Preventive Effects of Fucoidan on Mitochondrial Lipid Peroxidation Products and Mitochondrial Enzymes in Iso-Induced Myocardial Infarcted Rats
Received: 04-Jun-2021 / Accepted Date: 18-Jun-2021 / Published Date: 25-Jun-2021 DOI: 10.4172/2167-065X.1000224
Abstract
Cardiovascular diseases have a high prevalence in developing and developed countries and myocardial infarction (MI) accounts for majority of deaths and disabilities. It therefore attracts continuing Research into the biomedical activities of marine natural products has led to the discovery of many potentially active agents considered worthy of clinical application. Many of which exhibit structural or chemical features not found in terrestrial natural products. Marine bioactive compounds appear to be very beneficial and promising for biomedical studies to make clear many regular and pathological mechanism of movement within side the human frame in addition to withins ide the layout of very unique and effective new prescription drugs for a large form of diseases. A fucoidan with high ester sulfate content has been remoted in an industrial scale procedure from Laminaria japonica.
Keywords: Isoproterenol; Fucoidan; Myocardial infarction; Mitochondrial enzymes; Oxidative stress
Introduction
Myocardial infarction occurs as a result of imbalance between coronary blood supply and myocardial demand [1]. Isoproterenol (ISO)-induced myocardial necrosis involves membrane permeability alterations that bring about loss of function and integrity of myocardial membranes [2].
Administration of isoproterenol depletes the energy reserve of cardiac muscle cells and complex biochemical and structural changes causing cell damage, which is prelude to necrosis [3]. Targeting the mitochondrial respiration chain whose hobby is reduced in ischemic situations is a brand new method with large healing prospective. Mitochondria are important sub cellular organelles for cellular oxidative process and also the main source of reactive oxygen species (ROS) in the cell. Mitochondria are the main source of energy, which sustain cellular metabolism and integrity. The decrease in oxygen supply during myocardial infarction impairs energy production by mitochondria [4]. Prolonged oxidative stress in failing myocardium results in damage to mitochondrial deoxy ribonucleic acid, reactive oxygen species generation, and consequent cellular injury leading to functional decline [5,6].
Thus, mitochondria serve both as a source and target of reactive oxygen species mediated injury in failing heart. Defective mitochondrial function is a fundamental characteristic of the failing heart. Isoproterenol causes Ca2+ overload in myocardium, disruption of the mitochondria [7], and inactivation of tricarboxylic acid (TCA) cycle enzymes, and alterations in mitochondrial respiration and depletion of adenosine tri phosphate. The viability of the myocardial cell depends for most part on the integrity of several membrane systems. The major source of energy for contraction comes from the oxidative metabolism of mitochondria in the myocardial cell. For this reason, the characteristic of mitochondria in myocardial infarction is of specific interest.
The present study is undertaken to estimate the protective role of fucoidan on mitochondrial dysfunction in isoproterenol induced myocardial infarction in male albino Wistar rats. Rats subcutaneously injected with ISO (100 mg/kg) at an interval of 24 h for 2 days, resulting in significant (p<0.05) increase in the levels of serum mitochondrial lipid peroxides, declined antioxidant status and the activities of mitochondrial tricarboxylic acid cycle and respiratory chain enzymes. Pre- treatment with fucoidan (150 mg/kg) near normalized all the biochemical parameters and restored the normal mitochondrial function. Transmission electron microscopic observations also correlated with these biochemical findings. Thus, our findings revealed that fucoidan prevents the mitochondrial dysfunction during isoproterenol induced myocardial infarction in rats.
This is the first characterization of a fucoidan from this species of macro algae. Fucoidan is a poly anionic sulfated polysaccharide mainly composed of α-1, 3 backbones or repeating disaccharide units of α -1, 3 and α -1, 4 linked fucose residues with branching attached at C2 positions. It has been broadly studied in recent years, because it is endorsed with important biological properties such as antioxidant [8] anticoagulant, antithrombotic, anti-inflammatory, anti-angiogenic, and anti-adhesive activities [9]. Additionally, fucoidan administration has no adverse effects on rats. The effect of fucoidan against isoproterenol induced myocardial infarction, we evaluated the role of fucoidan on mitochondrial dysfunction in myocardial infarcted rats. Furthermore, the protective effect of fucoidan on the structure of heart mitochondria was confirmed by transmission electron microscopic study.
Materials and Methods
Isolation of heart mitochondrial fraction
Mitochondria from the heart tissues were isolated by the standard procedure of Takasawa et al., [10]. At first the heart tissues were put into ice cold 50 mMTris -HCl (pH 7.4) containing 0.25 M sucrose and homogenized. Then, the homogenates were centrifuged at 700 x g for 20 min. The supernatants obtained were centrifuged at 9,000 x g for 15 min. Thereafter, the pellets were washed carefully with 10 mMTris- HCl (pH 7.8) containing 0.25 M sucrose, and finally resuspended in the same buffer and used for mitochondrial studies.
Estimation of TBARS in the heart mitochondrial fraction
The level of heart mitochondrial thiobarbituric acid reactive substances (TBARS) was evaluated by the following method of Fraga et al., [11]. The level of TBARS was expressed in terms of n moles/mg protein.
Estimation of the heart mitochondrial LOOH
The level of heart mitochondrial lipid peroxidation was determined by the following method of Jiang et al., [12]. The levels of LOOH were expressed in terms of m moles /100 g wet tissue for heart mitochondrial fraction.
Assay of heart mitochondrial superoxide dismutase
In the heart mitochondrial fraction, the activity of superoxide dismutase was determined by the following method of Kakkar et al., [13]. The superoxide dismutase activity was expressed in terms Units/100 mg protein (Unit: One unit is defined as the enzyme concentration required inhibiting the optical density at 560 nm of chromogen production by 50% in 1 min).
Assay of heart mitochondrial glutathione peroxidation
The glutathione peroxidase activity in the heart mitochondrial fraction was assayed by the method of Rotruck et al., [14]. The enzyme activity was expressed in terms of n moles of GSH oxidized/min/mg protein.
Estimation of heart mitochondrial reduced glutathione
The reduced glutathione (GSH) level in the heart mitochondrial fraction was estimated by the method of Ellman [15]. The GSH values were expressed in terms of n moles/100 mg protein.
Estimation of glutathione reductase
Glutathione reductase (GRx) activity was assayed in the heart by the method of Horn and Burns, [16]. The activity of GRx was expressed in terms of nM of NADPH oxidized / min / 100 mg protein.
Assay of heart mitochondrial isocitrate dehydrogenase
The activity of heart mitochondrial isocitrate dehydrogenase was assayed by the way of method King [17]. The activity of isocitrate dehydrogenase was expressed in terms n moles of NADH oxidized /h /mg protein.
Assay of heart mitochondrial malate dehydrogenase
In the heart mitochondrial fraction, malate dehydrogenase activity was determined by the following method of Mehler et al., [18].
The malate dehydrogenase activity in the heart mitochondrial fraction was expressed as n moles of NADH oxidized/min/mg protein.
Assay of heart mitochondrial α-ketoglutarate dehydrogenase
The α-ketoglutarate dehydrogenase activity in the heart mitochondrial fraction was estimated by the method of Reed and Mukherjee [19]. The activity of α-ketoglutarate dehydrogenase in the heart mitochondria was expressed in terms n moles of ferrocyanide formed/h/mg protein.
Assay of succinate dehydrogenase
The activity of succinate dehydrogenase (SDH) in the heart mitochondrial fraction was assayed by the method of Slater and Borner, [20].
The activity of SDH was expressed in terms of n M of succinate oxidized /min/ mg protein.
Assay of heart mitochondrial NADH-dehydrogenase
The activity of NADH-dehydrogenase in the heart mitochondrial fraction was estimated by the method of Minakami et al., [21].
The NADH-dehydrogenase activity was expressed in terms n moles of NADH oxidized /min /mg protein in the heart mitochondria.
Assay of heart mitochondrial cytochrome-c-oxidase
The cytochrome-c-oxidase activity in the heart mitochondrial fraction was assayed by the method of Pearl et al., [22].
The activity of cytochrome-c-oxidase was expressed in terms of n moles/min/mg protein.
Transmission electron microscopic study on the structure of heart mitochondria
The ultra-structure of heart mitochondria was determined by transmission electron microscopic study according to the method of Lang [23].
The sections were then kept upon carbon grids and post-stained with combined uranyl and lead stain and thereafter rinsed with distilled H2O and dried. After drying, they were view and examined with TEM (Philip-420).
Statistical analysis
Statistical analysis was performed by one way analysis of variance followed by DMRT using Statistical Package for the Social Science software package version 12.00. Results were expressed as mean ± standard deviation for six rats in each group. P values <0.05 were considered significant.
Results
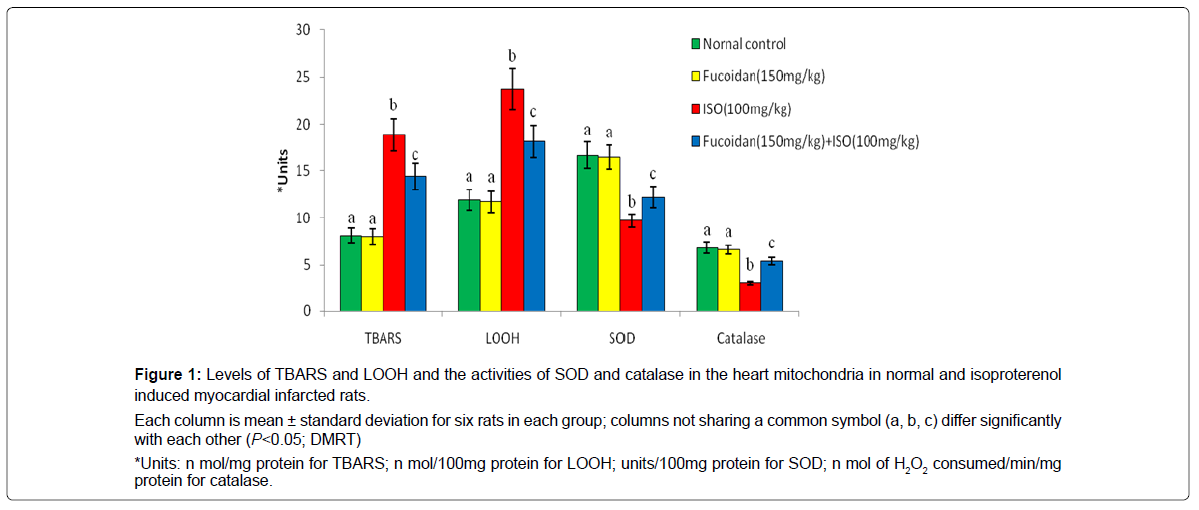
Figure 1 shows the effect of fucoidan on lipid peroxidation products and antioxidant enzymes such as superoxide dismutase and catalase in the mitochondrial fraction of normal and experimental rats. Isoproterenol induced myocardial infarcted rats shows the significant (P<0.05) increased levels of mitochondrial thiobarbituric acid reactive substances and lipid hydro peroxides with significant (P<0.05) decreased activities of superoxide dismutase and catalase in the heart mitochondria compared to normal control rats. Oral pretreatment with fucoidan significantly (P<0.05) decreased the levels of mitochondrial thiobarbituric acid reactive substances and lipid hydro peroxides and increased the activities of superoxide dismutase and catalase in the heart mitochondria of isoproterenol- induced rats compared to isoproterenol-alone induced rats.
Each column is mean ± standard deviation for six rats in each group; columns not sharing a common symbol (a, b, c) differ significantly with each other (P<0.05; DMRT)
*Units: n mol/mg protein for TBARS; n mol/100mg protein for LOOH; units/100mg protein for SOD; n mol of H2O2 consumed/min/mg protein for catalase.
Figure 1: Levels of TBARS and LOOH and the activities of SOD and catalase in the heart mitochondria in normal and isoproterenol induced myocardial infarcted rats.
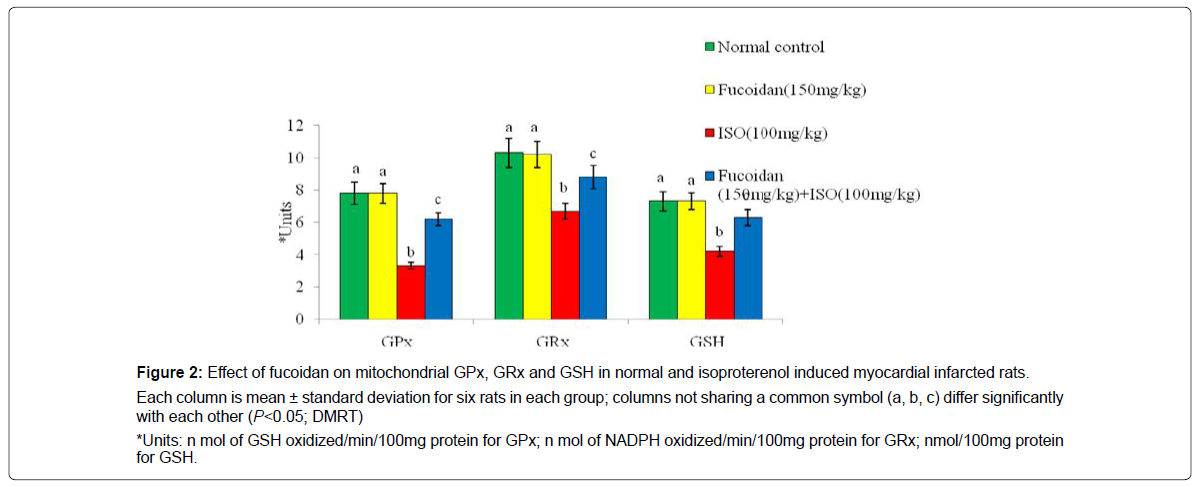
Figure 2 shows the effect of fucoidan on antioxidant enzymes such as glutathione peroxidase, glutathione reductase and reduced glutathione in the mitochondrial fraction of normal and experimental rats. Isoproterenol induced myocardial infarcted rats shows the significant (P<0.05) decreased activities of glutathione peroxidase, glutathione reductase and reduced glutathione in the heart mitochondria compared to normal control rats. Oral pretreatment with fucoidan significantly (P<0.05) increased the activities of glutathione peroxidase, glutathione reductase and reduced glutathione in the heart mitochondria of isoproterenol- induced rats compared to isoproterenol-alone induced rats.
Each column is mean ± standard deviation for six rats in each group; columns not sharing a common symbol (a, b, c) differ significantly with each other (P<0.05; DMRT)
*Units: n mol of GSH oxidized/min/100mg protein for GPx; n mol of NADPH oxidized/min/100mg protein for GRx; nmol/100mg protein for GSH.
Figure 2: Effect of fucoidan on mitochondrial GPx, GRx and GSH in normal and isoproterenol induced myocardial infarcted rats.
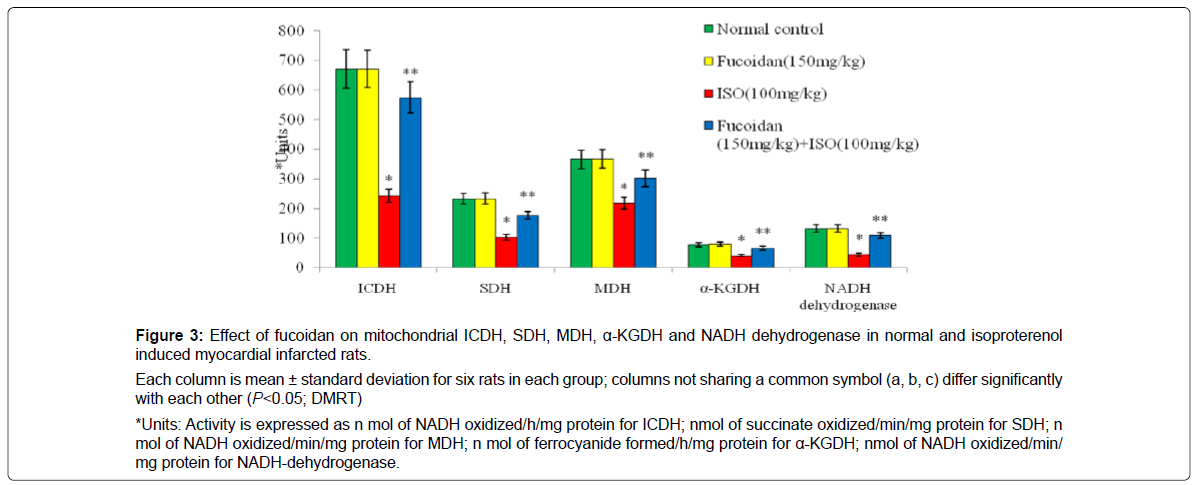
Figure 3 shows the activities of isocitrate dehydrogenase, succinate dehydrogenase, malate dehydrogenase, α-ketoglutarate dehydrogenase and NADH dehydrogenase in the heart mitochondria in normal and experimental rats. Isoproterenol induced rats showed significantly (P < 0.05) decreased activities of these mitochondrial marker enzymes in ISO-induced rats compared to normal control rats. Prior treatment with fucoidan significantly (P <0.05) increased the activities of these mitochondrial marker enzymes in isoproterenol -induced rats compared to isoproterenol -alone induced rats.
Each column is mean ± standard deviation for six rats in each group; columns not sharing a common symbol (a, b, c) differ significantly with each other (P<0.05; DMRT)
*Units: Activity is expressed as n mol of NADH oxidized/h/mg protein for ICDH; nmol of succinate oxidized/min/mg protein for SDH; n mol of NADH oxidized/min/mg protein for MDH; n mol of ferrocyanide formed/h/mg protein for α-KGDH; nmol of NADH oxidized/min/ mg protein for NADH-dehydrogenase.
Figure 3: Effect of fucoidan on mitochondrial ICDH, SDH, MDH, α-KGDH and NADH dehydrogenase in normal and isoproterenol induced myocardial infarcted rats.
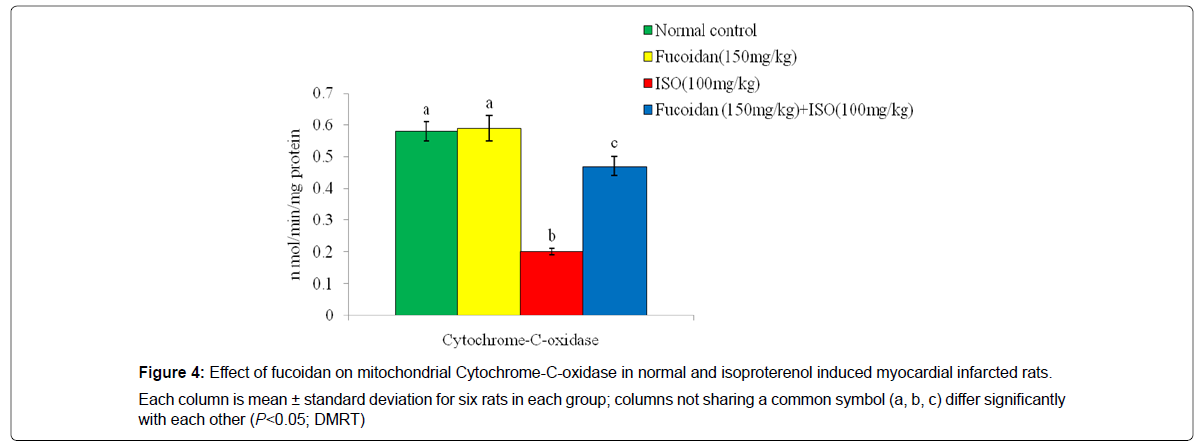
Figure 4 shows the effect of fucoidan on the activity of cytochrome- C-oxidase in the heart mitochondrial fraction in normal and experimental rats. Isoproterenol induced rats showed significant (P<0.05) decreased activity of cytochrome- C-oxidase in the heart mitochondria compared to normal control rats. Fucoidan pretreatment significantly (P<0.05) increased the activity of this enzyme in ISO induced rats compared to ISO-alone induced rats.
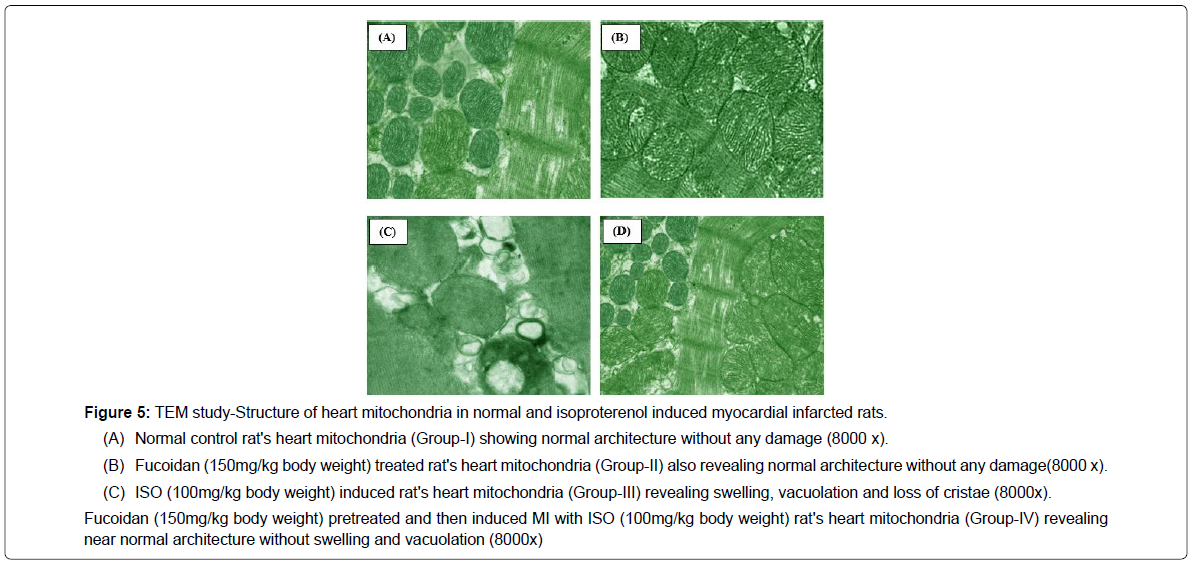
In the present study, the transmission electron microscopic images of the heart mitochondria in ISO-induced rats (Figure 5c) revealed swelling of mitochondria, loss of cristae with vacuolation and increased size. Fucoidan (150 mg/kg body weight) pretreated ISO-induced rats (Figure 5d) showed near normal architecture with mild separation of cristae without swelling and vacuolation. Fucoidan (150 mg/kg) treated normal rat's heart mitochondria (Group-II) showed normal mitochondrial structure without swelling without vacuolation (Figure 5a and 5b).
Fucoidan (150mg/kg body weight) pretreated and then induced MI with ISO (100mg/kg body weight) rat's heart mitochondria (Group-IV) revealing near normal architecture without swelling and vacuolation (8000x)
Figure 5: TEM study-Structure of heart mitochondria in normal and isoproterenol induced myocardial infarcted rats.
(A) Normal control rat's heart mitochondria (Group-I) showing normal architecture without any damage (8000 x).
(B) Fucoidan (150mg/kg body weight) treated rat's heart mitochondria (Group-II) also revealing normal architecture without any damage(8000 x).
(C) ISO (100mg/kg body weight) induced rat's heart mitochondria (Group-III) revealing swelling, vacuolation and loss of cristae (8000x).
Discussion
Experimental induction of myocardial infarction by isoproterenol in animals is a well-established model to study the protective role of different cardio protective agents [4]. Pharmacological induction of myocardial infarction by subcutaneous administration of isoproterenol in animals like rats has been found to be convenient because of relatively smaller size of coronary arteries [3]. The advantages of isoproterenol induced myocardial infarction that occur as a result of intense inotropic and chronotropic actions of isoproterenol compared to physical occlusion of the coronary artery as a less invasion accomplished without critical factors of common anesthesia and lack of foreign body remaining in the heart. Reperfusion is possible after isoproterenol, since there is no permanent overt. Occlusion and the survival rates with isoproterenol are consistent and reproducible after vessel occlusion.
Our study revealed that pretreatment with fucoidan prevents mitochondrial oxidative stress in isoproterenol induced myocardial infarcted rats. Isoproterenol induced myocardial infarction the release of cellular cardiac mitochondrial enzymes is correlated with changes in plasma membrane integrity and/or permeability as a response to β-adrenergic stimulation. This might be due to damage inflicted upon the sarcolemma by the β-agonist, rendering it leaky.
In this investigation, we observed increased lipid peroxidation and decreased antioxidant system in the mitochondrial heart of isoproterenol-induced rats. Lipid peroxide mediated cardiac mitochondrial damage has been observed in isoproterenol -induced myocardial infarcted rats. The enhanced levels of lipid peroxidation products such as TBARS and LOOH in the mitochondrial fraction of heart may decrease mitochondrial membrane fluidity, increase the negative surface charge distribution and alter membrane ionic permeability including proton permeability, which uncouple oxidative phosphorylation [4]. Thus, improved lipid peroxidation damages each the mitochondrial shape and feature of isoproterenol -prompted rats. Prior remedy with fucoidan reduced the stages of lipid peroxidation in isoproterenol -prompted rats.The decreased mitochondrial TBARS and LOOH observed in this study are due to anti-lipidperoxidative, anti-lipidaemic, anti-inflammatory, anti-oxidant and free radical scavenging effects of fucoidan. Thus, fucoidan lowers lipid peroxidation and improves cardiac mitochondrial structure and function.
The oxidative stress may be exerted through quinone metabolites of isoproterenol which react with oxygen to produce superoxide anions and other reactive oxygen species and interfere with antioxidant enzymes. Free radical scavenging enzymes such as superoxide dismutase and catalase are the first line of cellular defense against oxidative injury. The observed decreased activities of these enzymes in heart mitochondria is due to increased generation of reactive oxygen species, such as superoxide and hydrogen peroxide, which in turn leads to the inhibition of these enzymes. Pretreatment with fucoidan reduced the generation of reactive active species and increased the activities of free radical scavenging enzymes such as superoxide dismutase and catalase in the heart mitochondria by its antioxidant effect.
Reduced glutathione protects mitochondrial membrane from the damaging action of lipid peroxidation. Decreased concentration of mitochondrial reduced glutathione indicates a major mechanism of inducing an imbalance of mitochondrial function. The decreased activity of glutathione peroxidase makes mitochondria more susceptible to isoproterenol -induced cardiac damage that leads to mitochondrial dysfunction. A phase II enzyme such as glutathione-s-transferase not only catalyzes the conjugation of both hydro quinones and epoxides of polycyclic aromatic hydrocarbons with reduced glutathione for their excretion, but also shows low activity towards organic hydro peroxides for their detoxification from cells/tissues. Inactivation of glutathione reductase in the heart leads to accumulation of oxidized product of reduced glutathione (GSSG).
In mitochondria, the decreased reduced glutathione level and increased GSSG inactivate GSH-dependent enzymes such as glutathione reductase, glutathione peroxidase and glutathione-S- transferase in isoproterenol -induced myocardial infarcted rats. Our results show that fucoidan enhanced the activities of enzymatic antioxidants and the levels of non-enzymatic antioxidant in isoproterenol -induced rats. Mitochondrial and cellular damage can be prevented by increasing intracellular GSH content. The increased levels of GSH observed in fucoidan pretreated rats resulted in increased activities of GSH-related enzymes in the mitochondrial fraction of isoproterenol-induced rats. Thus, fucoidan acts as an antioxidant by scavenging reactive oxygen species and improving the antioxidant system in isoproterenol-induced rats.
Mitochondrial membrane lipid peroxidation results in irreversible loss of mitochondrial functions such as mitochondrial respiration, oxidative phosphorylation and ion transport [4]. Isocitrate dehydrogenase is mostly expressed in the heart and skeletal muscle mitochondria. It is NADP/NAD dependent and controls the mitochondrial redox balance and the subsequent oxidative damage. Succinate dehydrogenase is a component of electron chain and is bound to the inner mitochondrial membrane. The activities of these dehydrogenases were reduced in the heart mitochondria of isoproterenol induced rats. Malate dehydrogenase catalyzes the reversible NAD+- dependent-dehydrogenase reaction involved in central metabolism and redox homeostasis between organelle compartments. It is located in the outer membrane of mitochondria and vulnerable to free radical attack. The α-Ketoglutarate dehydrogenase catalyzes the conversion of α-ketoglutarate to succinyl-CoA and NADH in mitochondria. Isoproterenol induced rats significantly decreased the activities of tricarboxylic acid cycle enzymes, such as isocitrate dehydrogenase, Succinate dehydrogenase, Malate dehydrogenase and α-Ketoglutarate dehydrogenase. This is in agreement with an earlier study [8]. These dehydrogenases are located in the outer membrane of the mitochondria and are affected by increased levels of free radicals produced following isoproterenol administration. Fucoidan pretreatment increased the activities of these enzymes to near normal values in the isoproterenol-induced myocardial mitochondria. Increased activity of tri carboxylic acid cycle enzymes in fucoidan pretreated isoproterenol -induced rat’s shows better utilization of energy yielding intermediates by tricarboxylic acid cycle.
The decreased activities of respiratory chain enzymes such as NADH-dehydrogenase and cytochrome-C-oxidase observed in isoproterenol induced rats might be due to enhanced phospholipids degradation resulting in the non-availability of cardiolipin for their functional activity. Increased free radicals produced by isoproterenol also resulted in decreased activities of these enzymes [4]. Pretreatment with fucoidan showed significantly improved activities of respiratory chain enzymes in isoproterenol -induced rats. Thus, fucoidan protects tricarboxylic acid cycle and a respiratory chain enzyme from free radical attack and maintains the energy levels of mitochondria.
Summary and Conclusion
Alteration in the fine structure of mitochondria is the most prominent transmission electron microscopic images finding in cardiac damage induced by isoproterenol. Isoproterenol administration is characterized by increased mitochondrial swelling, disruption of cristae, vacuolation and increased size of the mitochondria.
Thus, the lack of mitochondrial enzymes in the end ends in cardiac tissue death. Fucoidan pretreatment showed normal architecture of mitochondria without swelling and vacuolation. The mitochondrial size also decreased. These observations agree carefully with the effects received via way of means of biochemical parameters withinside the study.Thus, fucoidan protects the structure of mitochondria.
Treatment with fucoidan (150 mg/kg body weight) in normal rats (Group-II) did not show any significant effect on the biochemical parameters and ultra-structure of mitochondria, which confirmed the safety assessment of fucoidan. The observed effects in this study are due to quenching free radicals, inhibiting lipid peroxidation, improving antioxidant system and mitochondrial marker enzyme activities and preventing oxidative stress thereby protecting cardiac mitochondria in myocardial infarcted rats. Transmission electron microscopic study also supports the biochemical findings of the study.
References
- Wang SB, Tian S, Yang F, Yang HG, Yang XY, et al. (2009) Cardioprotective effect of salvianolic acid. A on isoproterenol–induced myocardial infarction in rats. Eur J Pharmacol 615:125-132.
- Padmanabhan M, Prince PSM (2007) S-allylcysteine ameliorates isoproterenol-induced cardiac toxicity in rats by stabilizing cardiac mitochondrial and lysosomal enzymes. Life Sci 80:972-978.
- Rona G (1985) Catecholamine cardiotoxicity. J Mol Cell Cardiol 17:291-300.
- Kumaran KS, Prince PSM (2010) Preventive effect of caffeic acid on lysosomal dysfunction in isoproterenol-induced myocardial infarcted rats. J Biochem Mol Toxicol 24:115-122.
- Ilamathi J, Prince PSM (2016) Protective effects of Vanillic acid on mitochondrial antioxidant enzymes and lipids in isoproterenol induced myocardial infarction in rats. Int J Recent Sci Res 7:13143-13147.
- Ilamathi J, Prince PSM(2015) Vanillic Acid Prevents Lysosomal Membrane Damage In Isoproterenol Induced Myocardial Infarction In Rats. Int J Recent Sci Res 6:2667-2673.
- Xia T, Jiang C, Li L, Wu C, Chen Q, et al. (2002) A study on permeability transition pore opening and cytochrome-c-release from mitochondria, induced by caspase-3 in-vitro. FEBS Lett 510:62-66.
- Rocha de Souza MC, Marques CT, Dore CMG, da Silva FRF, Rocha HAO (2007)Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J Appl Phycol 19:153-160.
- Cumashi A, Ushakova NA, Preobrazhenskaya ME (2007) A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 17:541-552.
- Takasawa M, Hayakawa M, Sugiyama S, Hattori K, Ito T, et al.(1993) Age-associated damage in mitochondrial function in rat hearts. Exp Gerontol 28:269-280.
- Fraga CG, Leibovitz BE, Tappel AL(1988) Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155-161.
- Jiang ZY, Hunt JV, Wolff SP(1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384-389.
- Kakkar P, Das B, Viswanathan PN(1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130-132.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, et al.(1973) Selenium: biochemical role as a component of glutathione peroxidase. Sci 179:588-590.
- Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70-77.
- Horn HD, Burns FH (1978) Assay of glutathione reductase activity. In: Bergmeyer HV, editor. Methods of Enzymatic Analysis. New York: Academic Press p:142.
- King J (1965) Isocitrate dehydrogenase. In: King JC, Van D. Practical Clinical Enzymology. London: Nostrand Co p:363.
- Mehler AH, Kornberg A, Grisolia S, Ochoa S (1948) The enzymatic mechanism of oxidation-reductions between malate or isocitrate and pyruvate. J Biol Chem 174:961-977.
- Reed LJ, Mukherjee RB (1969) α-Ketoglutarate dehydrogenase complex from Escherichia coli. In: Lowenstein JM, editors, Methods in Enzymology. London: Academic Press pp:53-61.
- Slater EC, Borner WD (1952) The effect of fluoride on the succinic oxidase system. Biochem J 52:185-196.
- Minakami S, Ringler RL, Singer TP (1962) Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. J Biol Chem 237:569-576.
- Pearl W, Cascarano J, Zweifach BW (1963) Microdetermination of cytochrome oxidase in rat tissues by the oxidation on N-phenyl-p-phenylenediamine or ascorbic acid. J Histochem Cytochem 11:102-104.
- Lang RAD (1987) In mitochondria: A Practical Approach. Darely Usmar VM, Reckwood D, Wilson MT, editors. Washington: IRL Press Ltd pp:17-20.
Citation: Jayaraman I (2021) Preventive Effects of Fucoidan on Mitochondrial Lipid Peroxidation Products and Mitochondrial Enzymes in Iso-Induced Myocardial Infarcted Rats. Clin Pharmacol Biopharm, 10: 224. DOI: 10.4172/2167-065X.1000224
Copyright: © 2021 Jayaraman I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2960
- [From(publication date): 0-2021 - Dec 11, 2025]
- Breakdown by view type
- HTML page views: 2069
- PDF downloads: 891