Prognostic Value of the Expression of Endogenous Hypoxia Associated Proteins Hypoxia Inducible Factor-1 Alpha (HIF-1α) and Carbonic Anhydrase Isoform 9 (CAIX) Expressions in Breast Carcinoma
Received: 20-Oct-2017 / Accepted Date: 28-Nov-2017 / Published Date: 04-Dec-2017
Abstract
Background: Hypoxia has been found to be related to malignant initiation, progression, increasing the occurrence of metastasis and therapy resistance in many cancer types, which made a real need for discovering drugs that could antagonize the bad effect of hypoxia in cancer, decide which patients will have benefit from such anti-hypoxia therapy then to monitor response to therapy, especially in breast carcinoma.
It is important to detect degree of hypoxia in each cancer that could be done by evaluation of the expression of hypoxia-associated proteins in cancer biopsies e.g. hypoxia inducible factor-1 alpha (HIF-1α) and carbonic anhydrase IX (CAIX) and their detailed role in breast cancer is still uncertain and gives conflicting results.
Aim of the work: Aim of the work was to evaluate HIF-1α and CAIX expressions in breast carcinoma, correlating their expressions with each other, with presence of lymph node and distant metastases, with recurrence free and overall survival rates of female patients with breast cancer.
Methods: We evaluated HIF-1α and CAIX expressions in sections from 90 paraffin blocks of breast carcinoma using immunohistochemistry. We analyzed correlations between their levels of expressions, clinic-pathological and prognostic parameters of our patients.
Results: HIF-1α and CAIX positive expression in breast carcinoma was related to advanced stage, presence of lymph node metastases, HER2 amplified and triple negative molecular subtypes (p<0.001), higher tumor grade (p=0.001 and 0.02 respectively) and negative ER (p=0.005 and 0.008 respectively) and PR (p=0.009 and 0.027 respectively) hormonal receptors, The expression of both markers was significantly positively correlated with each other (p<0.001). HIF-1α and CAIX positive expression in breast carcinoma was associated with shortened recurrence free and overall survival rates (p<0.001).
Conclusion: HIF-1α and CAIX are markers of poor prognosis of breast carcinoma patients.
Keywords: Breast carcinoma; Hypoxia; HIF-1α; CAIX; Immunohistochemistry; Prognosis
Introduction
Breast carcinoma is considered the commonest cancer type and the 2nd leading cause of females’ cancer related mortality [1]. Breast carcinoma lymph node and distant metastasis are the most important prognostic factor for patients [2]. Because of perfusion deficits, solid tumors have heterogeneous regions of hypoxia (reduced pO2). Additionally, it has been reported that the altered tumor metabolism can also contribute to tumor hypoxia [3]. Hypoxia has been found to be related to malignant initiation, progression, increasing the occurrence of metastasis and therapy resistance in many cancer types, also it has a prognostic marker of poor patients’ survival rates [4]. The negative consequences of tumor hypoxia on cancer of various types, made a real need for discovering drugs that could antagonize the bad effect of hypoxia in cancer [5]. Also, hypoxia assessment in different cancer regions which can help to decide which patients will have benefit from such anti-hypoxia therapy then to monitor response to therapy, especially in breast carcinoma. An easy method of hypoxia detection could be done by assessment of endogenous hypoxia-associated proteins expression in tumor biopsies using immunohistochemistry e.g. hypoxia inducible factor-1 alpha (HIF-1α) and carbonic anhydrase IX (CAIX) [6]. HIF- 1α protein is destroyed and removed within minutes in conditions of normal oxygen concentration, while it is stabilized and up regulated during hypoxia. When it is stabilized, it is translocated to the nucleus, activated and forming active transcription complex. After that it binds to hypoxia response element in promoters of different target genes that could allow increase in oxygen availability and/or increase metabolic adaptation to hypoxia [7]. CAIX is a glycoprotein that is considered a major HIF-1α downstream target; its expression has been related to prognosis in some types of cancer [8]. So it is considered an attractive endogenous marker of detection of hypoxia and evaluating its role in cancer prognosis [9]. Relation between HIF-1α and CAIX expression in cancer cells, the underlying mechanism of actions of both markers and their roles in induction by hypoxia remain unclear. Although several studies have evaluated expression of both markers in many cancers including breast carcinoma but up till now no accurate role has been detected regarding their clinicopathological and prognostic role in breast carcinoma patients and also, there is no previous studies that have studied them both in a large number of Egyptian females [4].
Aim of the Work
Aim of the work was to evaluate HIF-1α and CAIX expressions in breast carcinoma, correlating their expressions with each other, with presence of lymph node and distant metastases, with recurrence free and overall survival rates of female patients with breast cancer.
Patients and Methods
We started our prospective cohort study in July 2014 finished it in July 2017, where we included ninety female patients that are having breast carcinoma that were admitted to general surgery department oncology unit, faculty of medicine Zagazig university, Zagazig Egypt, where mastectomy were surgeons that are sharing in the study performed modified radical mastectomy with axillary clearance for all cases, then sent the biopsies to Pathology Department, Faculty of Medicine, Zagazig University where they are processed and diagnosed as breast carcinoma by routine H and E histopathological examination pathologests from Department of Pathology, Faculty of Medicine, Benghazi University, Benghazi, Libya revise the diagnosis of all slides. Pathologists from Pathology Department, Faculty of Medicine, Zagazig University Zagazig, Egypt and from Department of Pathology, Faculty of Medicine, Benghazi University, Benghazi, Libya used the American Joint Committee on Cancer staging system classification (8th edition) for cancer staging and the Nottingham (Elston–Ellis) modification of the [Scarff. Bloom Richardson] grading system for cancer grading [10,11]. We identified age, tumor size, histopathological subtype, grade, stage of cancer by examination of the patient’s and the slide files of the Pathology Department. ER, PR hormonal receptors and Her2 neu expressions and Ki67 labeling index were evaluated for all cases. All cases are followed up for therapy response, recurrence and survival in clinical oncology and laboratory medicine department, faculty of medicine, Zagazig University. We followed up our patients until death or until the last seen alive with the median follow-up period of 30 month with range from (15-36 month).
The technique of immunohistochemical staining
We used the common technique of streptavidin-biotin immunoperoxidase for staining [12]. We cut sections of five-μm thichness of formalin-fixed, paraffin-embedded tissues blocks prepared from surgically excised breast carcinoma tissue; we placed sections on positively charged slides, de-wax and rehydrate them. We block the activity of endogenous peroxidase; we exposed the sections to heat for antigen retrieval in the autoclave, incubated them overnight with primary mouse monoclonal anti-HIF-1α (Calbiochem, Germany, diluted 1:300), and primary rabbit polyclonal anti-CAIX (Santa Cruz Bioscience, Santa Cruz, CA, USA, diluted 1:100) antibodies at 4°C. We used the chromogen diaminobenzidine substrate (DAB). Lastly we counterstained sections with hematoxylin. We included positive and negative controls of both markers in all cases. We considered sections from cervical squamous cell carcinoma that was positive for HIF-1α, and CAIX as positive control for both markers [13]. And we have omitted the primary antibodies and replaced the by non-immune serum for the negative controls.
Evaluation of immunohistochemical expression of HIF-1α and CAIX
We considered any dark stained nuclei and positive membranous and cytoplasmic stain in>1% of the tumor cells as positive for HIF-1α and CAIX respectively [14].
Results
Ninety females’ patients were included in our study with 49 (54.4%) patients were >55 years. All detailed clinicopathological criteria are included in Table 1.
| Clinicopathological feature | No. (%) | |
|---|---|---|
| Age group | <55y | 41 (45.6%) |
| >55y | 49 (54.4%) | |
| Pathology | IDC (NST) | 70 (77.8%) |
| ILC | 20 (22.2%) | |
| Grade | 1 | 20 (22.2%) |
| 2 | 40 (44.4%) | |
| 3 | 30 (33.3%) | |
| LVI | Absent | 26 (28.9%) |
| Present | 64 (71.1%) | |
| ER | Negative | 42 (46.7%) |
| Positive | 48 (53.3%) | |
| PR | Negative | 42 (46.7%) |
| Positive | 48 (53.3%) | |
| ER/PR | Positive/Positive | 44 (48.9%) |
| Positive/Negative | 4 (4.4%) | |
| Negative/Positive | 4 (4.4%) | |
| Negative/Negative | 38 (42.2%) | |
| HER2 | Negative | 54 (60.0%) |
| Positive | 36 (40.0%) | |
| KI 67 | Low | 31 (34.4%) |
| High | 59 (65.6%) | |
| Molecular | Luminal A | 34 (37.8%) |
| Luminal B | 12 (13.3%) | |
| HER2 amplified | 24 (26.7%) | |
| Triple -ve | 20 (22.2%) | |
| LN | Negative | 26 (28.9%) |
| Positive | 64 (71.1%) | |
| DM | Absent | 69 (76.7%) |
| Present | 21 (23.3%) | |
| T classification | T1 | 19 (21.1%) |
| T2 | 37 (41.1%) | |
| T3 | 21 (23.3%) | |
| T4 | 13 (14.4%) | |
| N classification | N0 | 26 (28.9%) |
| N1 | 18 (20.0%) | |
| N2 | 27 (30.0%) | |
| N3 | 19 (21.1%) | |
| Stage | Stage I | 14 (15.6%) |
| Stage II | 30 (33.3%) | |
| Stage III | 25 (27.8%) | |
| Stage IV | 21 (23.3%) | |
Table 1: The clinicopathological features of our 90 patients.
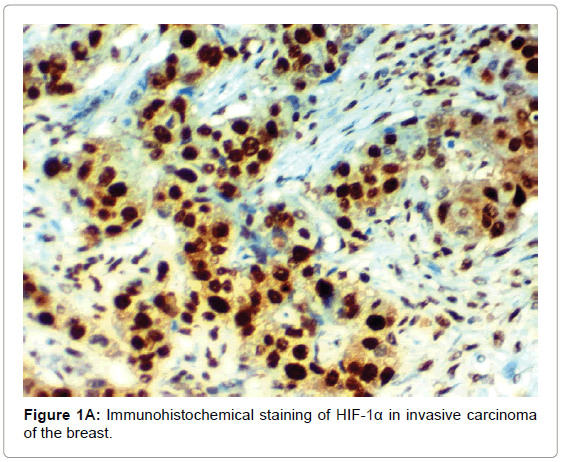
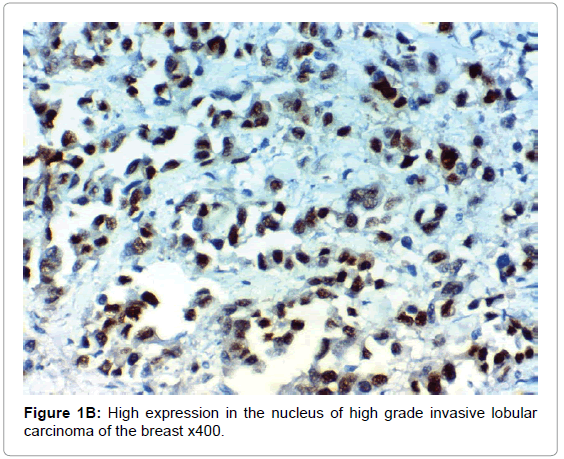
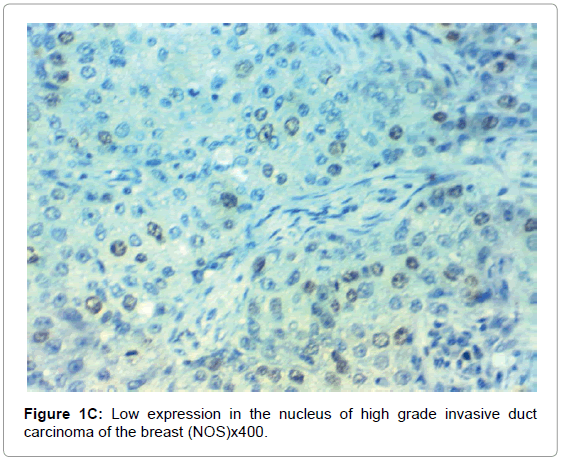
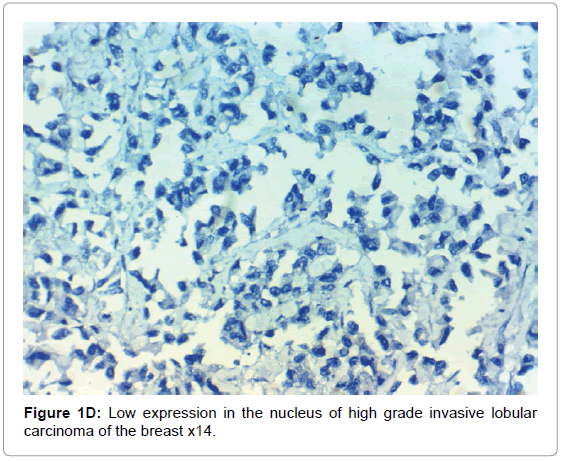
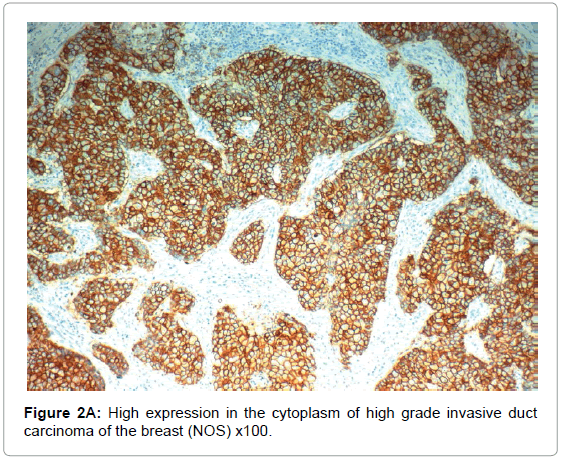
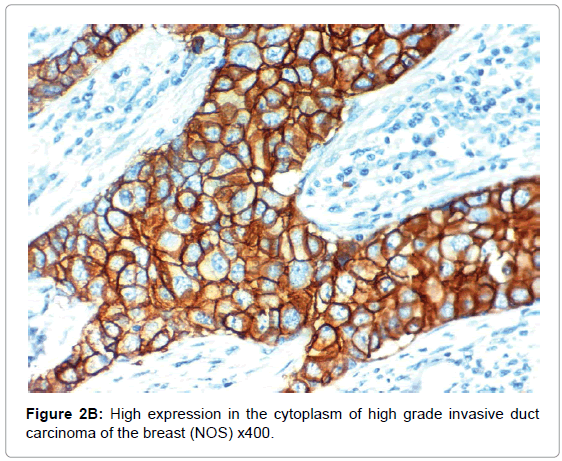
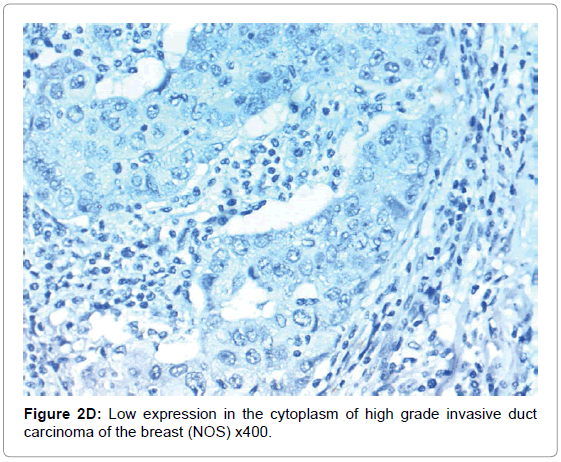
HIF-1α expression, correlation to clinical and histopathological findings, HIF-1α positive expression in breast carcinoma was significantly correlated with older age of the patients, higher grade and advanced stage of the tumor. HIF-1α positive expression was significantly correlated with aggressive molecular subtypes as HER2 amplified and triple negative subtypes, presence of lymph node metastases, high KI67 index (p<0.001 for all of them), presence of distant metastasis (p=0.041), negative ER (p=0.005) and PR (p=0.009), but it had no significant correlation with histopathological subtype of breast cancer (Tables 2, 3 and Figure 1).
| Markers | No. (%) | |
|---|---|---|
| CAIX | Negative | 39 (43.3%) |
| Positive | 51 (56.7%) | |
| HIF-1α | Negative | 32 (35.6%) |
| Positive | 58 (64.4%) | |
| CAIX/HIF-1α | Positive/Positive | 51 (56.7%) |
| Negative/Positive | 7 (7.8%) | |
| Negative/Negative | 32 (35.6%) | |
Table 2: Frequency of HIF-1α and CAIX expressions in our 90 patients.
| CAIX | P | HIF-1α | P | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||
| N=51 | N=39 | N=58 | N=32 | ||||
| Age group | <55y | 13 (25.5%) | 28 (71.8%) | <0.001 | 18 (31.0%) | 23 (71.9%) | <0.001 |
| >55y | 38 (74.5%) | 11 (28.2%) | 40 (69.0%) | 9 (28.1%) | |||
| Pathology | IDC (NST) | 40 (78.4%) | 30 (76.9%) | 0.865 | 45 (77.6%) | 25 (78.1%) | 0.953 |
| ILC | 11 (21.6%) | 9 (23.1%) | 13 (22.4%) | 7 (21.9%) | |||
| Grade | 1 | 8 (15.7%) | 12 (30.8%) | 0.02 | 9 (15.5%) | 11 (34.4%) | 0.001 |
| 2 | 20 (39.2%) | 20 (51.3%) | 22 (37.9%) | 18 (56.3%) | |||
| 3 | 23 (45.1%) | 7 (17.9%) | 27 (46.6%) | 3 (9.4%) | |||
| LVI | Absent | 5 (9.8%) | 21 (53.8%) | <0.001 | 5 (8.6%) | 21 (65.6%) | <0.001 |
| Present | 46 (90.2%) | 18 (46.2%) | 53 (91.4%) | 11 (34.4%) | |||
| ER | Negative | 30 (58.8%) | 12 (30.8%) | 0.008 | 34 (58.6%) | 8 (25.0%) | 0.005 |
| Positive | 21 (41.2%) | 27 (69.2%) | 24 (41.4%) | 24 (75.0%) | |||
| PR | Negative | 29 (56.9%) | 13 (33.3%) | 0.027 | 33 (56.9%) | 9 (28.1%) | 0.009 |
| Positive | 22 (43.1%) | 26 (66.7%) | 25 (43.1%) | 23 (71.9%) | |||
| ER/PR | Positive/Positive | 18 (35.3%) | 26 (66.7%) | 0.017 | 21 (36.2%) | 23 (71.9%) | 0.01 |
| Positive/Negative | 3 (5.9%) | 1 (2.6%) | 3 (5.2%) | 1 (3.1%) | |||
| Negative/Positive | 4 (7.8%) | 0 (0.0%) | 4 (6.9%) | 0 (0.0%) | |||
| Negative/Negative | 26 (51.0%) | 12 (30.8%) | 30 (51.7%) | 8 (25.0%) | |||
| HER2 | Negative | 21 (41.2%) | 33 (84.6%) | <0.001 | 27 (46.6%) | 27 (84.4%) | <0.001 |
| Positive | 30 (58.8%) | 6 (15.4%) | 31 (53.4%) | 5 (15.6%) | |||
| KI 67 | Low | 8 (15.7%) | 23 (59.0%) | <0.001 | 10 (17.2%) | 21 (65.6%) | <0.001 |
| High | 43 (84.3%) | 16 (41.0%) | 48 (82.8%) | 11 (34.4%) | |||
| Molecular | Luminal A | 11 (21.6%) | 23 (59.0%) | <0.001 | 13 (22.4%) | 21 (65.6%) | <0.001 |
| Luminal B | 9 (17.6%) | 3 (7.7%) | 10 (17.2%) | 2 (6.3%) | |||
| HER2 amplified | 21 (41.2%) | 3 (7.7%) | 21 (36.2%) | 3 (9.4%) | |||
| Triple -ve | 10 (19.6%) | 10 (25.6%) | 14 (24.1%) | 6 (18.8%) | |||
| LN | Negative | 5 (9.8%) | 21 (53.8%) | <0.001 | 5 (8.6%) | 21 (65.6%) | <0.001 |
| Positive | 46 (90.2%) | 18 (46.2%) | 53 (91.4%) | 11 (34.4%) | |||
| DM | Absent | 36 (70.6%) | 33 (84.6%) | 0.113 | 41 (70.7%) | 28 (87.5%) | 0.041 |
| Present | 15 (29.4%) | 6 (15.4%) | 17 (29.3%) | 4 (12.5%) | |||
| T | T1 | 3 (5.9%) | 16 (41.0%) | <0.001 | 5 (8.6%) | 14 (43.8%) | <0.001 |
| T2 | 18 (35.3%) | 19 (48.7%) | 22 (37.9%) | 15 (46.9%) | |||
| T3 | 20 (39.2%) | 1 (2.6%) | 21 (36.2%) | 0 (0.0%) | |||
| T4 | 10 (19.6%) | 3 (7.7%) | 10 (17.2%) | 3 (9.4%) | |||
| N | N0 | 5 (9.8%) | 21 (53.8%) | <0.001 | 5 (8.6%) | 21 (65.6%) | <0.001 |
| N1 | 9 (17.6%) | 9 (23.1%) | 11 (19.0%) | 7 (21.9%) | |||
| N2 | 21 (41.2%) | 6 (15.4%) | 26 (44.8%) | 1 (3.1%) | |||
| N3 | 16 (31.4%) | 3 (7.7%) | 16 (27.6%) | 3 (9.4%) | |||
| Stage | Stage I | 0 (0.0%) | 14 (35.9%) | <0.001 | 0 (0.0%) | 14 (43.8%) | <0.001 |
| Stage II | 13 (25.5%) | 17 (43.6%) | 16 (27.6%) | 14 (43.8%) | |||
| Stage III | 23 (45.1%) | 2 (5.1%) | 25 (43.1%) | 0 (0.0%) | |||
| Stage IV | 15 (29.4%) | 6 (15.4%) | 17 (29.3%) | 4 (12.5%) | |||
| HIF-1α | Negative | 0 (0.0%) | 32 (82.1%) | <0.001 | |||
| Positive | 51 (100.0%) | 7 (17.9%) | |||||
Table 3: Association of clinicopathological features with HIF-1α and CAIX expressions in our 90 patients.
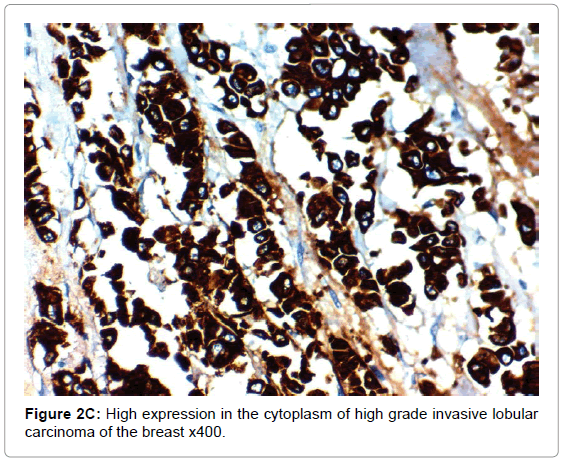
CAIX expression, correlation to clinical and histopathological findings the positive expression of CAIX in breast carcinoma was significantly correlated with older age of the patients, advanced stage of the tumor, aggressive molecular type, presence of lymph node metastases, high KI67 index, aggressive molecular subtypes as HER2 amplified and triple negative subtypes, (p<0.001 for all of them), higher grade (p=0.02) negative ER (p=0.008) and PR (p=0.027) hormonal receptors, But it had no significant correlation with histopathological subtype of breast cancer or presence of distant metastasis. The expression of HIF-1α and CAIX in breast carcinoma was significantly positively correlated with each other (p<0.001) (Figure 2).
Survival analysis
After the follow-up period of 30 months 27.8% of patients died; The 3-year overall survival rate was 74.4% with a mean of 32.6 ± 0.62 months (95% CI; 31.4-33.8 months) while the median OS was not detected. The 3-year RFS rate was 56.1% with a mean of 30.3 ± 0.8 months (95 % CI; 28.7-32.2 months); however the median RFS was not detected. At the end of follow up there was 38.9% of patients [35/90 patients] developed cancer recurrence.
In multi variant analysis LN metastasis is the most significant factor of RFS and OS rates (Tables 4, 5 and Figure 3).
| Variables | 3-year overall survival Rate (%) | p-value | 3-year Recurrence Free survival Rate (%) | p-value | |
|---|---|---|---|---|---|
| Age group | <55y | 78.9% | 0.001 | 69.3% | 0.002 |
| >55y | 54.5% | 46.8% | |||
| Pathology | IDC (NST) | 67.6% | 0.141 | 60.1% | 0.029 |
| ILC | 59.5% | 39.7% | |||
| Grade | 1 | 100.0% | <0.001 | 90.0% | <0.001 |
| 2 | 45.7% | 56.6% | |||
| 3 | 47.5% | 25.3% | |||
| LVI | Absent | 78.5% | 0.032 | 80.2% | 0.001 |
| Present | 59.4% | 44.8% | |||
| ER | Negative | 54.3% | 0.004 | 38.9% | <0.001 |
| Positive | 74.7% | 70.9% | |||
| PR | Negative | 57.6% | 0.010 | 40.7% | <0.001 |
| Positive | 74.3% | 70.0% | |||
| ER/PR | Positive/Positive | 73.8% | 0.016 | 68.3% | <0.001 |
| Positive/Negative | 100.0% | 100.0% | |||
| Negative/Positive | 100.0% | 100.0% | |||
| Negative/Negative | 52.2% | 32.8% | |||
| HER2 | Negative | 70.2% | 0.039 | 61.1% | 0.027 |
| Positive | 60.7% | 50.1% | |||
| KI 67 | Low | 69.8% | 0.135 | 63.5% | 0.094 |
| High | 64.8% | 54.4% | |||
| Molecular | Luminal A | 69.6% | <0.001 | 62.7% | <0.001 |
| Luminal B | 100.0% | 100.0% | |||
| HER2 amplified | 33.5% | 20.8% | |||
| Triple -ve | 71.6% | 59.0% | |||
| LN | Negative | 78.5% | 0.032 | 80.2% | 0.001 |
| Positive | 59.4% | 44.8% | |||
| DM | Absent | 73.9% | <0.001 | 69.9% | <0.001 |
| Present | 36.1% | 0.0% | |||
| T | T1 | 88.9% | <0.001 | 83.3% | <0.001 |
| T2 | 70.1% | 70.5% | |||
| T3 | 45.4% | 25.9% | |||
| T4 | 35.2% | 0.0% | |||
| N | N0 | 78.5% | <0.001 | 80.2% | <0.001 |
| N1 | 74.7% | 68.8% | |||
| N2 | 69.9% | 44.1% | |||
| N3 | 25.9% | 23.7% | |||
| Stage | Stage I | 85.7% | <0.001 | 85.7% | <0.001 |
| Stage II | 72.8% | 76.7% | |||
| Stage III | 68.6% | 44.8% | |||
| Stage IV | 36.1% | 0.0% | |||
| CAIX | Negative | 70.6% | 0.043 | 69.6% | 0.005 |
| Positive | 60.5% | 45.5% | |||
| HIF-1α | Negative | 74.5% | 0.037 | 72.5% | 0.007 |
| Positive | 60.1% | 46.2% | |||
| CAIX/HIF-1α | Positive/Positive | 60.5% | 0.102 | 45.5% | 0.016 |
| Negative/Positive | 40.0% | 40.0% | |||
| Negative/Negative | 74.5% | 72.5% | |||
Table 4: Univariate analysis of overall and Recurrence-Free Survival in relation to clinicopathological parameters of our 90 patients.
| Variables | 3 Years RFS | 3 Years OS | ||
|---|---|---|---|---|
| HR (95% CI) | Sig. | HR (95% CI) | Sig | |
| Age >55y | 1.2 (0.3-5.03) | 0.770 | 3.7 (0.68-20.06) | 0.130 |
| Pathology | 1.1 (0.42-2.8) | 0.870 | 0.7 (0.23-1.89) | 0.430 |
| Grade | 2.7 (1.17-6.2) | 0.020 | 6.4 (1.98-20.76) | <0.001 |
| LVI | 0.1 (0.01-1.15) | 0.060 | 0.01 (0.001-0.33) | 0.010 |
| ER | 2.1 (0.03-154.62) | 0.740 | 34.6 (0.39-3081.22) | 0.120 |
| PR | 0.1 (0.01-0.94) | 0.040 | 0.2 (0.01-2.23) | 0.180 |
| HER2 | 0.3 (0.06-1.37) | 0.120 | 0.2 (0.03-1.07) | 0.060 |
| KI67 | 0.1 (0.01-0.85) | 0.040 | 0.1 (0.01-2.62) | 0.180 |
| Molecular | 0.7 (0.14-3.81) | 0.710 | 1.2 (0.28-5.39) | 0.790 |
| DM | 2.3 (0.29-17.67) | 0.430 | 2.0 (0.2-19.1) | 0.570 |
| T | 2.1 (0.74-6.04) | 0.160 | 1.7 (0.55-5.41) | 0.350 |
| N | 10.6 (2.32-47.88) | <0.001 | 56.3 (5.52-574.02) | <0.001 |
| Stage | 0.4 (0.07-2.69) | 0.360 | 0.1 (0.02-1.11) | 0.060 |
| CAIX | 0.9 (0.17-5.01) | 0.920 | 0.3 (0.05-2.17) | 0.240 |
| HIF1α | 3.1 (0.47-20.54) | 0.240 | 6.5 (0.65-65.63) | 0.110 |
HR: Hazard Ratio; 95%CI: 95% Confidence Interval, p<0.05 is significant. OS: Overall Survival, RFS: Recurrence-Free Survival.
Table 5: Multivariate analysis of overall and Recurrence-Free Survival in relation to clinicopathological parameters of our 90 patients.
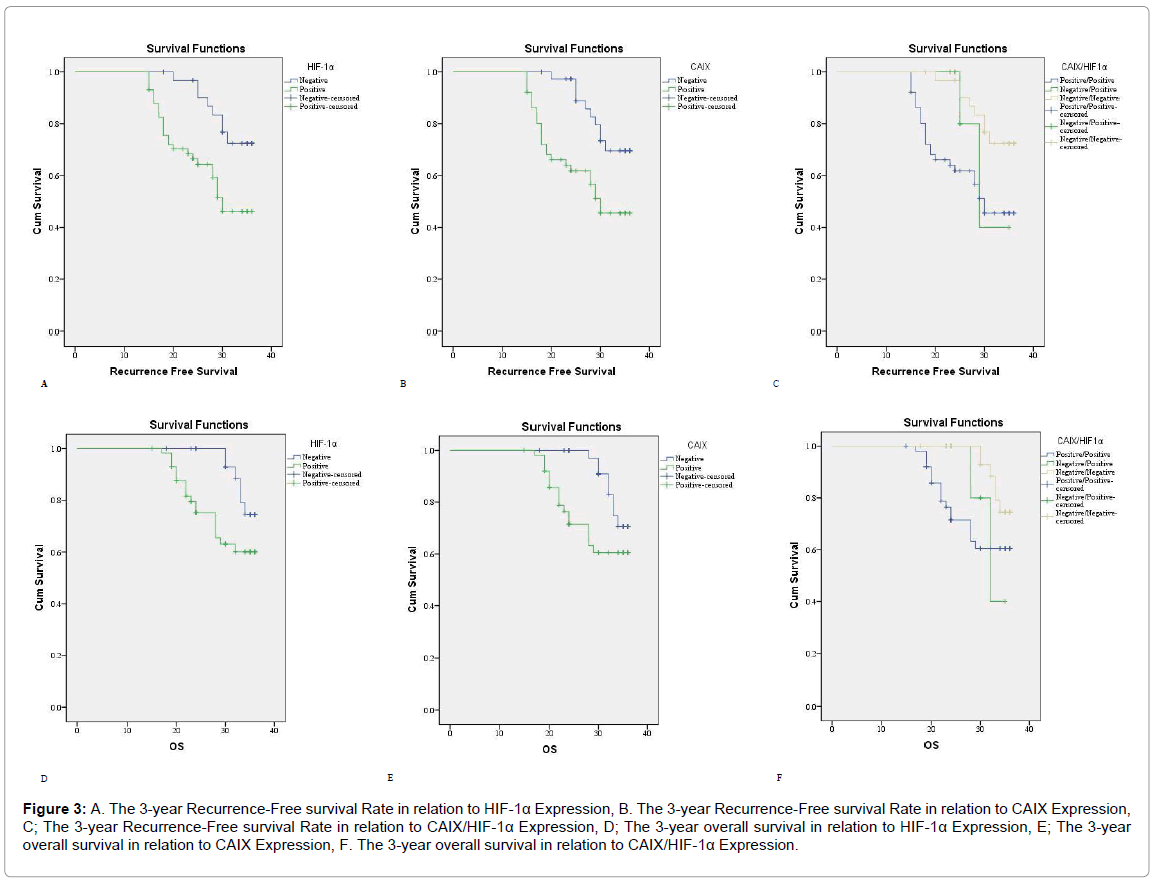
Figure 3: A. The 3-year Recurrence-Free survival Rate in relation to HIF-1α Expression, B. The 3-year Recurrence-Free survival Rate in relation to CAIX Expression, C; The 3-year Recurrence-Free survival Rate in relation to CAIX/HIF-1α Expression, D; The 3-year overall survival in relation to HIF-1α Expression, E; The 3-year overall survival in relation to CAIX Expression, F. The 3-year overall survival in relation to CAIX/HIF-1α Expression.
Progression follow-up and survival results in correlation to HIF-1α and CAIX expression
Cases with positive HIF-1α and CAIX expression had a higher rate of carcinoma recurrence (p<0.001). In univariant analysis patients with positive HIF-1α and CAIX expression had poor RFS and 3 year OS rates (p=0.007). We found a significant correlations between HIF-1α and CAIX positive expressions in carcinoma of the breast (p<0.001) (Table 4 and Figure 3).
Discussion
Former researchers had explored the role of HIF-1α is involved in breast carcinogenesis [15], and detected that it could influence its growth rate and metastatic ability and subsequently could be associated with poor patient prognosis [16], but proved results are still conflicting and lacking accurate sharp data. Our present results detected that when HIF-1α positively expressed in breast carcinoma that will be significantly related to worse clinic pathological findings like older age of the patients, higher grade and advanced stage of the tumor, aggressive molecular subtypes, presence of LN and distant metastasis, also we found that cases with positive HIF-1α expression had a higher rate of carcinoma recurrence, poor RFS and 3 year OS rates. Our results were near results of former research that was done by Lee et al. [17] evaluate the expression of HIF-1α in relation to markers of angiogenesis and other clinicopathological criteria in a cohort of breast cancer from Africa and they detected that positive HIF-1α expression was associated with increased tumor angiogenesis, high cancer cell proliferation rate that was evidenced by increased Ki-67 labeling index, high cancer grade that points to HIF-1α as a poor prognostic marker of breast carcinoma and a therapeutic target for breast cancer patients. We detected an association between HIF-1α expression and the presence of LN and distant metastasis in breast carcinoma; our results were near results of Lakhani et al. [16]. Who had proved that proved that HIF-1α is a regulator of cell hypoxia response and focused on HIF-1α role in increasing breast carcinoma etastasis, as they explored that HIF-1 had several roles in metastasis, e.g. increasing malignant cells invasion, upregulating epithelial-mesenchymal transition (EMT), and formation of metastatic niche [16]. Also discuss the values of therapeutic benefits of targeting the HIF-1α for management of breast cancer patients that is considered a recent therapeutic approach that could be used in combination with currently used therapies [4]. HIF-1α protein expression is present in malignant tumors of many organs and that is associated with poor prognosis of carcinoma of cervix, endometrium and ovary. They stated that HIFs had many roles in cancer cells growth, proliferation, differentiation, angiogenesis, cancer cell metabolism, local invasion, lymph nodes and distant metastasis. Subsequently, HIFs could be responsible for to chemo- and radiotherapy resistance, so they are associated with poor prognosis of cancer patients. In addition HIF-1α could increase the expression of PD-L1 that is an immune checkpoint protein, which could be responsible for immune suppression [18].
But Ward et al. [4] stated that the prognostic value of HIF-1α in breast carcinoma patients has conflicting results in many follow-up studies [19], showed results similar to ours that there is strong association between HIF-1α expression and presence of distant metastases in breast carcinoma, and also HIF-1α increased the extravasation of breast carcinoma cells in the lung, that was explained by Noman et al. [20] by the ability of HIF-1 in regulation of metastatic niche formation at distant sites before cancer cell arrival. Solid malignant tumors, like carcinoma of the breast contain hypoxic areas due to presence of vascularization defects in these rapidly growing cancer cells. HIF-1α plays an essential role in cancer cells adaptation to hypoxia by increasing transcription of many genes that could regulate angiogenesis, proliferation, invasion, and metastasis [21]. Another mechanism by which HIF-1α can act is by up-regulating epithelialmesenchymal transition (EMT) process which is essential for tumor progression. EMT could be stimulated by hypoxia, by many mechanisms like HIF-1α pathways in several human malignancies. HIF-1α induce EMT by up-regulation of EMT transcription factors e.g. Twist, Snail, Slug and Zeb in many cancer types [22]. In addition, hypoxia is an angiogenesis stimulating agent via production of many HIF-1 transcription factors, moreover during the EMT process HIF-1α stimulated angiogenesis by up-regulating VEGF transcription, and associated with micro vessel growth which is an evidence of activated angiogenesis [4]. As we found that increased positive HIF-1α expression shows strong association with poor outcome and dismal survival rates of breast carcinoma patients, so that hypoxia is considered a hallmark of aggressive behavior of many solid tumors and responsible for metastases and therapy resistance, so it is considered a cancer attractive therapeutic targets like the recently discovered HIF-1α inhibitors [16]. Targeting hypoxic cancer cells have been explored by many approaches e.g. hypoxia-activated prodrugs, and HIF-1α specific targeting [21]. HIF-1α inhibitors, like digoxin and acriflavine, had potential therapeutic roles in decreasing cancer growth, invasion, metastasis and vascularization in breast cancer [20]. HIF-1α targeting is considered as a novel therapeutic modality for management of breast cancer patients and improving their prognosis which could be used in combination with currently used therapies. Many researchers have studied CAIX expression in a plethora of human malignancies and stated that it was associated with poor patient’s outcome, but its role in breast carcinoma patients still needs further clarifications [23]. Here we proved that positive CAIX expression in breast carcinoma tissues was correlated related to worse clinic pathological findings like older age of the patients, higher grade and advanced stage of the tumor, aggressive molecular subtypes, presence of LN metastasis, also we found that cases with positive CAIX expression had a higher rate of carcinoma recurrence, poor RFS and 3 year OS rates [24]. Also found nearly the same, that CAIX expression was related to aggressive pathological phenotype, chemotherapy resistance and poor prognosis of patients with breast cancer. We proved that the positive expression of CAIX in breast carcinoma was related to the presence of LN metastases, that was like results of Sobhanifar et al. [25] who proved the same results, and results of Sugie et al. [26] who found that positive CAIX expression was strongly correlated with sentinel LN metastasis in addition to invasion of lymphatic vessels by the primary tumors, also many previous studies proved results similar to us [25], breast cancer patients with positive expression of CAIX had lower pathologic complete response (PCR) rates when treated with neoadjuvant chemo-therapy. We proved that the positive expression of CAIX in breast carcinoma was correlated to larger tumor size, higher tumor grade, stage and aggressive molecular type similar to our results detected upregulation of CAIX expression in breast carcinoma patients with advanced stages [27]. And results of Supuran and Thiry et al. [28,29] found a positive correlation between CAIX expression, aggressive phenotype of breast carcinoma and poor patient prognosis. In our study we found that patients with high CAIX expression had shorter RFS and 3 year OS rates. Similar to our results [30], observed that that positive CAIX expression was related to chemo-resistance and shorter survival rates in breast cancer patients. Thus, these data suggest that CAIX is a predictive and a prognostic marker for breast cancer patients. Different from our results, [29] found no association between CAIX expression in breast cancer tissues and patients’ nodal status that could be explained by different number of patients, variable technique of staining and different antibody clone which gives different results. There are multiple theories which could explain the association between positive CAIX expression and poor patients’ outcome in breast cancer. That, CAIX expression is linked to cancer tissue hypoxia and acidosis and its upregulation is a step in cancer cells adaptation to survive under hypoxic conditions [29]. Also, CAIX is related to cancer hypoxia and stimulates cancer cell spread and invasion, which incriminated tumor hypoxia to increase cancer cells invasion and metastasis. Tumors with upregulation of CAIX could be able to maintain their intracellular but it increased acidification in extracellular space, which leads to extracellular matrix breakdown which could increase malignant cells invasive ability [29,31], in addition increased hypoxia in the malignant cells leads to genome instability. Also, CAIX could influence breast cancer stem cells growth and survival under hypoxic conditions [32] That association of CAIX positive expression with aggressive clinicopathological and prognostic parameters of breast cancer patients that proved by our results and results of previous studies, could support the theory that discovering selective CAIX inhibitors could be used to manage cancer patients and improving their prognosis, moreover some of such inhibitors are in the preclinical setting and still under evaluation [33,34]. We found positive correlation between HIF-1α and CAIX expression in breast cancer tissue, that was similar to results of [35] different from [29,30]. Who found no an association between both markers expression, which could be explained by that they have done their studies on tissue microarray that allow analyses of results based on only minute tissue samples and their tissue sections were acquired from only non-necrotic areas [29]. Explained the absence of association between both markers expression in their study by different half-lives of HIF-1α and CAIX, as HIF-1α was found to be rapidly destroyed and removed within minutes of reoxygenation [36]. While, CAIX has a longer half-life of 2-3 days [37], so they stated that they could not be present together [38]. found positive correlation between HIF-1α and CAIX expression in breast carcinoma tissue, which was similar to ours but they detected CAIX expression only without HIF-1α expression in peri-necrotic regions in cancers, which is also due to differences in half-lives of HIF-1α and CAIX [30]. Summary, conclusions and future suggestionshif-1 and its downstream target HIF-1α are considered regulators of cancer cell response to hypoxic stress and play important roles in breast carcinoma cells growth, invasion and metastasis. Both markers, mainly HIF-1α, is involved in the key step of the metastatic process e.g. EMT, malignant cell invasion, and metastatic niche formation. As we demonstrated that breast carcinogenesis is stimulated by cells adaptation to hypoxia and acidosis, moreover the glycolytic, acid-resistant phenotype that has HIF-1α and CAIX positive expression is an aggressive phenotype. Hence it will be better that tumor management strategies should aim at antagonizing the sequence of hypoxia, glycolysis and cidosis. Moreover, identification of the metabolic phenotype of breast carcinoma will allow discovering to novel therapeutic modalities. Also, the aggressive triple negative molecular subtype that is difficult to treat, as they are both chemo-resistant and hormonal non-responsive, and as we detected that such subtype showed positive expression of both HIF-1α and its downstream target CAIX, so targeting them both e.g. targeting HIF-1α with its inhibitors, gene therapies and CAIX inhibitors could be of particular importance in managing this aggressive cancer and improving patients prognosis [9]. The combination of HIF-1α and CAIX inhibitors with existing therapeutic modalities might be found to be useful clinically. Clinical therapeutic trials are needed to determine if they could increase the survival of patients having breast cancer alone or in addition to currently used therapies. Future studies are needed to discover more specific HIF-1α and CAIX inhibitors, to study their detailed mechanism of action, and to include them in clinical therapeutic trials of breast cancer patients.
References
- Aomatsu N, Yashiro M, Kashiwagi S, Kawajiri H, Takashima T, et al. (2014) Carbonic anhydrase 9 is associated with chemosensitivity and prognosis in breast cancer patients treated with taxane and anthracycline. BMC Cancer 14: 400.
- Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, et al. (2006) CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res 12: 6421-6431.
- Bussink J, Kaanders JH, van der Kogel AJ (2003) Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol 67: 3-15.
- Ward C, Langdon SP, Mullen P, Harris AL, Harrison DJ, et al. (2013) New strategies for targeting the hypoxic tumour microenvironment in breast cancer, cancer Review Treatment 39: 171-179.
- Chen CL, Chu JS, Su WC, Huang SC, Lee WY (2010) Hypoxia and metabolic phenotypes during breast carcinogenesis: expression of HIF-1alpha, GLUT1, and CAIX, virchows archiv 457: 53-61.
- Schutze D, Milde-Langosch K, Witzel I, Rody A, Karn T, et al. (2013) Relevance of cellular and serum carbonic anhydrase IX in primary breast cancer, Journal of Cancer Research and Clinical Oncology 139: 747-754.
- Tan EY, Yan M, Campo L, Han C, Takano E, et al. (2009) The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy, British Journal of Cancer, 100: 405-411.
- Elston CW, Ellis IO (2002) Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19: 403-410.
- Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, et al. (2013) Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche, Oncogene 32: 5210-5219.
- Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, et al. (2017) Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67: 290-303.
- Glaberman UB, Marron M, Chalasani P, Livingston R, Iannone M, et al. (2016) Circulating Carbonic Anhydrase IX and Antiangiogenic Therapy in Breast Cancer Disease Markers. 7 pages
- Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577-580.
- Jiang BH, Semenza GL, Bauer C, Marti HH (1996) Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 271: C1172-C1180.
- Eom KY, Jang MH, Park SY, Kang EY, Kim SW, et al. (2016) The Expression of Carbonic Anhydrase (CA) IX/XII and Lymph Node Metastasis in Early Breast Cancer Cancer Res Treat 48: 125-132.
- Kronblad A, Jirstrom K, Ryden L, Nordenskjold B, Landberg G (2006) Hypoxia inducible factor-1alpha is a prognostic marker in premenopausal patients with intermediate to highly differentiated breast cancer but not a predictive marker for tamoxifen response. Int J Cancer 118: 2609-2616.
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ (2012) WHO Classification of Tumours of the Breast.
- Lee WY, Huang SC, Hsu KF, Tzeng CC, Shen WL (2008) Roles for hypoxia-regulated genes during cervical carcinogenesis: somatic evolution during the hypoxia-glycolysis-acidosis sequence. Gynecol Oncol 108: 377-384.
- Liu Z-J Semenza GL, Zhang H-F (2015) Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B 16: 32-43.
- Nalwoga H, Ahmed L, Arnes JB, Wabinga H, Akslen LA (2016) Strong Expression of Hypoxia-Inducible Factor-1α (HIF-1α) Is Associated with Axl Expression and Features of Aggressive Tumors in African Breast Cancer PLOS ONE.
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, et al. (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211: 781-790.
- McDonald PC, Winum JY, Supuran CT, Dedhar S (2012) Recent developments in targeting carbonic anhydrase IX for cancer therapeutics, Oncotarget 3: 84-97.
- Rafajova M, Zatovicova M, Kettmann R, Pastorek J, Pastorekova S (2004) Induction by hypoxia combined with low glucose or low bicarbonate and high posttranslational stability upon reoxygenation contribute to carbonic anhydrase IX expression in cancer cells. Int J Oncol 24: 995-1004.
- Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29: 625-634.
- Semenza GL (2012) Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 33: 207-214.
- Sobhanifar S, Aquino-Parsons C, Stanbridge EJ, Olive P (2005) Reduced expression of hypoxia-inducible factor-1alpha in perinecrotic regions of solid tumors. Cancer Res 65: 7259-7266.
- Sugie T, Sawada T, Tagaya N, Kinoshita T, Yamagami K, et al. (2013) Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol 20: 2213-2218.
- Sun JD, Liu Q, Wang J, Ahluwalia D, Ferraro D, et al. (2012) Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res 18: 758-770.
- Supuran CT (2008) Development of small molecule carbonic anhydrase IX inhibitors. BJU Int 101: 39-40.
- Thiry A, Dogne JM, Masereel B, Supuran CT (2006) Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 27: 566-573.
- Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, et al. (2007) HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer 120: 1451-1458.
- Müller V, Riethdorf S, Rack B, Janni W, Fasching PA, et al. (2011) Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IXin correlation to circulating tumor cells in patients with metastatic breast cancer, Breast Cancer Research 13: 4-71.
- Wigerup C, PÃ¥hlman S, Bexell D (2016) Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer Pharmacology & Therapeutics 152-169.
- Wojtkowiak JW, Cornnell HC, Matsumoto S, Saito K, Takakusagi Y, et al. (2015) Pyruvate sensitizes pancreatic tumors to hypoxia-activated prodrug TH-302. Cancer Metabol 3: 2.
- Wong CC, Zhang H, Gilkes DM, Chen J, Wei H, et al. (2012) Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med 90: 803-815.
- Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, et al. (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors, Cancer Research 71: 3364-3376.
- Zatovicova M, Jelenska L, Hulikova A, Csaderova L, Ditte Z, et al. (2010) Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr Pharm Des 16: 3255-3263.
- Zhang W, Shi X, Peng Y, Wu M, Zhang P, et al. (2015) HIF-1alpha Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS One.
- Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, et al. (2012) HIF-1-dependent expression of angiopoietin-like 4 and L1CAMmediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene 31: 1757-1770.
Citation: Harb OA, Ibtesam E, Amal MAA, Warda MS, Sharifa A, et al. (2017) Prognostic Value of the Expression of Endogenous Hypoxia Associated Proteins Hypoxia Inducible Factor-1 Alpha (HIF-1α) and Carbonic Anhydrase Isoform 9 (CAIX) Expressions in Breast Carcinoma. J Oncol Res Treat 2: 116.
Copyright: © 2017 Harb OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 5947
- [From(publication date): 0-2017 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 4946
- PDF downloads: 1001