Protective Effect of Ginger (Zingiber officinale) on Alzheimer's disease Induced in Rats
Received: 11-Mar-2014 / Accepted Date: 04-Jun-2014 / Published Date: 12-Jun-2014
Abstract
The possible protective effect of Ginger on Alzheimer’s disease induced in rats was investigated. Ninety rats were used as follows: control group, AD protective group using AlCl3, 3rd, 4th, and 5th groups rats were received orally Rivastigmine, Ginger (108 and 216 mg/kg/day) respectively, for two weeks followed by combination of each treatment for another 4 weeks. 6th group is a therapeutic AD group, while 7th, 8th & 9th groups are AD rats treated with the same doses of Rivastigmine and Ginger for 12 weeks. At baseline and after each treatment, behavioral stress tests, Rotarod and T-Maze tests were done. At the end of all experiments rats' brains were dissected and prepared for determination of acetylcholine (Ach), acetycholinesterase (AchE) levels and histopathologic examination. This study indicated that AD-induced rats exhibited reduction in behavior, Rotarod and T-Maze tests, reduction in brain Ach and increase AchE levels. While rats treated with Rivastigmine and Ginger in protective and therapeutic groups exhibited significant improvement in behavior, Rotarod and T-Maze, significant increase in brain Ach and decrease AchE levels. These results were consistent with the histopathological findings and revealed Rivastagmine, and Ginger ameliorates neurodegeneration characters of Alzheimer’s diseases in rats.
Keywords: Alzheimer’s disease; Aluminum chloride; Ginger; Therapeutic; Protective; Rats
411779Introduction
Alzheimer's disease (AD), which represents one of the most economically costly diseases to the society is a neurodegenerative disorder characterized by progressive degeneration of hippocampal and cortical neurons that leads to impairment of memory and cognitive ability. Impairment of short-term memory is usually the first clinical feature, whereas retrieval of distant memories is preserved relatively well into the course of the disease. When the condition progresses, additional cognitive abilities are impaired, as the ability to calculate, and use common objects and tools. The pathological hallmarks of AD are senile plaques, which are spherical accumulations of the protein ß-amyloid accompanied by degenerating neuronal processes, and neurofibrillary tangles, composed of paired helical filaments and other proteins. This corresponds to the clinical features of marked impairment of memory and abstract reasoning, with preservation of vision and movement [1].
The selective deficiency of acetylcholine in AD, has given rise to the "cholinergic hypothesis," which proposes that a deficiency of acetylcholine is critical in the genesis of the symptoms of AD [2]. Therefore a major approach to the treatment of AD has involved attempts to augment the cholinergic function of the brain. This involves the use of inhibitors of acetyl cholinesterase as tacrine, donepezil, rivastigmine, and galantamine [3]. Also other hypotheses state that inflammation plays a key role in the pathogenesis of AD. In addition excessive reactive oxygen species (ROS) levels are implicated in the aetiology of AD [4].
Medicinal plants have been traditionally used in the treatment of several human diseases and their pharmacological and therapeutic properties have been attributed to different chemical constituents isolated from their crude extracts. Of particular importance, chemical constituents with antioxidant activity can be found at high concentrations in plants and can be responsible for their preventive effects in various degenerative diseases; including cancer, neurological and cardiovascular diseases [5]. Thus, the antioxidant properties of plants have a full range of perspective applications in human healthcare [6].
Ginger has been listed in “Generally Recognized as Safe” (GRAS) document of the United States Food and Drug Administration (FDA) [7]. Ginger extract can prevent the increase in cholesterol levels following intake of cholesterol-rich diet by rats and rabbits thus protects against atherosclerosis, therefore, ginger acts as a hypolipidemic factor. Ginger also inhibits platelet aggregation. It has anti-oxidative properties and scavenges superoxide anion and hydroxyl radicals due to its high content of gingerol which is a polyphenolic compound. Ginger also has anti-inflammatory properties due to inhibition of prostaglandin and leukotriene biosynthesis owing to its content of Gingerols and diarylhepatanoids [8].
The purpose of this experimental work was to investigate the possible prophylactic and curative effects of aqueous infusion of Ginger (Zingiber officinale), in Alzheimer’s disease induced in rats by using AlCl3.
Materials and methods
Materials
Aluminium Chloride (AlCl3) with molecular weight (M.wt) 133.34 was purchased from Sigma-Aldrich Co., Munich, Germany, Rivastigmine 0.3 mg, was purchased from Novartis Co., Cairo, Egypt, while Ginger powder was purchased from Mepaco (Arab Company for Pharmaceutical and Medicinal plant) , .
Animals
The present study was conducted on 90 adult male Wistar rats weighing from 150 to 200 gm obtained from the Animal House Colony of the National Research Centre, , . The animals were maintained on standard laboratory diet and water ad libitum. After an acclimation period of one week, the animals were housed in stainless steel cages in a temperature controlled (23 ± 1ºC) and artificially illuminated (12 h dark/light cycle) room free from any source of chemical contamination. All animals received human care and use according to the guide lines for Animal Experiments which were approved by the Ethical Committee of Medical Research, National Research Centre, Egypt.
Preparation of aqueous infusion of ginger
Fifty ml of boiling distilled water was poured on 1250 mg of the plant powder in a beaker. The mixture was allowed to stand for 30 minutes before it was filtered with a filter paper. An equivalent of extract from 25 mg dried plant material per ml aqueous infusion was obtained. The dose was determined according to Ajith et al.
Experimental design
The animals used were classified into nine groups (10 rats each) as follows:
1st group: normal control rats were given 1ml tab water orally daily throughout the experiment.
Protective study groups
2nd group (positive control AD-group): induction of animal model mimicking AD by using AlCl3 orally in a dose of 17 mg/kg b.wt daily for 4 successive weeks was done according to Krasovskii et al [9].
3rd group: rats given rivastigmine aqueous infusion orally in a dose of 0.3 mg/kg b.wt/day according to Carageorgious et al. [10] for two successive weeks followed by combination of Rivastigmine and AlCl3 for four successive weeks.
4th group: rats given ginger aqueous infusion orally in a dose of 108 mg/kg b.wt /day for two successive weeks followed by combination of ginger and AlCl3 for four successive weeks.
5th group: rats given ginger aqueous infusion orally in a dose of 216 mg/kg b.wt /day for two successive weeks followed by combination of ginger and AlCl3 for four successive weeks.
Therapeutic study groups
6th: (positive control AD group): as in 2nd group.
7th group: AD-induced rats treated daily for 12 weeks with rivastigmine orally in a dose of 0.3 mg/kg b.wt/day according to Carageorgious et al. [10]
8th group: AD-induced rats treated daily with ginger aqueous infusion orally for 12 weeks, in a dose of 108 mg/kg b.wt/day.
9th group: AD-induced rats treated daily with ginger aqueous infusion orally for 12 weeks, in a dose of 216 mg/kg b.wt/day.
Assessment of psychological state using the Grid floor Activity Cage test:
Activity was measured by detecting rat movements by using grid floor activity cage (Ugo Basile, Varese, Italy, Model 7430) according to Pavic et al. [11].
Assessment of motor coordination using the Accelerating speed rotarod test:
Motor coordination in this study was assessed by using the accelerating speed rotarod (Ugo Basile, Varese, Italy, Model 7750) according to Vijitruth et al. [12].
Assessment of cognitive abilities using Rewarded T-Maze test:
A cognitive ability of rats in this study was assessed by using the rewarded T-Maze test (locally constructed in the National Research Centre, Giza, Egypt) according to Deacon and Rawlins [13].
Brain tissue sampling and preparation
At the end of each experimental period, the animals were kept fasting for 12 hours and sacrificed by decapitation, then the whole brain of each animal was rapidly dissected, thoroughly washed with isotonic saline, dried and weighed. Then each brain was sagitally divided into two portions. The first portion of each brain was homogenized immediately to give 10% (w/v) homogenate in ice-cold medium containing 50 mM Tris-Hcl (pH 7.4) and 300 mM sucrose [14]. The homogenate was centrifuged at 3000 rpm for 10 min at 4ºC. The supernatant (10%) was separated for biochemical analysis (Ach, AchE and total protein). The second portion of each brain was fixed in formaline buffer (10%) for histopathological examination.
Biochemical analyses
Brain Acetylcholine (Ach) and Acetyl cholinesterase (AChE) levels were determined using quantification ELISA kits purchased from BioVision Co, California, USA, according to the method of Engvall and Perlman [15]. Quantitative estimation of total protein level in the brain homogenate was carried out according to the method of Lowry et al. [16].
Histopathological examination
The second portion of each brain was fixed in formaline buffer (10%) for 24 hours. Washing was done in tap water then serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56ºC in hot air oven for 24 hours. Paraffin bees wax tissue blocks were prepared for sectioning at 4 microns by microtome. The obtained tissue sections were collected on glass slides, deparaffinized and stained by hematoxylin and eosin stains [17] for histopathological examination using the light microscope.
Statistical Analysis
In activity cage and rotarod tests, a percent (%) of change of behaviour was calculated, it was considered 100% for normal rats, then square root transformed % was calculated according to Jones et al. [18] and considered to be 1 for normal rats, these calculations were done in order to avoid normal biological variations in activity of normal rats in all groups (provided that each group contains rats with approximately similar activity). All values were presented as means ± standard error (mean ± S.E).Comparison of square root transformed percent of more than two different groups was carried out using the non-parametric one-way analysis of variance (ANOVA) followed by Dunn's multiple comparisons test. All values of T-maze test were presented as mean of seconds± S.E. Also values of the biochemical parameters were presented as means of levels in brain homogenates± S.E, then comparison between more than two different groups was carried out using the ANOVA followed by Tukey–Kramer multiple comparisons test. Difference was considered significant at P<0.05.
Results
Protective study (Tables 1-4)
Grid floor activity cage:
The results obtained in Table 1 exhibited significant reduction in activity (denoting deteriorated psychological state) of group receiving AlCl3 for 4 weeks (positive control AD-group), in comparison with baseline (before administration of AlCl3) of the same group. While groups treated with rivastigmine (0.3 mg/kg b.wt/day), or ginger aqueous infusions (108 and 216 mg/kg b.wt/day), exhibited a significant increase in activity (denoting improved psychological state), after 2 weeks of administration of rivastigmine or ginger aqueous infusions alone and after 4 weeks of administration of both rivastigmine (0.3 mg/kg b.wt/ day) or ginger aqueous infusion (216 mg/kg b.wt/day) in combination with AlCl3 when compared to positive control AD-group of rats, but the group that received ginger aqueous infusion (108 mg/kgb.wt/day) in combination with AlCl3 exhibited a significant reduction in activity compared to the baseline of the same group.
| Time Duration Group | Baseline, (0 weeks) | 2 weeks Pre-treatment | 2 weeks pre-treatment, then 4 weeks treatment with AlCl3 |
|---|---|---|---|
| Control | 100* | 98.9+2.3 | 97.2+1.8 |
| 1** | 0.99+0.06b | 0.98+0.05b | |
| AD-group AlCl3 (17 g/kg) | 100* | 51.7+ 6.72 | |
| 1** | 0.71+0.04a | ||
| Rivastigmine (0.3 mg/kg) | 100* | 95.11+1.57 | 93.44+1.55 |
| 1** | 0.97+0.008b | 0.96+0.08b | |
| Ginger (108 mg/kg) | 100* | 81.46+3.22 | 66.49+3.99 |
| 1** | 0.9+0.01b | 0.81+0.02a | |
| Ginger (216 mg/kg) | 100* | 96.8+1.9 | 86.65+6.96 |
| 1** | 0.98+0.01b | 0.92+0.03b |
Table 1: Evaluation of protective effects of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) using grid floor activity cage in AD-disease induced in rats by AlCl3. All data are expressed as Means of movements +SEM. *: % change, **: Square root transformed % change: (a) Significantly different from baseline of the same group at P < 0.05. (b) Significantly different from AD group at P<0.05.
Accelerating speed rotarod
The results obtained in Table 2 exhibited significant reduction in duration of balance on the rotaroad (denoting deteriorated motor coordination), for positive control AD-group and the group treated with ginger aqueous infusion (108 mg/kg b.wt/ day) in combination with AlCl3 in comparison to the baseline of the same group. While all the other groups exhibited insignificant changes (denoting unaffected motor coordination).
| Time Duration Group | Baseline, (0 weeks) | 2 weeks Pre-treatment | 2 weeks pre-treatment, then 4 weeks treatment with AlCl3 |
|---|---|---|---|
| Control | 100* | 96.4+3.1 | 97.3+2.8 |
| 1** | 0.981+0.17 | 0.986+0.16 | |
| AD-group AlCl3 (17 g/kg) | 100* | 83.07+2.69 | |
| 1** | 0.91+0.01 | ||
| Rivastigmine (0.3 mg/kg) | 100* | 95.02+1.34 | 92.32+1.4 |
| 1** | 0.97+0.006 | 0.96+0.007 | |
| Ginger (108 mg/kg) | 100* | 77.52+5.57 | 70.53+3.43 |
| 1** | 0.87+0.03 | 0.83+0.02a | |
| Ginger (216 mg/kg) | 100* | 77.24+3.93 | 74.38+2.73 |
| 0.87+0.02 | 0.86+0.01 |
Table 2: Evaluation of protective effects of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) using accelerating speed rotarod in AD-disease induced in rats by AlCl3. All data are expressed in seconds as Means +SEM, *: %change, **: Square root transformed %change (a) Significantly different from baseline of the same group at P<0.05.
Rewarded alternation T-Maze test:
The results obtained in Table 3 showed significant increase in time in seconds (denoting deteriorated cognitive abilities), taken by rats to reach food in the T-Maze for the following groups: positive control AD group, groups treated with ginger aqueous infusions (108 and 216 mg/kg b.wt/ day) alone as well as when ginger aqueous infusions were given in combination with AlCl3, in comparison with baseline of each of these groups (before giving AlCl3). Moreover the groups treated with rivastigmine (0.3mg/kg b.wt/ day) or ginger aqueous infusions (108 and 216 mg/kg b.wt/day) in combination with AlCl3 for 4 successive weeks exhibited a significant reduction in time in seconds (denoting improved cognitive abilities), taken by rats to reach food in the T-Maze in comparison with positive control AD-group, however the groups treated with ginger aqueous infusions (108 and 216 mg/kg b.wt/day) in combination with AlCl3 for 4 successive weeks revealed significant increase in time (seconds) taken by rats to reach food in the T-Maze compared to the group of rats treated with rivastigmine in combination with AlCl3 for 4 successive weeks.
| Time Duration Group | Baseline, (0 weeks) | 2 weeks Pre-treatment | 2 weeks pre-treatment, then 4 weeks treatment with AlCl3 |
|---|---|---|---|
| Control | 13.44+0.91 | 14.1+0.88 | 15.56+1.3b |
| AD-group AlCl3 (17 g/kg) | 15.66+1.07 | 115+4.83a | |
| Rivastigmine (0.3 mg/kg) | 15.33+1.63 | 13.16+1.5 | 18.5+1.4b |
| Ginger (108mg/kg) | 12.33+1.7 | 50.2+13.6a | 52.2+2.95abc |
| Ginger (216 mg/kg) | 8.57+0.48 | 43.6+3.73 | 48+3.47abc |
Table 3: Evaluation of protective effects of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) using Rewarded T-Maze test in AD-disease induced in rats by AlCl3. All data Results are in seconds expressed as Means +SEM. (a) Significantly different baseline duration of the same group at P < 0.05. (b) Significantly different from AlCl3 after 4 weeks induction at P<0.05. (c) Significantly different from Rivastigmine group after 6 weeks at P<0.05.
Biochemical parameters
The results obtained in Table 4 exhibited both significant decrease in ACh level, and significant increase in AChE level in brains of positive control AD group in comparison to the normal control group. Rivastigmine (0.3 mg/kg b.wt/ day) and ginger aqueous infusions (108 and 216 mg/kg b.wt/ day) groups exhibited both a significant increase in Ach, and a significant decrease in AchE levels in comparison with positive control AD group of rats.
| Time Duration Group | Acetylcholine, (ACh) (µmol/mg tissue protein) | Acetylcholinesterase, (AChE) (u/mg tissue protein) |
|---|---|---|
| Control | 5.54+0.13 | 0.52+0.008 |
| AD-group AlCl3 (17 g/kg) | 0.83+0.04a | 0.79+0.01a |
| Rivastigmine (0.3 mg/kg) | 5.3+0.12b | 0.55+0.02b |
| Ginger (108 mg/kg) | 1.21+0.11ac | 0.74+0.01ac |
| Ginger (216 mg/kg) | 5.34+0.22bd | 0.52+0.02bd |
Table 4: Evaluation of Protective effect of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) on brain Acetylcholine and acetylcholinesterase activities in AD-disease induced in rats by AlCl3. All data are expressed as Means of +SEM. (a) Significantly different from negative control group at P <0.05. (b) Significantly different from AlCl3 group at P <0.05. (c) Significantly different from Rivastigmine group at P <0.05. (d) Significantly different from Ginger 108 mg/kg P <0.05.
Therapeutic study (Tables 5-8)
Grid floor activity cage
The results obtained in Table 5 exhibited significant reduction in activity (denoting deteriorated psychological state) of untreated AD group which was given AlCl3 for 4 successive weeks and left without therapy for 12 successive weeks, the same was observed for rivastigmine (0.3 mg/kg b.wt/ day) and ginger aqueous infusions (108 and 216 mg/kg b.wt/ day) groups that were given AlCl3 for 4 successive weeks before starting therapy, in comparison with baselines of each of these groups. However there was a significant increase in activity (denoting improved psychological state) of induced AD group that was treated with rivastigmine for 12 successive weeks in comparison with baseline of the same group before treatment and with untreated AD group. On the other hand, induced AD rats that were treated with both doses of ginger for 12 successive weeks exhibited significant increase in the activity compared to the same group before treatment, but significant reduction in activity compared to that of rats treated with rivastigmine for 12 weeks.
| Time Duration Group | Baseline, (0 weeks) | 4 weeks Alcl3 induction |
12 weeks (after stopping Alcl3 induction† or after treatment) |
|---|---|---|---|
| Control | 100* | 97.2+1.8 | 97.9+3.4 |
| 1** | 0.98+0.05 | 0.99+0.07cd | |
| AD-group AlCl3 (17 g/kg) | 100* | 51.71+6.72 | 22.4+0.6 |
| 1** | 0.71+0.04a | 0.47+0.006ab | |
| Rivastigmine (0.3 mg/kg) | 100* | 31.7+ 5.15 | 232.72+27.18 |
| 1** | 0.54+0.04a | 1.5+0.09abc | |
| Ginger (108 mg/kg) | 100* | 46.08+6.03 | 99.9+8.67 |
| 1** | 0.66+0.04a | 0.99+0.04bcd | |
| Ginger (216 mg/kg) | 100* | 35.41+7.86 | 123.19+7.42 |
| 1** | 0.58+0.06a | 1.1+0.03bcd |
Table 5: Evaluation of therapeutic effects of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) using grid floor activity cage in AD-disease induced in rats by AlCl3. All Results are expressed in as Means of movements +SEM, *: %change, **: Square root transformed %change (a) Significantly different from baseline of the same group at P<0.05. (b) Significantly different from AD-group of rats before treatment in the same group at P<0.05. (c) Significantly different from the AlCl3 group 12 weeks after stopping AlCl3 (P<0.05). (d) Significantly different from Rivastigmine group after 12 weeks of treatment at P<0.05.
Accelerating speed rotarod
The results obtained in Table 6 exhibited significant reduction in duration of sustained balance of rats on the rotarod (denoting deteriorated motor coordination), after administration of AlCl3 for 4 successive weeks only for the group of AD induced rats that would be treated with ginger (108 mg/kg b.wt/ day) in comparison with the baseline of the same group. Later on when AD induced group was treated with Ginger (108 mg/kg b.wt/ day) for 12 weeks exhibited significant increase in duration of sustained balance on the rotaroad (denoting improved motor coordination), when compared with the baseline of the same group, untreated AD-group, and both groups treated with rivastigmine (0.3 mg/kg b.wt/ day) and ginger (216 mg/kg b.wt/ day) for 12 successive weeks. On the other hand the untreated AD, as well as the rivastigmine treated group showed significant reduction in duration of sustained balance of rats on the rotarod in comparison with the baseline of each of these groups.
| Time Duration Group | Baseline, | 4 weeks | 12 weeks (after stopping Alcl3 induction† or after treatment) |
|---|---|---|---|
| (0 weeks) | Alcl3 induction | ||
| Control | 100* | 97.3+2.8 | 98.7+2.5 |
| 1** | 0.986+0.16 | 0.99+0.09cde | |
| AD-group AlCl3 (17 g/kg) | 100* | 83.07+2.69 | 48.42+11.32 |
| 1** | 0.91+0.01 | 0.67+0.07ab | |
| Rivastigmine (0.3 mg/kg) | 100* | 91.04+3.67 | 70.45+3.39 |
| 1** | 0.95+0.01 | 0.83+0.02ace | |
| Ginger (108 mg/kg) | 100* | 58.46+12.6 | 184.28+43.29 |
| 1** | 0.71+0.09a | 1.28+0.15abcd | |
| Ginger (216 mg/kg) | 100* | 73.65+3.64 | 80.64+1.74 |
| 1** | 0.85+0.02 | 0.89+0.009ce |
Table 6: Evaluation of therapeutic effects of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) using Accelerating speed rotarod in AD-disease induced in rats by AlCl3. All data are expressed in seconds as Means +SEM, *: %change, **: Square root transformed %change (a) Significantly different from base line of the same group (P<0.05). (b) Significantly different from AD-group of rates before treatment in the same group at P<0.05. (c) Significantly different from the AlCl3 group after 12 weeks of stopping AlCl3 (P < 0.05). (d) Significantly different from Rivastigmine group after 12 weeks of treatment at P<0.05. (e) Significantly different from Ginger 108 mg/kg after 12 weeks of treatment at P<0.05.
Rewarded alternation T-Maze test
The results obtained in Table 7 exhibited significant deterioration in cognitive abilities (manifested by increased duration in seconds to reach food), by rats given AlCl3 for 4 successive weeks and left without treatment for 12 successive weeks (untreated AD-group), as well as by AD induced groups before treatment with rivastigmine (0.3 mg/kg b.wt/ day) and with both doses of ginger (108 and 216 mg/kg b.wt/ day) in comparison with baseline of each of these groups, while the groups of AD induced rats that were treated with rivastigmine and with both doses of ginger for 12 successive weeks showed significant improvement in cognitive abilities when tested by T-Maze (manifested by reduced duration in seconds to reach food), in comparison to each of these groups before treatment, and with untreated AD-group, yet the improvement caused by rivastigmine treatment was superior to those of both doses of ginger.
| Time Duration Group | Baseline, (0 weeks) | 4 weeks Alcl3 induction |
12 weeks (after stopping Alcl3 induction† or after treatment) |
|---|---|---|---|
| Control | 13.44+0.91 | 15.56+1.3 | 16.2+1.2cd |
| AD-group AlCl3 (17 g/kg) | 15.66+1.07 | 115+4.83a | 120+0a |
| Rivastigmine (0.3 mg/kg) | 18.33+0.83 | 96.87+7.57a | 8.4+0.73bc |
| Ginger (108 mg/kg) | 14.33+0.17 | 99.16+8.97a | 61+4.4abcd |
| Ginger (216 mg/kg) | 18.57+0.48 | 114.5+5.09a | 83.33+1.9abcd |
Table 7: Evaluation of therapeutic effects of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) using Rewarded TMaze test in AD-disease induced in rats by AlCl3. All data are expressed in seconds as Means +SEM. (a) significantly different baseline duration of the same group at P<0.05. (b) Significantly different from AD-group of rats before treatment in the same group at P<0.05. (c) Significantly different from AlCl3 group after 12 weeks at P < 0.05. (d) Significantly different from Rivastigmine group after 12 weeks at P<0.05.
Biochemical parameters
The results obtained in Table 8 exhibited significant reduction in ACh levels of brain homogenates of untreated AD group, and both ginger (108 and 216 mg/kg b.wt/day) groups after treatment for 12 successive weeks, while significant increase in AChE activity was detected in brain homogenates of untreated AD-group only, in comparison to the control group. Treatment with rivastigmine (0.3 mg/kg b.wt/ day) and ginger (216 mg/kg b.wt/ day) for 12 successive weeks exhibited a significant increase in Ach levels in comparison to untreated AD group, and significant decrease in AchE activity was reported in rivastigmine and both groups of ginger-treated rats for 12 successive weeks in comparison to untreated AD group of rats.
| Time Duration Group | Acetylcholine, (ACh) (µmol/mg tissue protein) | Acetylcholinesterase, (AChE) (u/mg tissue protein) |
|---|---|---|
| Control | 6.54+0.13 | 0.49+0.02 |
| AD-group AlCl3 (17 g/kg) | 0.68+0.05a | 1.76+0.04a |
| Rivastigmine (0.3 mg/kg) | 6.17+0.01b | 0.37+0.01b |
| Ginger (108 mg/kg) | 0.35+0.03ac | 0.63 + 0.16b |
| Ginger (216 mg/kg) | 5.48+0.32abd | 0.63 + 0.16b |
Table 8: Evaluation of therapeutic effects of Ginger (108 and 216 mg/kg b.wt/day) and Rivastigmine (0.3 mg/kg b.wt/day) on brain acetylcholine and acetylcholinesterase activities in AD-disease induced in rats by AlCl3. All data are expressed as Means +SEM. (a) Significantly different from negative control group at P <0.05. (b) Significantly different from AlCl3 group at P <0.05. (c) Significantly different from Rivastigmine group at P<0.05. (d)Significantly different from Ginger 108 mg/kg P<0.05.
Histopathological results for protective and therapeutic groups:
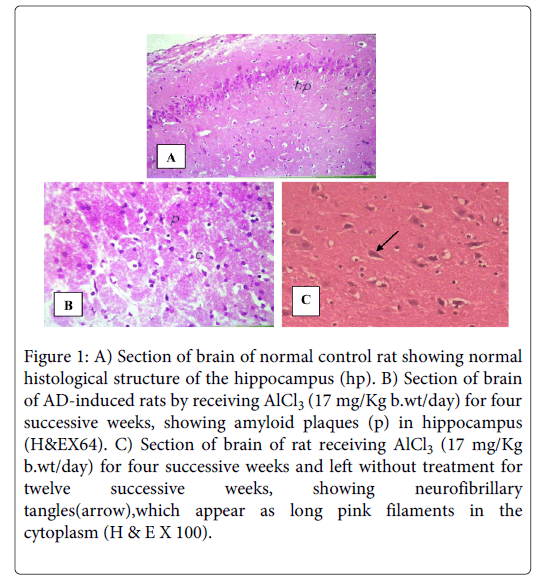
Examination of hippocampal tissue stained with Haematoxylin and Eosin (H & E), for negative control rats, shows highly active nerve cells with huge nuclei with relatively pale-stained, disappeared nuclear chromatin and prominent nuclei. The surrounding relatively inactive support cells have small nuclei with densely stained, condensed chromatin and no visible nucleoli indicative of normal cerebral tissue (Figure 1A).While sections of brains of positive control groups receiving AlCl3 (17 mg/kg) only for four successive weeks show necrosis of the brain, spongy appearance, plaques, and loss of the normal structure and the outlines of the cells and their nuclei. Some nuclei appear ring shape and the recently dead ones appear dark (Figure 1B). Sections of brains of rats receiving AlCl3 (17 mg/Kg) for four successive weeks and left without treatment for twelve successive weeks, show neurofibrillary tangles which appear as long pink filaments in the cytoplasm, as well as fatty change and necrosis of the brains (Figure 1C).
Figure 1: A) Section of brain of normal control rat showing normal histological structure of the hippocampus (hp). B) Section of brain of AD-induced rats by receiving AlCl3 (17 mg/Kg b.wt/day) for four successive weeks, showing amyloid plaques (p) in hippocampus (H&EX64). C) Section of brain of rat receiving AlCl3 (17 mg/Kg b.wt/day) for four successive weeks and left without treatment for twelve successive weeks, showing neurofibrillary tangles(arrow),which appear as long pink filaments in the cytoplasm (H & E X 100).
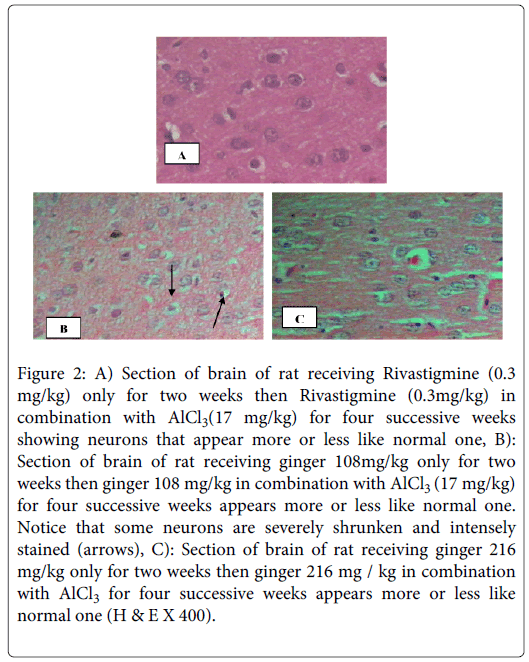
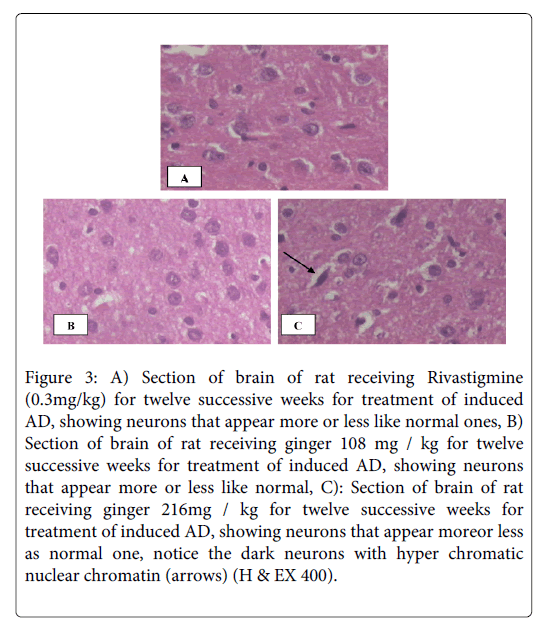
On the other hand sections of brains of rats receiving rivastigmine (0.3 mg /kg b.wt/day) or ginger in a dose of 216 mg /kg b.wt/day in combination with AlCl3 (17 mg/kg b.wt/day) for four weeks, in the protective study, appear more or less like normal sections (Figures 2A and 2C).The same appearance when ginger in a dose of 108 mg / kg b.wt/day was used for protection from AD, but in addition some neurons are severely shrunken and intensely stained (Figure 2B). While sections of brains of rats in the therapeutic study, that received rivastigmine (0.3 mg/kg b.wt/day), as well as sections of brains of rats that received both ginger 108 and 216 mg /kg b.wt/day for twelve successive weeks show neurons that appear more or less like normal ones (Figures 3A-C), in addition Figure 3C shows dark neurons with hyper chromatic nuclear chromatin.
Figure 2: A) Section of brain of rat receiving Rivastigmine (0.3 mg/kg) only for two weeks then Rivastigmine (0.3mg/kg) in combination with AlCl3(17 mg/kg) for four successive weeks showing neurons that appear more or less like normal one, B): Section of brain of rat receiving ginger 108mg/kg only for two weeks then ginger 108 mg/kg in combination with AlCl3 (17 mg/kg) for four successive weeks appears more or less like normal one. Notice that some neurons are severely shrunken and intensely stained (arrows), C): Section of brain of rat receiving ginger 216 mg/kg only for two weeks then ginger 216 mg / kg in combination with AlCl3 for four successive weeks appears more or less like normal one (H & E X 400).
Figure 3: A) Section of brain of rat receiving Rivastigmine (0.3mg/kg) for twelve successive weeks for treatment of induced AD, showing neurons that appear more or less like normal ones, B) Section of brain of rat receiving ginger 108 mg / kg for twelve successive weeks for treatment of induced AD, showing neurons that appear more or less like normal, C): Section of brain of rat receiving ginger 216mg / kg for twelve successive weeks for treatment of induced AD, showing neurons that appear moreor less as normal one, notice the dark neurons with hyper chromatic nuclear chromatin (arrows) (H & EX 400).
Discussion
Alzheimer’s disease (AD) is now the most common cause of dementia [19]. The incidence of AD increases with age [20]. Impairment of short-term memory usually is the first clinical feature. When the condition progresses, additional cognitive abilities are impaired, as the ability to calculate, and use common objects and tools [1]. Anders and Martin mentioned that there were 35.6 million people living with dementia worldwide, which will increase to 65.7million by 2030 and 115.4 million by 2050 [21]. Nearly two-thirds live in low and middle income countries, where the sharpest increases in numbers occur. Atherosclerotic diseases, deficiency of acetylcholine, inflammation of the brain and oxidative stress accompanied by depletion of endogenous antioxidants levels are implicated in the etiology of AD [2,4,22]
Aluminum is found in our daily life as in drinking water,soil and tooth pastes, moreover, it is used to manufacture cooking utensils.Aluminium causes oxidative deterioration of cellular lipids, proteins and DNA [23]. Lipid peroxidation can cause tissue damage under chronic condition [24] Nourooz-Zadeh et al. [25] therefore, Aluminum can be considered as a contributing factor in AD.
Acetylcholine esterase inhibitors are the only agents approved by the Food and Drug Administration (FDA) for the treatment of AD. All other agents prescribed for the treatment of AD are used on an off-label basis. Current research into new drugs is focused on agents that will prevent, slow down and/or halt the progress of the disease process. Hence, the potential for developing medicinal herb-derived and food plant-derived prophylactic agents directed at neurodegenerative disorders especially memory dysfunction has increased in importance. That's why in the present study we tried to investigate the protective and therapeutic effects of ginger (108 and 216 mg/kg b.wt/day) aqueous infusions compared to rivastigmine (as a reference drug) by assessing their effects on the behavioural status of rats involved in this work that represent animal model mimicking AD (by using AlCl3) and on their brain Ach level and AchE activities.
In the present study AlCl3 caused significant deterioration in psychological state when tested by grid floor activity cage, deterioration in motor coordination when tested by rotaroad, deterioration in cognitive abilities when tested by rewarded T-Maze test, in addition to significant decrease in level of Ach, and elevation in AchE activities in brain homogenates of rats involved in this experimental work. These findings were confirmed by the histopathologic examination of the hypocampus of the same rats which revealed the presence of amyloid plaques. On the other hand treatment of AD group of rats with rivastigmine as protective or as therapeutic medication exhibited an improvement in behavioural status as represented by a significant increase in activity (improved psychological state), duration of sustained balance on rotarod (improved motor coordination), and reduction in time taken to reach food in T-Maze test (improved cognition), as well as increased brain Ach level and significant decrease in AchE activities than AD-Induced rats. These results were confirmed with the histopathological finding in brain, where the amyloid plaques that were formed under the influence of AlCl3 administration disappeared. Rivastigmine might have acted through the glutameric mechanism, decrease the oxidative stress and restoring the antioxidant defense [26,27] to protect against the Aß-induced oxidative stress [28].
Ginger plant is commonly used worldwide in household; in addition, it is used as an important ingredient for various medicinal purposes in traditional medicine. The present study showed that in the protective and therapeutic groups of Alzheimer's like disease induced in rats, treatment with ginger in doses of 108 or 216 mg/kg, exhibited a significant improvement in Alzheimer's like disease status in rats as evidenced by increases in activity, brain Ach level and significant decreases in time (seconds) taken by rats to reach food in T-Maze test, as well as reduction in brain AchE activity more than untreated Alzheimer's like disease induced rats in both studies. However the high dose of Ginger (216 mg/kg) exhibited a better effect than the low dose (108 mg/kg). Histopathological finding in brain cells appeared more or less like the normal control group and the amyloid plaques disappeared.
These findings can be enforced by what was previously reported by Wattanathorn et al. who demonstrated that alcohol extract of ginger could reduce cognitive deficits and protect against brain damage in rats [29]. Also, Ghayur and Gilani reported that ginger induced vasodilatation. Therefore, the improvement of spatial memory in our study might be due to the ability of ginger to enhance cerebral blood flow as well as due high polyphenols which are potent antioxidants in aqueous ginger infusion [30]. Moreover Joshi and Parle reported that ginger ethanol extract significantly increased whole brain Acetyl cholinesterase inhibition activity. Which was reflected in improved learning and potential memory in young mice as the central cholinergic system plays an important role in learning and memory [31]. Also reversed the amnesia induced by diazepam and scopolamine. Furthermore, it reversed aging induced amnesia due to natural aging of mice. They also revealed that all ginger extracts are potential anti-cholinesterase agents and possess nootropic activity in view of its facilitator effect on retention of acquired learning. Also, Wang et al. [32] stated that ginger aqueous extract increased the function of cholinergic neuron, inhibited AChE activity and increased the ratio of super oxide dismutase/malondialdehyde (SOD/MDA) and decrease MDA content in brain, and another study undertaken by Ghayur et al. showed that 70% aqueous/methanolic extract of ginger had a combination of muscarinic, Ca++ antagonist and Butrylcholine esterase (BuChE) inhibitory activities, which are clues to its benefit in dementia, including AD as their effects were similar to physostigmine, which is a cholinesterase inhibitor. These studies support our findings in both the protective and therapeutic studies [33].
The protective and therapeutic effects of ginger aqueous infusion on AD in this study might be due to its polyphenolic ingredients which are gingerols and gingerol analogs as shogaols and paradols that directly inhibit prostaglandins and leukotriene synthesis [34]. These results might have been due to the antinflammatory effect of ginger which had been previously described by Hassan Abbad et al. [35] in their study that revealed that the addition of aqueous extract of ginger to drinking water reduced inflammation in diabetic mice , as well as Tripathi et al. [36] who indicated that several doses of 6-gingerol selectively inhibited production of pro-inflammatory cytokines such as tumour necrosis factor (TNF-a) and interleukins (IL-1, and IL-12) by murine peritoneal macrophages in the presence of lipopolysaccarides (LPS) stimulation.
The study conducted by Aydin et al. [37] revealed that ginger caused inactivation of lipid peroxidation reactions, and reduction in thiobarbituric acid reactive substance (TBARS) levels which also might increase the activity of glutathione peroxidase (GSH-PX) in rats, this supported the antioxidant effect of ginger which might be the base of our results. Some clinicians attribute Alzheimer to be due to atherosclerosis or hardening of blood vessels ; therefore the anti-hypercholesterolaemic effect of ginger might also be contributing to the observed memory-enhancing activity [38].
The effects of ginger manifested in the current study by improvement in the cognition, psychological state as well as in the locomotor activity, may be due to the presence of polyphenolic compounds and vitamin C in ginger [39-41]. Shirin and Jamuna [42] demonstrated that the highest total phenolic content was in aqueous ginger extract followed by ethanol, then methanol, hexane and least in acetone extracts. Flavonoid content of methanolic and ethanolic extracts are less than aqueous extract. This is due to higher solubility of ginger flavonoids in water than other solvents. The highest tannins (1.34 and 1.51 g/100 g of sample) are present in aqueous extract. In addition the study of El-Ghorab et al. [43] revealed that, the analysis of volatile oils of ginger showed that camphene, p-cineole, alpha-terpineol, zingiberene and pentadecanoic acid were major components. These oils decelerated degradation of lipids and yielded nearly the same antioxidant activity toward lipid peroxidation as did the synthetic antioxidant butylhydroxanisole (BHA) [44]. Histopathologic examination results in our study were in accordance with Kim et al. who found that aqueous ginger extracts, effectively protected cells from beta amyloid (1-42) insult [45].
There is growing evidence that suggests a potential beneficial effect of vitamin C which is a constituent of ginger, against the damaging effects of neurodegenerative disorders such as AD [46]. This may also explain why in this study ACh was increased in brains of rats treated with ginger which consequently led to the neuro-protective effect and that was reflected in the net memory enhancement and improved cognition.
Conclusion
It is concluded from this study that ginger has a protective and therapeutic effect on AD, which is more effective in therapy than in prophylaxis. This assumption may be attributed to the difference in treatment durations (4 weeks and 12 weeks for prophylactic and therapeutic regimens respectively), which lead to insufficient level of ginger to reach the protective level due to short duration of usage. The short duration of therapy in the protective study was accompanied rapid deterioration caused by AlCl3. Further clinical trials in humans are required to determine the efficacy of ginger, or one or more of its constituents, on neurodegenerative disorders.
Acknowledgements
This work is a part from a project number 251 entitled “Development of new drugs for treatment of Alzeheimer's disease”, (from 2009 - 2011) supported by STDF, of and Technology, to whom the authors are deeply thanks prof. Ahmed Hussein (The principal investigator of the project) for his kind help and their financial support.
References
- Kimura R, Ohno M (2009) Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis 33: 229-235.
- Terry AV Jr, Buccafusco JJ (2003) The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J PharmacolExpTher 306: 821-827.
- Lon AD, Elena KF, Brian RO (2008) Cholinesterase inhibitors improve visual attention in drivers with Alzeheimer's disease. Alzeheimer and Dementia.4: 498.
- Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, et al. (2006) Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis 9: 147-153.
- Mentreddy S (2007) Medicinal plant species with potential antidiabetic properties. J Sci Food Agric. 87: 743-750.
- Silva CG, Herdeiro RS, Mathias CJ, Panek AD, Silveira CS, et al. (2005) Evaluation of antioxidant activity of Brazilian plants. Pharmacol Res 52: 229-233.
- Ajith TA, Aswathy MS, Hema U (2008) Protective effect of Zingiberofficinale roscoe against anticancer drug doxorubicin-induced acute nephrotoxicity. Food ChemToxicol 46: 3178-3181.
- Kota N, Krishna P, Polasa K (2008) Alterations in antioxidant status of rats following intake of ginger through diet. Food Chem. 106: 991-996.
- KrasovskiÄ GN, Vasukovich LY, Chariev OG (1979) Experimental study of biological effects of leads and aluminum following oral administration. Environ Health Perspect 30: 47-51.
- Carageorgiou H, Sideris AC, Messari I, Liakou CI, Tsakiris S (2008) The effects of rivastigmine plus selegiline on brain acetylcholinesterase, (Na, K)-, Mg-ATPase activities, antioxidant status, and learning performance of aged rats. Neuropsychiatr Dis Treat 4: 687-699.
- Pavic R, Tvrdeic A, Tot OK, Heffer-Lauc M (2007) Activity cage as a method to analyze functional recovery after sciatic nerve injury in mice. Somatosens Mot Res 24: 213-219.
- Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, et al. (2006) Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson's disease. J Neuroinflammation 3: 6.
- Deacon RM, Rawlins JN (2006) T-maze alternation in the rodent. Nat Protoc 1: 7-12.
- Tsakiris S, Schulpis KH, Marinou K, Behrakis P (2004) Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+ -ATPase activities induced by the in vitro galactosaemia. Pharmacol Res 49: 475-479.
- Engvall E, Perlmann P (1971) Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8: 871-874.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J BiolChem 193: 265-275.
- Banchroft JD, Steven A, Turner DR (1996) (4th edtn )Theory and practice of histological technique. Churchil Livingstone, New york, London, San Francisco, Tokyo.
- Jones M, Onslow M, Packman A, Gebski V (2006) Guidelines for statistical analysis of percentage of syllables stuttered data. J Speech Lang Hear Res 49: 867-878.
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, et al. (2006) Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5: 228-234.
- Maccioni RB, Muñoz JP, Barbeito L (2001) The molecular bases of Alzheimer's disease and other neurodegenerative disorders. Arch Med Res 32: 367-381.
- Anders W, Martin P (2010) Alzheimer’s Disease International World Alzheimer Report. The Global Economic Impact of Dementia.Alzheimer’s Disease International (ADI)
- Mahdy K, Shaker O, Wafay H, Nassar Y, Hassan H, et al. (2012) Effect of some medicinal plant extracts on the oxidative stress status in Alzheimer's disease induced in rats. Eur Rev Med PharmacolSci 16 Suppl 3: 31-42.
- Sargazi M, Shenkin A, Roberts NB (2006) Aluminium-induced injury to kidney proximal tubular cells: Effects on markers of oxidative damage. J Trace Elem Med Biol 19: 267-273.
- Nourooz-Zadeh J, Rahimi A, Tajaddini-Sarmadi J, Tritschler H, Rosen P, et al. (1997) Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia 40: 647-653.
- Campbell A (2002) The potential role of aluminium in Alzheimer's disease. Nephrol Dial Transplant 17 Suppl 2: 17-20.
- Xiao XQ, Wang R, Han YF, Tang XC (2000) Protective effects of huperzine A on beta-amyloid(25-35) induced oxidative injury in rat pheochromocytoma cells. NeurosciLett 286: 155-158.
- Shah S, Reichman WE (2006) Treatment of Alzheimer's disease across the spectrum of severity. ClinInterv Aging 1: 131-142.
- Kumar P, Kumar A (2009) Protective effect of rivastigmine against 3-nitropropionic acid-induced Huntington's disease like symptoms: possible behavioural, biochemical and cellular alterations. Eur J Pharmacol 615: 91-101.
- Wattanathorn J, Jittiwat J, Tongun T, Muchimapura S, Ingkaninan K (2011) Zingiberofficinale Mitigates Brain Damage and Improves Memory Impairment in Focal Cerebral Ischemic Rat. Evid Based Complement Alternat Med 2011: 429505.
- Ghayur MN, Gilani AH (2005) Ginger lowers blood pressure through blockade of voltage-dependent calcium channels. J CardiovascPharmacol 45: 74-80.
- Joshi H, Parle M (2006) ZingiberOfficinale: Evaluation of its nootropic effect in mice. Afr J Trad. CAM 3: 64-74.
- Wang J, Hang Q , Jia S (2008) Effect of Aqueous Extract of Ginger on Vascular Dementia in Rats. Journal of Medical Research;.8.
- Ghayur MN, Gilani AH, Ahmed TK., Nawaz SA, Agbedahunsi JM, (2008) Muscarinic, Ca(++) antagonist and specific butyrylcholinesterase inhibitory activity of dried ginger extract might explain its use in dementia. J Pharm Pharmacol. 60: 1375-1383.
- Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, Tran VH, Duke CC (2003) Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res 111: 259-265.
- Hassan AZF, Gholamnezhad Z, Jafarzadeh M, Fatehi M, (2005). The anti-inflammatory effects of aqueous extracts of Ginger root in diabetic mice. DARU, J Pharm Sci,. 13: 70-73.
- Tripathi S, Maier KG, Bruch D, Kittur DS (2007) Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J Surg Res 138: 209-213.
- Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, et al. (2001) Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. ClinBiochem 34: 65-70.
- Nair A, Green RC (2006) Alzheimer's and Pitfalls, Practical Neurology. 22-29.
- Benzie IF, Szeto YT (1999) Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem 47: 633-636.
- Jayakumar SM, Nalini (1999) Antioxidant activity of ginger (ZingiberofficinaleRosacoe.) in rats fed a high fat diet. Med Sci Res.27: 341.
- Yen G, Chang Y, Su SW (2003) Antioxidant activity and active compounds of rice koji fermented with Aspergillus candidus. Food Chem. 83: 49–54.
- Adel, PRS, Jamuna P (2010) Chemical composition and antioxidant properties ofginger root (Zingiberofficinale). JMPR 4: 2674-2679.
- El-Ghorab AH, Nauman M, Anjum FM, Hussain S, Nadeem M (2010) A comparative study on chemical composition and antioxidant activity of ginger (Zingiberofficinale) and cumin (Cuminumcyminum). J Agric Food Chem 58: 8231-8237.
- Murcia MA, Egea I, Romojaro F, Parras P, Jiménez AM, MartÃnez-Tomé M (2004) Antioxidant evaluation in dessert spices compared with common food additives. Influence of irradiation procedure. J Agric Food Chem. 52:1872-1881.
- Kim DS, Kim JY, Han YS (2007) Alzheimer's disease drug discovery from herbs: neuroprotectivity from beta-amyloid (1-42) insult. J Altern Complement Med 13: 333-340.
- Cantuti-Castelvetri I, Shukitt-Hale B, Jos eph JA (2000) Neurobehavioral aspects of antioxidants in aging. Int J DevNeurosci 18: 367-381.
Citation: Karam AM, Nadia AMG, Abd El-FHM, Nemat AZY, Siham MAES, et al. (2014) Protective Effect of Ginger (Zingiber officinale) on Alzheimer's disease Induced in Rats. J Neuroinfect Dis 5:159.
Copyright: © 2014 Karam AM et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 16898
- [From(publication date): 6-2014 - Sep 04, 2025]
- Breakdown by view type
- HTML page views: 12083
- PDF downloads: 4815