4.8 | 0.912 |
| BMI at delivery (kg/m2) | 26.0 ± 4.0 | 26.1 ± 4.4 | 1.000 |
| Primipara (no.) | 37 | 41 | 0.322 |
| History of PE or GH (no.) | 7 | 14 | 0.173 |
| Impaired glucose tolerance (no.) | 7 | 6 | 0.353 |
| Infertility treatment (no.) | 4 | 4 | 0.527 |
*There were no significant differences between the two groups
Table 1: Comparison of background between the groups.
We also examined the relationship between PE or GH prevalence and the confirmed risk factors: Impaired glucose tolerance, primipara, history of PE or GH and infertility treatment. There were no significant differences observed between the 2 groups.
The cutoff point of the TSH/FT4 ratio derived from the ROC curve was 1.9 (sensitivity 0.45, specificity 0.81). We performed multiple logistic regression analysis to examine the relationship between PE or GH prevalence measured the first time after the second trimester and the confirmed risk factors mentioned above. The adjusted odds ratio of the prevalence of PE or GH was 3.60 (95% CI: 1.62-8.02) (Table 2 and Figure 2).
| Variable | Unadjusted or 95% CI | Adjusted or 95% CI | ||
|---|---|---|---|---|
| Primipara | 1.27 | (0.63-2.52) | 0.98 | (0.41-2.37) |
| BMI before pregnancy | 0.66 | (0.29-1.50) | 0.54 | (0.22-1.36) |
| Infertility treatment | 1.09 | (0.23-5.15) | 1.09 | (0.23-5.15) |
| History of PE or GH | 0.56 | (0.21-1.48) | 0.68 | (0.21-2.25) |
| TSH/fT4>1.9 | 3.45 | (1.59-7.47) | 3.6 | (1.62-8.02) |
| Impaired glucose tolerance | 1.48 | (0.47-4.65) | 1.94 | (0.55-6.86) |
| Pregnancy after age 35 | 1.39 | (0.70-2.75) | 1.41 | (0.66-3.02) |
*The adjusted odds ratio of PE or GH prevalence was 3.60 (95% CI: 1.62-8.02)
Table 2: Predictor of GH or PE.
TSH/FT4 ratios were measured the first time at a median of 26 weeks and 6 days (14 weeks, from 0 days to 39 weeks, 5 days) and the median duration from the week TSH/FT4 ratio was measured for the first time to the week PE or GH was diagnosed was 45 days (from 1 day to 169 days).
Discussion
The findings of this study indicate that >1.9 of the TSH/FT4 ratio could be a risk factor of PE or GH prevalence and this test can be performed easier than for the measurement of the sFlt-1/PlGF ratio.
To date, it has been reported that, in hyperthyroidism, TSH stimulates TSH receptors (TSHR) at the thyroid follicles, resulting in increased cAMP and VEGF mRNA expression and VEGF is bound to Flt-1 on vascular endothelial cells; therefore, it follows that the proliferation and adhesion of vascular endothelial cells causes the angiogenesis [12].
TSH is a thyroid-specific growth factor and it has been reported that VEGF, basic fibroblast growth factor and their receptors express in human thyroid cells [13]. TSHR exists in extrathyroid tissues, such as adipose tissue, muscle cells, fibroblasts and red blood cells as well as in endothelial cells obtained from the human aorta [14-17]. Studies performed in a rat model of thyroid goitrogenesis found that TSH treatment increases thyroid capillary density [18].
TSHR has been reported to exist in human dermal microvascular endothelial cell line (HMEC-1) cells and that intracellular cAMP concentrations increase after TSH treatment. TSH stimulates capillary network formation in HMEC-1, whereas antibodies against VEGF and TSHR inhibit this effect, which shows that TSH induces angiogenesis in extrathyroid tissues and is inhibited by antibodies against VEGF [19].
In placenta with PE or GH, the signal cannot adequately transmit, because angiogenic factors, such as VEGF and PlGF cannot adequately bind to VEGF receptors (Flt-1) in the vascular walls due to the increased production of sFlt-1. Therefore, angiogenesis in the placenta is inhibited, which leads to a vicious cycle of hypoxia in the fetal-placental circulation.
A relative decrease in an angiogenic factor such as VEGF leads to the oversecretion of TSH and promotes VEGF production in the thyroid. Antiangiogenic factors inhibit this effect. Therefore, elevated TSH levels may reflect the increased effect of antiangiogenic factors such as sFlt-1.
As reported in previous studies, the maternal circulation, increase in sFlt-1 levels in late pregnancy and the increasing rate of PE or GH is higher than in normal pregnancy. Moreover, high sFlt-1 levels in PE or GH are associated with the prevalence of hypothyroidism and women with PE or GH are at a higher risk of postpartum hypothyroidism [20].
Thyroxine (T4) and thyroid hormone nearly (about 99%) bind to thyroid-binding protein (TBG, albumin). T4 binding to thyroidbinding protein does not have a hormonal effect on the body. A small amount of free T4 (FT4), which does not bind to thyroid-binding protein, exhibits a hormonal effect. Moreover, thyroid hormones have an angiogenic effect on vascular endothelial cells and vascular muscles through the hormone receptors at the surface of integrin αvβ3 [21-24]. Recently, a new mechanism reported that T4 secreted from the thyroid glands is converted to T3 by deiodinase type 2 (D2) and it promotes vascular endothelial cell migration throughout the regulation of gene expression [25]. Therefore, FT4 reflects not only angiogenic effects but also vascular endothelial cell migration.
We considered that TSH reflected antiangiogenic factors and FT4 reflected angiogenic factors and showed that the TSH/FT4 ratio after the second trimester was associated with the risk of prevalence of PE or GH.
Researchers have reported that sFlt-1, sEng and PlGF are effective in predicting early onset and severe PE but are less effective in predicting late-onset PE [26-29].
In the present study, we examined TSH/FT4 ratio after the second trimester, because there were small cases. We believe that the TSH/ FT4 ratio may predict the prevalence of late-onset PE but larger-scale studies are required.
We also examined TSH/FT4 ratio at the first trimester, but it had no relation to the prevalence of PE or GH.
D2, which converts T4 to T3 and deiodinase type 3 (D3), which inactivates thyroid hormones, is expressed in human placenta. Placental D3 activity is 200-fold higher than that of D2 activity and most of the T4 from the mother is metabolized in the placenta. D2 and D3 activity decrease, but the weight of the placenta increases with advancing gestation [30].
Therefore, in early pregnancy, T4 is converted to T3 by D2, T3 affects the thyroid hormones, but the placenta avoids exposure of the maternal thyroid hormones by inactivating most thyroid hormones from the mother, as D3 activity, which inactivates thyroid hormones, is strong during the entire duration of the pregnancy.
It is known that human chorionic gonadotropin (hCG) has the same subunit as TSH and stimulates the thyroid glands, which leads to transient hyperthyroidism in pregnancy. One of the potential reasons is that hCG has little effect on the thyroid glands after the second trimester, as hCG levels peak at 9-12 gestational weeks and subsequently decrease.
This was a retrospective study, thus, few cases were measured in early pregnancy. We intend to examine more cases in early pregnancy when we measure the TSH/FT4 ratio and when the ratio changes prior to the prevalence of PE or GH.
Conclusion
We found that the TSH/FT4 ratio after the second trimester may predict the prevalence of PE or GH. We will continue our research in this area with a higher number of cases moving forward.
Acknowledgement
We are grateful to members of Department of Endocrinology and Metabolism for their helpful suggestions. We thank Dr. Mariko Fukumoto for recruiting patients and for collecting data.
References
- Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, et al. (1991) Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 98: 648-655
- Pijnenborg R, Dixon G, Robertson WB, Brosens I (1980) Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1: 3-19.
- Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, et al. (2004) Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145: 4838-4845
- Khaliq A, Dunk C, Jiang J, Shams M, Li XF, et al. (1999) Hypoxia down regulates placenta growth factor, whereas fetal growth restriction up-regulates placenta growth factor expression: Molecular evidence for “placental hypoxia†in intrauterine growth restriction. Lab Invest 79: 151-170.
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, et al. (2003) Overexpression of the soluble vascular endothelial growth factor receptor in preeclampsia patients: Pathophysiological consequences. J Clin Endocrinol Metab 88: 5555-5563.
- Lash GE, Taylor CM, Trew AJ, Cooper S, Anthony FW, et al. (2002) Vascular endothelial growth factor and placental growth factor release in cultured trophoblast cells under different oxygen tensions. Growth Factors 20: 189-196.
- Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, et al. (2010) Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989-999.
- Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, et al. (2003) Neutralization of circulating vascular endothelial growth factor (VEGF) by anti VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605-12608.
- Männistö T, Mendola P, Grewal J, Xie Y, Chen Z, et al. (2013) Thyroid disease and Adverse pregnancy outcomes in a contemporary US cohort. J Clin Endocrinol Metab 98: 2725-2733.
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, et al. (2016) Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. NEJM 371: 13-22.
- Korevaar TI, Steegers EA, Schalekamp-Timmermans S, Ligthart S, De Rijke YB, et al. (2014) Soluble Flt1 and placental growth factor are novel determinants of newborn thyroid (Dys)function: The generation R study. J Clin Endocrinol Metab 99: 1627-1634.
- Sato K, Yamazaki K, Shizume K, Kanaji Y, Obara T, et al. (1995) Stimulation by TSH and Graves` IgG of vascular endothelial growth factor mRNA expression in human thyroid follicles in vitro and flt mRNA expression in the rat thyroid in vivo. J Clin Invest 96: 1295-1302.
- Hoffmann S, Hofbauer LC, Scharrenbach V, Wunderlich A, Hassan I, et al. (2004) Thyrotropin (TSH)-induced production of vascular endothelial growth factor in thyroid cancer cells in vitro: Evaluation of TSH signal transduction and of angiogenesis-stimulating growth factors. J Clin Endocrinol Metab 89: 6139-6145.
- Porcellini A, Messina A, De Gregorio G, Feliciello A, Carlucci A, et al. (2003) The expression of the thyroid-stimulating hormone (TSH) receptor and the cAMP-dependent protein kinase RIIß regulatory subunit confer TSH-cAMP-dependent growth to mouse fibroblasts. J Biol Chem 278: 40621-40630.
- Agretti P, Chiovato L, De Marco G, Marcocci C, Mazzi B, et al. (2002) Real-time PCR provides evidences for thyrotropin receptor mRNA expression in orbital as well as in extra-orbital tissues. Eur J Endocrinol 147: 733-739.
- Balzan S, Del Carratore R, Nicolini G, Forini F, Lubrano V, et al. (2009) TSH induces co-localization of TSH receptor and Na/K-ATPase in human erythrocytes. Cell Biochem Funct 27: 259-263.
- Donnini D, Ambesi-Impiombato FS, Curcio F (2003) Thyrotropin stimulates production of procoaugulant and vasodilatative factors in human aortic endothelial cells. Thyroid 13: 517-521.
- Reisinger K, Baal N, McKinnon T, Münstedt K, Zygmunt M (2007) The gonadotropins: Tissue-specific angiogenic factors? Mol Cell Endocrinol 269: 65-80.
- Balzan S, Del Carratore R, Nicolini G, Beffy P, Lubrano V, et al. (2012) Proangiogenic effect of TSH in human microvascular endothelial cells through its membrane receptor. J Clin Endocrinol Metab 97: 1763-1770.
- Levine RJ, Vatten LJ, Horowitz GL, Qian C, Romundstad PR, et al. (2009) Pre-eclampsia, soluble FMS-like tyrosine kinase 1 and the risk of reduced thyroid function: Nested case-control and population based study. BMJ.
- Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB (2005) Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146: 2864-2871.
- Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, et al. (2004) Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res 94: 1500-1506.
- Mousa SA, O'Connor LJ, Bergh JJ, Davis FB, Scanlan TS, et al. (2005) The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J Cardiovasc Pharmacol 46: 356-360.
- Mousa SA, Davis FB, Mohamed S, Davis PJ, Feng X (2006)) Pro-angiogenesis action of thyroid hormone and analogs in a three-dimensional in vitro microvascular endothelial sprouting model. Int Angiol 25: 407-413.
- Aoki T, Katsuhiko T, Araki O, Ogiwara T, Nara M, et al. (2015) Type 2 iodothyronine deiodinase activity is required for rapid stimulation of PI3K by thyroxine in human umbilical vein endothelial cells. Endocrinology 156: 4312-4324.
- Levine RJ, Lam C, Qian C (2006) CPEP Study Group: Soluble endoglin and other circulating anti-angiogenic factors in preeclampsia. N Engl J Med 355: 992-1005.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, et al. (2004) Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672-683.
- Simas TAM, Crawford SL, Solitro MJ, Frost SC, Meyer BA, et al. (2007) Angiogenic factors for the prediction of preeclampsia in high-risk woman. Am J Obstet Gynecol 197: 244-248.
- Erez O, Romero R, Espinoza J, Fu W, Todem D, et al. (2008) The change in concentration of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Mtarn Fetal Neonatal Med 21: 279-2-87.
- Patel J, Landers K, Li H, Mortimer RH, Richard K (2011) Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab 22: 164-170.
Citation: Fudaba M, Tanaka K, Matuski A, Komori M, Matsuki T, et al. (2017) Predictive Value of the TSH/FT4 Ratio in Women with Suspected PE or GH. J Preg Child Health 4: 357. DOI: 10.4172/2376-127X.1000357
Copyright: ©2017 Fudaba M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6595
- [From(publication date): 0-2017 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 5597
- PDF downloads: 998