Prevention of Peroxide-Induced Biochemical Damage to the Neural Retina by Caffeine: A Preliminary Report
Received: 21-Feb-2019 / Accepted Date: 27-Mar-2019 / Published Date: 04-Apr-2019 DOI: 10.4172/2168-9652.1000250
Abstract
Oxidative stress is one of the significant factors in the pathogenesis of several retinal diseases, viz. age-related macular degeneration, diabetic retinopathy, etc. Available treatments are not fully effective in attenuating tissue damage and the associated vision loss. Hence development of newer therapeutic compounds is highly desirable. We have previously demonstrated the effectiveness of metabolic and nutritional antioxidants such as pyruvate and caffeine in preventing oxidative damage to the lens. However, so far, studies investigating the protective effect of caffeine on the neural retina exposed to direct oxidative stress are lacking. The primary goal of this study was therefore to examine the efficacy of caffeine in preventing biochemical damage to the neural retina exposed to oxyradicals, in terms of maintaining the concentration of glutathione (GSH), a major endogenous antioxidant reserve. In vitro short-term tissue culture studies were conducted using freshly isolated neural retinas exposed to H2O2 in a medium with/without caffeine supplementation. Bovine neural retinas were incubated in medium 199 for 4 hours. Oxidative stress was induced by incubating the tissue with 9 mM H2O2. Caffeine group was incubated with 9 mM H2O2+ caffeine (5mM). Controls were incubated without H2O2 and caffeine. Tissue damage was assessed by measuring GSH content following incubation. Incubation of neural retina with H2O2 decreased GSH level to ~40% of the controls. Caffeine, however, maintained it at ~95% of the controls, indicating its effectiveness in preventing retinal oxidative stress. This novel effect of caffeine in the neural retina has been shown for the first time. The results are highly encouraging with regard to pursuing further studies investigating its other possible mechanisms of action, and its potential neuroprotective effect.
Keywords: Oxidative stress; Caffeine; Antioxidant; Glutathione; Neural Retina
Introduction
Utilization of oxygen to generate ATP (Adenosine Triphosphate) is associated with simultaneous production of partially reduced species of oxygen, especially during cellular respiration in the mitochondria, even under physiological conditions. These oxygen species, commonly known as Reactive Oxygen Species (ROS), Reactive Oxygen Intermediates (ROI) or oxyradicals, are the singlet oxygen (1O2), superoxide (O2 •-), Hydrogen Peroxide (H2O2), and Hydroxyl radical (OH•). The hydroxyl radical is known to be the most reactive, while H2O2, although not a radical, is nonetheless highly reactive and potentially damaging to biological cells by generating the hydroxyl radical via Fenton’s reaction (H2O2+Fe2+ → Fe3++OH−+OH•) [1,2]. The cellular components susceptible to damage by ROS are the enzymatic and non-enzymatic proteins, lipids (including membrane lipids), nucleic acids as well as carbohydrates. Oxidation of proteins can lead to modification of their conformation and eventual loss of ability to perform their normal function, such as maintaining cell structure if cytoskeletal proteins are affected. Similar oxidative modification of enzymatic proteins can lead to their inactivation and consequent metabolic aberrations adversely affecting ATP generation and hence many biological processes. Oxidation of membrane lipids and proteins can alter membrane permeability and cause disturbances in ionic balance inside and outside the cells. All the above changes have the potential to cause cell death [3,4]. Fortunately, the small amounts of ROS generated physiologically are scavenged by endogenous cellular enzymatic and non-enzymatic defense mechanisms. Enzymatic defenses consist of superoxide dismutase which dismutates O2 .- to the less reactive H2O2, catalase which catalyzes the conversion of H2O2 to H2O, and glutathione peroxidase which also scavenges H2O2. Additional antioxidant enzymes such as thioredoxin reductase, glutathione reductase, etc. also contribute to the neutralization of ROS. The major non-enzymatic defense molecule is the tripeptide glutathione (GSH, λ-glutamylcysteinylglycine), which is present in high concentration in many tissues, especially in the skin and the eye which are constantly exposed to solar radiation [5,6]. Exposure to UV rays is known to induce a cascade of reactions initiated by endogenous photosensitizers resulting in cyclic and excessive ROS generation [5]. High concentration of GSH in these tissues is nature’s own way of protecting them from damage. The above defense systems are considerably effective in preventing oxidative damage to the cells under physiological conditions. However, when ROS generation increases, as with aging, as well as in certain diseases viz. diabetes, or with excessive exposure to ultraviolet (UV) radiation, these defenses become overwhelmed, leaving un-scavenged radicals available to react with cell components [7,8]. In addition, the activities of defense enzymes are known to decrease with aging. Hence the use of exogenous ROS-scavenging compounds can be potentially beneficial in protecting the tissues from such damage and help in preventing age-associated cell damage as well as retarding disease progression [9-13].
ROS-induced damage is one of the known factors involved in the pathogenesis of many ocular diseases, such as cataract, diabetic retinopathy (DR), age-related macular degeneration (AMD), glaucoma etc., causing significant debility due to vision impairment and even blindness [5,14-16]. Most of these conditions are multifactorial, hence effective treatments to prevent/retard their progression are limited. It is desirable to identify newer and more effective pharmacological compounds that could be effective in preventing oxidative damage to the retina, and can become potentially useful for therapeutic purpose. Caffeine, or 1,3,7 trimethyl xanthine, is an efficient free radical scavenger previously shown to be effective in preventing oxidative damage to the lens and in preventing cataract formation [17]. It is therefore hypothesized that it will be effective in preventing oxidative stress to the neural retina as well, the neural component therein being primarily dependent on oxidative metabolism for its ATP needs. The hypothesis was verified by inducing oxidative stress in neural retina by incubating it with H2O2 with and without caffeine supplementation. The extent of biochemical damage was assessed by measuring the level of the major antioxidant glutathione (GSH) in the tissue.
Materials and Methods
Freshly enucleated bovine eyes were obtained from the local abbatoir (Ruppersberger, Baltimore). Care was taken to keep the eyes on ice until they were brought to the laboratory. Neural retinas were then dissected out and divided into 3 incubation groups. Control group was incubated in medium 199 (11043-023, Gibco, Grand Island, NY) without any additions. Experimental group was incubated in medium 199 containing 9 mM H2O2 (H325, Fisher, Fair Lawn, NJ) as the oxidant, whereas caffeine group was incubated in medium 199 containing 9 mM H2O2 and 5 mM caffeine (C0750, Sigma, St. Louis, MO). Incubations were conducted in a humidified incubator maintained at 37°C and 5% CO2. Duration of incubation was 4 hours.
The retinas were then removed from the medium and processed for biochemical studies following their homogenization in ice-cold dH2O. The homogenate was centrifuged at 34000 rpm and the supernatant used for determination of protein and glutathione content.
Protein determination
Water-soluble protein concentration in the supernatant was determined by Bradford’s assay using Coomassie blue dye-based reagent (B6916, Sigma, St. Louis, MO). An aliquot of the supernatant was reacted with the dye and the resulting color read spectrophotometrically at 595 nm. Bovine serum albumin standards of known concentration were run simultaneously. The concentration of water-soluble protein in the sample was then calculated by comparing the absorbance of the standards with absorbance of the tissue samples.
Statistical analysis to assess the significance of the data was done using Student’s t test and determining the p value.
Glutathione assay
The supernatant obtained as described above was deproteinized with the addition of 100% trichloroacetic acid (TCA) to a final concentration of 5%. The sample was then centrifuged to remove the protein precipitate, and the protein-free supernatant was used for GSH assay.
GSH was determined spectrophotometrically using Ellman’s reaction as done previously [4,11]. The assay is based on the reaction of -SH with Ellman’s reagent containing 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) to produce a yellow-colored product thionitrobenzoate which absorbs at 412 nm. The reagent was prepared by dissolving 4 mg DTNB in 10 ml of 1% trisodium citrate solution. An aliquot of the acidified supernatant prepared as above was mixed with 0.6M Na2HPO4 to a neutral pH. Ellman’s reagent was then added and the yellow color produced was measured spectrophotometrically at 412 nm. Standards with known concentrations of GSH were run simultaneously.
Results
The primary purpose of this study was to determine whether caffeine can prevent oxidative damage to the neural retina. Since glutathione is a major antioxidant in the tissue, concentration of this tripeptide was measured in the incubated retinas exposed to ROS in the absence and presence of caffeine.
The level of GSH was calculated as nanomoles/mg protein. Due to the difference in the protein concentration among the individual retinas and the inherent physiological and biochemical variations in the tissue obtained from the abattoir, the final GSH results are expressed relative to the controls, the controls considered as 100%.
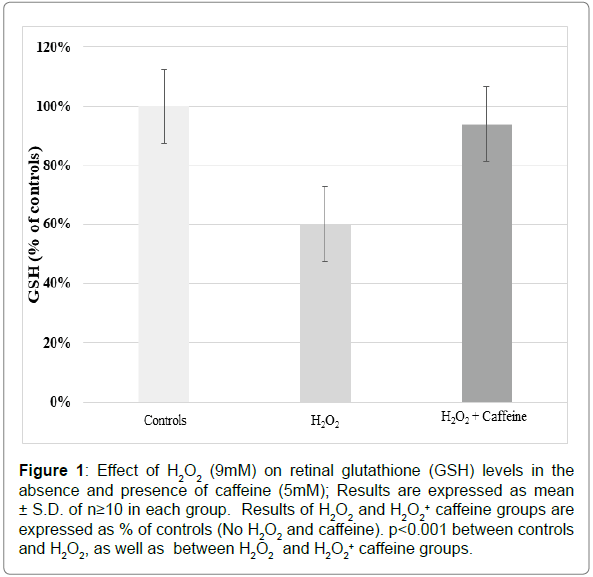
As shown in Figure 1, the level of GSH decreased substantially to ~60% of the controls in the tissues incubated with 9mM H2O2. However, such decrease was prevented to a significant extent when the incubation medium contained 5 mM caffeine in addition to 9 mM H2O2. As evident, GSH in the caffeine group was maintained close to the controls. p<0.001 between controls and H2O2, as well as between H2O2 and H2O2+ caffeine groups.
Figure 1: Effect of H2O2 (9mM) on retinal glutathione (GSH) levels in the absence and presence of caffeine (5mM); Results are expressed as mean ± S.D. of n=10 in each group. Results of H2O2 and H2O2 + caffeine groups are expressed as % of controls (No H2O2 and caffeine). p2O2, as well as between H2O2 and H2O2 + caffeine groups.
Caffeine was thus found to exert a significant protective effect against peroxide-induced depletion of GSH. Such an effect of caffeine in the neural retina has been demonstrated for the first time.
Discussion
Ocular diseases such as DR & AMD are known to be multifactorial in origin, oxidative insult being one of the major inducers of tissue damage. Both these diseases are associated with the onset of early (often pre-symptomatic) indications of retinal changes. For example, preclinical signs of DR include decrease in contrast sensitivity with associated electrophysiological abnormalities as determined by electroretinography and visually evoked potentials. These aberrations suggest damage to the neural retina very early in the disease even before the onset of ophthalmoscopically visible microvascular changes characteristic of clinical DR [18-22]. Early AMD is characterized by the appearance of drusen between the retinal pigment epithelium (RPE) and Bruch’s membrane visible ophthalmoscopically in the macula and also often in the peripheral retina. This is called the dry AMD, which can eventually progress to formation of excessive drusen, degeneration of the overlying RPE, degeneration of the photoreceptors which depend on the RPE for their maintenance, and consequent damage to the neural retina, choroidal neovascularization with leaky blood vessels (wet AMD) and scarring of the retina. The associated vision loss is permanent and is usually progressive. The Age-related Eye Disease Study (AREDS) concluded that an antioxidant formulation consisting of ascorbate, beta-carotene, vitamin E, copper, zinc, lutein and zeaxanthin may have some role in retarding progression of dry AMD (atrophic AMD) [23]. However, even early intervention with this treatment has limited benefit which is seen only in a small percentage of patients. This could be attributable to the possible tendency of some of these antioxidants to become pro-oxidant, especially in the presence of trace metals, and also due to the involvement of other factors such as genetic predisposition and smoking in the pathogenesis of this disease. Hence there is a need for the development of newer antioxidants which will not have a pro-oxidant effect under physiological conditions and are effective in preventing oxidative stress to the retina. Such therapies would perhaps be most beneficial if initiated at the pre-clinical stages.
Caffeine has been shown to be an effective scavenger of reactive oxygen, especially OH• The rate constants of its reaction with OH• and 1O2, as determined by electron spin resonance and pulse radiolysis are ~5.9 to 6.9× 109 M-1 S-1 and 2.9×107 M−1S−1, respectively. It also scavenges O2 .-, albeit less efficiently, with a rate constant of 7.5×10 M-1S-1 [24,25]. We have previously demonstrated its effectiveness in protecting the lens against UV-damage in vitro. It was also effective in preventing cataract formation in vivo in the selenite cataract model, as well in galactoseinduced cataract in rats [17,26].
Results in the present study demonstrating the effectiveness of caffeine in preventing the decrease in GSH level in the neural retina exposed to peroxide are highly encouraging. Such an effect of caffeine in the neural retina has been shown for the first time. Further studies are in progress to investigate its other possible effects on the neural retina, such as its effect on neural retinal metabolism.
Acknowledgements
The authors are thankful to the financial support of Wilson H. Elkins Professorship awarded to Dr. Kavita Hegde by the University System of Maryland for this research.
References
- Davies KJA (1995) Oxidative stress: the paradox of aerobic life. Biochem Soc Symp 61: 1-31.
- Fenton HJH, Jones HO (1900) The oxidation of organic acids in presence of ferrous iron. J Chem Soc Trans 77: 69-76.
- Halliwell B (1978) Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase. Cell Biol Int Rep 2: 113-128.
- Hegde KR, Kovtun S, Varma SD (2007) Induction of UV cataracts in vitro. Prevention by pyruvate. J Ocul Pharmacol Ther 23(5): 492-502.
- Varma SD, Hegde KR (2007) Oxidative stress and cataract formation. Horizons on its medical prevention. Expert Rev Ophthalmol 2: 779-801.
- Hegde KR, Varma SD (2005) Combination of Glycemic and Oxidative Stress in Lens: Implications in Augmentation of Cataract Formation in Diabetes. Free Radic Res 39(5): 513-517.
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, et al (2004) Global data on visual impairment in the year. Bulletin WHO 82: 844-851.
- Varma SD, Chand D, Sharma YR, Kuck JF Jr, Richards RD (1984) Oxidative stress on lens and cataract formation. Role of light and oxygen. Curr Eye Res 3: 35-57.
- Hegde KR, Varma SD (2008) Prevention of oxidative stress to the retina by pyruvate. A preliminary report. Ophthalmologica 222(3): 194-198.
- Varma SD, Hegde KR (2010) Prevention of oxidative damage to lens by caffeine. J Ocul Pharmacol Ther 26(1): 73-77.
- Varma SD (1987) Ascorbic acid and the eye with special reference to the lens. Ann N Y Acad Sci 498: 280-306.
- Trevithick JR, Linklater HA, Mitton KP, Dzialoszynski T, Sanford SE (1989) Modeling cortical cataractogenesis: IX. Activity of vitamin E and esters in preventing cataracts and gamma-crystallin leakage from lenses in diabetic rats. Ann N Y Acad Sci 570: 358-371.
- Hegde KR, Varma SD (2004) Morphogenetic and apoptotic changes in diabetic cataract: prevention by pyruvate. Mol Cell Biochem 262: 233-237.
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. (2004) Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol 137(1): 62-69.
- Beatty S1, Koh H, Phil M, Henson D, Boulton M (2000) The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv Ophthalmol 45(2): 115-134.
- Ceriello A (2003) New Insights on Oxidative Stress and Diabetic Complications May Lead to a “Causal†Antioxidant Therapy. Diabetes Care 26(5): 1589-1596.
- Varma SD, Hegde KR, Kovtun S (2010) Inhibition of selenite-induced cataract by caffeine. Acta Ophthalmol 88(7): 245-249.
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70-77.Â
- Kizawa J, Machida S, Kobayashi T, Gotoh Y1, Kurosaka D (2006) Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol 50(4): 367-373.
- Yonemura D, Aoki T, Tsuzuki K (1962) Electroretinogram in diabetic retinopathy. Arch Ophthalmol 68: 19-24.
- Chakravarthy H, Devanathan V (2018) Molecular Mechanisms Mediating Diabetic Retinal Neurodegeneration: Potential Research Avenues and Therapeutic Targets. J Mol Neurosci 66(3): 445-461.
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, et al (1998) Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest 102(4): 783-791.
- Age-Related Eye Disease Study Research Group (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8.  Arch Ophthalmol 119(10): 1417-1436.
- Devasagayam TP, Kamat JP, Mohan H, Kesavan PC (1996) Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochim et Biophys Acta Biomembranes 1282(1), 63-70.
- Kumar SS, Devasagayam TP, Jayashree B, Kesavan PC (2001) Mechanism of protection against radiation-induced DNA damage in plasmid pBR322 by caffeine. Int J Radiat Biol 77: 617-623.
- Varma SD, Kovtun S, Hegde K (2010) Effectiveness of topical caffeine in cataract prevention: studies with galactose cataract. Mol Vis 16: 2626-2633.
Citation: Hegde KR, Brown DD (2019) Prevention of Peroxide-Induced Biochemical Damage to the Neural Retina by Caffeine: A Preliminary Report. Biochem Physiol 8: 250. doi: 10.4172/2168-9652.1000250 DOI: 10.4172/2168-9652.1000250
Copyright: © 2019 Hegde KR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3521
- [From(publication date): 0-2019 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 2608
- PDF downloads: 913