Protective Effect of XPJY Decoction on Inflammation and Mitochondrial Function/Structure of Skeletal Muscle in Depressive Rats
Received: 03-Dec-2018 / Accepted Date: 08-Jan-2019 / Published Date: 11-Jan-2019 DOI: 10.4172/2314-7326.1000284
Abstract
Muscle soreness or pain and fatigues are important physical symptoms in patients with depression, which could negatively affect the movement desire and daily functioning of patients. The main pathological mechanism of muscle soreness and fatigue is not only related to the injury of muscle fibers and connective tissue, but also related to inflammation and mitochondrial damage. At present, the traditional SSRIs have no obvious effect on the improvement of somatic symptoms such as muscle soreness and fatigues in depression. Xing Pi Jie Yu (XPJY) Decoction is one of the most widely used clinical formulas of traditional Chinese medicine. Our study aims to exploring whether it has Anti-inflammatory and mitochondrial effects on skeletal muscle. The rat model of depression was established by CUMS (chronic unpredictable mild stress, CUMS) for 6 weeks. They were randomly divided into four groups: control group, CUMS group, CUMS+XPJY group, CUMS+ sertraline group. We used sucrose preference test, forced swimming test and Open field exploratory behavior test to verify the success of the depression model. The contents of CK in orbital blood were measured weekly as well as the following assay index were measured on 14th day, 28th day and 42th day, including TNF-α, IL-6, ATP and mitochondrial respiratory chain complex I, II, III, IV in gastrocnemius muscle, the mitochondrial ultrastructure was observed by transmission electron microscope. Research shows that, CUMS could induce inflammation, damage of mitochondrial function and structure in skeletal muscle. Early application of sertraline could prevent the increase of pro-inflammatory factors in skeletal muscle tissue, but it couldn’t improve the function and structure of mitochondria. XPJY decoction could significantly reduce the content of pro-inflammatory factors in skeletal muscle tissue and serum CK, prevent the damage of mitochondrial function and structure of skeletal muscle. This research provides an important theoretical basis for the clinical application of XPJY.
Keywords: ATP; CK; Depression; Respiratory chain complex; XPJY decoction
Abbreviations
CUMS: Chronic Unpredictable Stress; XPJY: Xing Pi Jie Yu Decoction; MS/P: Muscle Soreness or Pain; CK: Creatine Kinase; ATP: Adenosine Triphosphate; mtDNA: Mitochondrial DNA; ETC: Electron Transport Chain
Introduction
Depression is a kind of psychosomatic disease characterized by significant and lasting depression, lack of interest, slow thinking and cognitive function. It has four characteristics: high morbidity, high disability rate, high suicide rate and high recurrence rate. According to WHO statistics, depression is expected to become the second largest burden of disease after cardiovascular disease by 2020. Therefore, depression seriously endangers people’s physical and mental health, and has become the focus and hot spot of the world’s common concern [1].
Depression can cause physical symptoms such as muscle soreness and fatigue, which could negatively affect the movement desire and daily functioning of patients. Symptoms of muscle soreness or pain (MS/P) in depression include headache and the pain in multiple parts such as back, neck, shoulder, orofacial area, abdomen, joints, and chest [2,3]. Researches [4-6] show that depression and MS/P are closely related and interacted. Among patients with depression, muscle soreness is one of the independent prognostic factors for slow remission of baseline damage in depression [7]. Besides, the physical manifestations of typical fatigue (tiredness, low energy, weakness, heaviness, slowness) also occur in many depressed patients. A Sequenced Treatment Alternatives to Relieve Depression (STAR*D) clinical trial in 2014 was found that greater than 90% of 2868 patients had substantial fatigue on the baseline. Another important finding of the STAR*D trial was that 60.8% of patients had residual fatigue after 14 weeks of treatment with a selective serotonin reuptake inhibitor [8]. In summary, MS/P and fatigues are important physical symptoms in patients with depression, which persist easily after SSRIs anti-depressant treatment.
Many studies have shown that the main pathological mechanism of muscle soreness and fatigue is not only related to the injury of muscle fibers and connective tissue, but also to inflammation and mitochondrial damage. Jounger found the levels of IL-6, IL-7, IL-8 and IL-13 were elevated in patients with temporomandibular (TMD) myalgia [8,9]. It is known that the lack in ATP will lead to muscle dysfunction of contraction and diastolic due to the dependence of ATP on muscle contraction and relaxation. However, studies have confirmed that ATP in cells rarely drops below 70% of the pre-exercise level even in high-intensity exercise fatigue. Therefore, it is believed that the cause of exercise fatigue is not ATP itself, but other factors that reduce the activity of ATPase resulting in low utilization rate, or damage to the mitochondrial function leading to ATP re-synthesis disorder. In addition, some scholars have pointed out that, when the utilization rate of ATP exceeds its production rate, the rate of oxygen passing through the cell membrane is greatly increased, which will significantly accelerate the production of oxygen free radicals in mitochondria and in vascular endothelial cells. The oxygen free radicals will attack the organelles, reduce the viability of cells and cause necrosis, eventually leading to the occurrence of MS/P. Therefore, skeletal muscle inflammation and mitochondrial damage may be the important mechanism of somatic symptoms such as MS/P and fatigues in depression. However, the current research on depression mostly focuses on the level of serum inflammatory factors or neurotransmitters in brain tissue. Few people have studied the specific mechanism of MS/P in depression and its relationship with inflammation, mitochondria and whether SSIRs could regulate it. In this study, the depressive rat model was established by CUMS. The inflammatory factors, mitochondrial respiratory function and ATP, CK content in skeletal muscle of CUMS depressed rats were observed in different periods. Besides, the morphology of muscle fibers and mitochondria was observed by electron microscope to explore the mechanism and sequence of muscle injury in depression.
At present, the traditional SSRIs have no obvious effect on the improvement of somatic symptoms such as muscle soreness in depression. However, in our previous studies, XPJY decoction at 14.4 g/kg improved the depressive behavior and increased the content of 5-HT in depressive rats, obviously improving mitochondria morphology and oxidative stress in many parts of mitochondria but lacking in the specific effects and mechanism on skeletal muscle. Consequently, this paper aims to exploring the mechanism of XPJY prescription on muscle symptoms of depressed rats and seeking new medications for treatment of depressive muscle symptoms.
Methods
Animals and grouping
Adult male Wistar rats, weighing 160-180 g, were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. These animals are raised in single cages in 12 hours of light/12 hours of darkness and are allowed free access to water and food. Before the study, rats were pretreated for 7 days, with 3 minutes of adjustment each day. All animal procedures are in line with the guidelines for the care and use of laboratory animals.
74 rats were randomly divided into 4 groups: control, model, XPJY decoction group and sertraline group. There were 18 rats in each group. Rats in the control group were given normal saline daily for 42 days; rats in the model group were exposed to unpredictable sources of stress and were given normal saline daily for 42 days; rats in the XPJY decoction group were exposed to unpredictable sources of stress and given orally once a day at doses of 14.4 g/kg for 42 days; rats in sertraline group were exposed to unpredictable stressors, sertraline (Pfizer) was intra-gastrically injected once a day of 4.5 mg/kg/d for 42 days. Sertraline and XPJY granules were dissolved in normal saline.
Animal model preparation
The stress scheme was slightly modified from previous study. Briefly, a 6-week chronic unpredictable mild stress (CUMS) model of depressive rats was established. The methods are as follows: inclined rat cage + water deprivation (30 degrees, 12 hours), high frequency flash (12 hours), restraint + tail clamping (5 minutes), noise (12 hours of squeal), cold water swimming (4 degrees, 5 minutes), fasting (12 hours), water deprivation (12 hours), wet mattress (12 hours), warm and hot environment (30-32 degrees, 12 hours), light (24 hours), dark-ness (24 hours), mixed culture (24 hours), 1. Two kinds of stimulation were arranged by random number table method for 42 days. One or two kinds of stress were given daily, and the same kind of stimulation appeared discontinuously. Except for the blank control group, both the model group and the drug group were subjected to stress.
Sucrose preference test
Two bottles of 0.8% sucrose water were given to rats two days before the sugar preference test, and one bottle of water and one bottle of 0.8% sucrose water were given to rats one day before the test. On the test day, rats were given a bottle of water and 0.8% sucrose water for 20 hours, so that rats could drink water freely for 24 hours and change the bottle position at the midpoint. After 24 hours, the sugar preference rate was calculated.
Forced swimming test
Forced swimming is a typical depressive behavior experiment. It is mainly used to investigate animal behavioral despair. Rat swimming barrels are 25 cm in diameter and 45 cm in diameter depth. After swimming in water for 6 minutes, the statistical analysis was made on the immobility time of rats in water for 4 minutes. The immobility criterion is that mice will only float their heads out of the water, and their bodies will not struggle. The longer the immobility time, the more desperate the animals are. During the experiment, the rats were not allowed to receive audio-visual interference. Before releasing one rat at a time, the floating feces in the water should be cleaned up. After removing the mice from the water, the hair of the rats should be wiped with a towel as far as possible to prevent the rats from catching a cold.
Open field exploratory behavior test
Open field experiment can detect spontaneous activity and exploratory behavior of mice. It is a classical behavioral experiment for evaluating spontaneous activity and anxiety state of mice. Xeye Aba V3.2 video analysis system was used to analyze the behavior of rats. The behavioral changes of rats in each group were observed on the 1st day, the 14th day, the 28th day and the 42nd day, respectively. A single rat was placed in a 100 cm × 100 cm circular clean open box. The inner wall of the open box was coated with black paint. The locomotion trajectory of rats was observed and the distance of movement, the number of times of entering the central area and the time of staying in the central area within 5 minutes were recorded. Each rat was tested only once. After each measurement, the open box was thoroughly cleaned, and the next observation was carried out.
Detection of blood and CK contents from the orbit
At 2 and 6 weeks, 1.5 mL blood was taken from the orbit after inhalation of ether. At room temperature, centrifuge was centrifuged at 4°C for 3000 r/min centrifugation 10 min to separate serum and cryopreserved at -80°C. Serum CK levels were measured with CK kit instructions. CK units were expressed in U/L.
Tissue sampling and content determination of ATP, TNF-α and IL-6
After 10% chloral hydrate was injected intraperitoneally at the end of the 2nd, 4th and 6th weeks, the head was broken after glutaraldehyde perfusion and fixation. The skeletal muscle of right lower extremity was taken out immediately, washed with normal saline, drained with filter paper and frozen with liquid nitrogen (-196°C). Specific operation: Take frozen tissue from refrigerator at -80°C, add pre-cooled homogenate medium (including sucrose 250 mmol/L, EDTA 0.5 mmol/L, Tris-HCL 10 mmol/L, adjust PH 7.4) at the ratio of 1:9 (W/V), and prepare 10% homogenate. After that, the electric homogenizer was used to homogenizing for 8 times, with 10 seconds each time. Then centrifuged in a cryogenic high-speed centrifuge at 4°C for 3000 r/ min centrifugation 10 min. The supernatant was extracted as skeletal muscle homogenate. ATP content in skeletal muscle was detected by ATP content test kit (chemical method) of Nanjing Jiancheng Institute of Bioengineering, expressed by mmol/g; The content of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in skeletal muscle tissue was detected by Elisa method with reference to the kit instruction. ELISA kits were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Measurement of respiratory chain complexes activities
At the end of the 2nd, 4th and 6th weeks, after centrifuging the remaining skeletal muscle homogenate for 15 minutes at 2000 r/min, extracting the supernatant; centrifuging the obtained supernatant for 15 minutes at 2000 r/min, extracting the supernatant; centrifuging the obtained supernatant for 12000 r/min, 15 min, discarding the supernatant, adding a proper amount of buffer solution to the precipitate, after fully mixing, centrifuging for 15 minutes at 12000 r/min, the precipitate is mitochondria. Mitochondrial isolation was carried out at 0-4°C. The prepared mitochondria were suspended in suspension (30 mmol/L sucrose, 20 mmol/LTris-HCl, 0.1% BSA, PH 7.2), and the aliquots stored at -80°C. The activity of mitochondrial respiratory chain complex I ~ IV (C I ~ IV) was determined by Spectrophotometry with reference to Vyatlina’s method [10]. Mitochondrial proteins of 10 ml were added to the buffer with the final volume of 2 mL. Distilled water was used as blank tube. The absorbance was corrected to 0. The absorbance values at 340, 600, 550 and 550 nm were measured for 3 minutes, respectively. All samples were determined with a corresponding assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Ultrastructure observation of mitochondria
At the end of the 2nd, 4th and 6th weeks, rats were anesthetized and then decapitated after perfusion fixation. The skeletal muscle (gastrocnemius) was separated from the right hind limb. Tissue blocks about 1 mm × 1 mm × 3 mm in size were taken. The skeletal muscle specimens were fixed in 3% glutaraldehyde solution for 48 hours, then treated with 1% osmium acid for 2 hours, gradient acetone dehydration (50%, 70%, 90% and 100% acetone for 10-15 minutes), pure acetone + Epon 812 embedding agent (1:1) for 1 h, pure acetone + Epon 812 embedding agent (1:2) for 1 h, it was cured in oven at 37°C for 48 h and in oven at 60°C for 24 h. The LKB-V ultrathin slice machine was sliced. The thickness was 50-60 nm and stained with 3% uranium acetate-lead citrate.
Statistical Analysis
Data are presented as mean ± SEM. Differences between groups were analyzed by one-way analysis of variance followed by Dunnett multiple comparisons. The significant level was set at p<0.05.
Results
Sucrose preference test
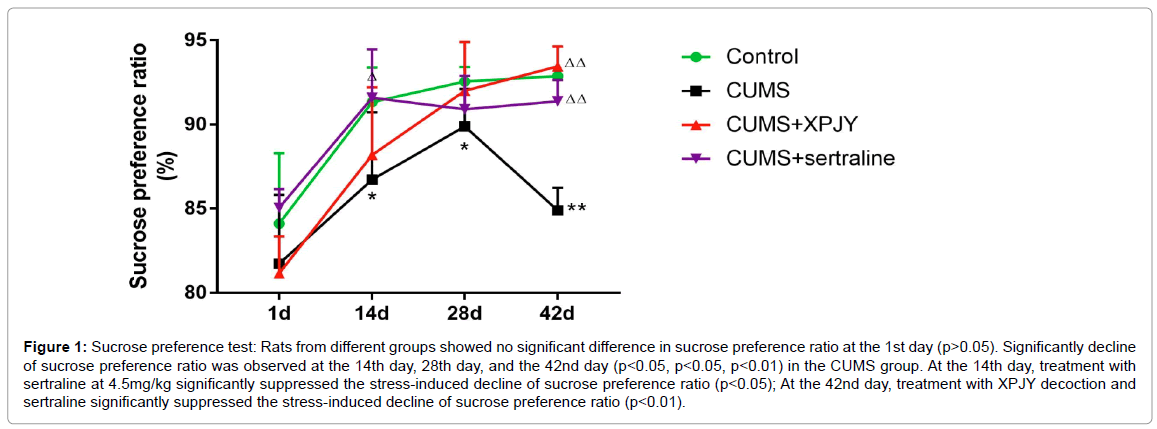
Rats from different groups showed no significant difference in sucrose preference ratio at the 1st day (p>0.05). Significantly decline of sucrose preference ratio was observed at the 14th day, 28th day, and the 42nd day (p<0.05, p<0.05, p<0.01) in the CUMS group. At the 14th day, treatment with sertraline at 4.5 mg/kg significantly suppressed the stress-induced decline of sucrose preference ratio (p<0.05); At the 42nd day, treatment with XPJY decoction and sertraline significantly suppressed the stress-induced decline of sucrose preference ratio (p<0.01) (Figure 1).
Figure 1: Sucrose preference test: Rats from different groups showed no significant difference in sucrose preference ratio at the 1st day (p>0.05). Significantly decline of sucrose preference ratio was observed at the 14th day, 28th day, and the 42nd day (p<0.05, p<0.05, p<0.01) in the CUMS group. At the 14th day, treatment with sertraline at 4.5mg/kg significantly suppressed the stress-induced decline of sucrose preference ratio (p<0.05); At the 42nd day, treatment with XPJY decoction and sertraline significantly suppressed the stress-induced decline of sucrose preference ratio (p<0.01).
Forced swimming test
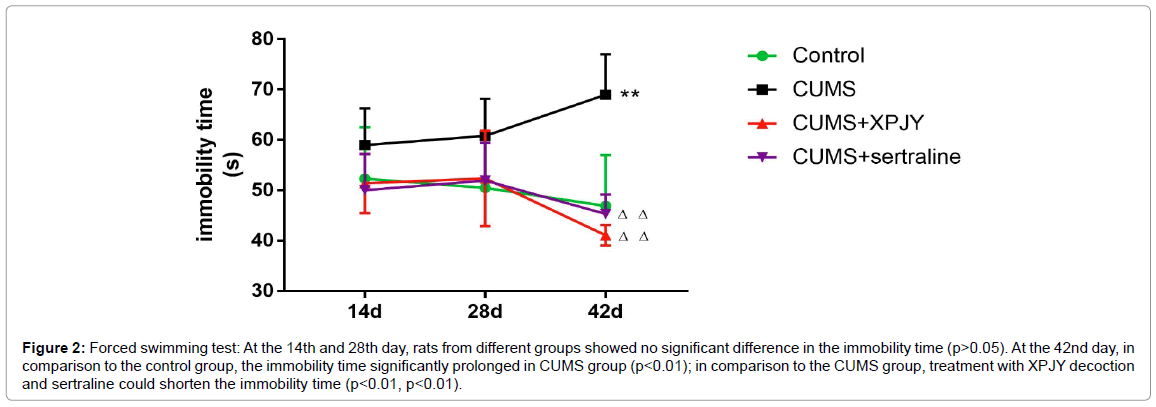
At the 14th and 28th day, rats from different groups showed no significant difference in the immobility time (p>0.05). At the 42nd day, in comparison to the control group, the immobility time significantly prolonged in CUMS group (p<0.01); in comparison to the CUMS group, treatment with XPJY decoction and sertraline could shorten the immobility time (p<0.01, p<0.01) (Figure 2).
Figure 2: Forced swimming test: At the 14th and 28th day, rats from different groups showed no significant difference in the immobility time (p>0.05). At the 42nd day, in comparison to the control group, the immobility time significantly prolonged in CUMS group (p<0.01); in comparison to the CUMS group, treatment with XPJY decoction and sertraline could shorten the immobility time (p<0.01, p<0.01).
Open field exploratory behavior test
At the 14th day, rats from different groups showed no significant difference in the total distance of exercise, number of times of entering central area and residence time in central area (p>0.05). At the 28th day, compared with the blank control group, the total distance of exercise in the CUMS group was reduced (p<0.05); compared with the CUMS group, the total distance of exercise in the XPJY and sertraline group was increased (p<0.05); but there was no significant difference in the number of times entering the central area and residence time in central area among the groups. (p>0.05). At the 42nd day, compared with the blank control group, the total distance of exercise in the CUMS group was reduced (p<0.05); compared with the CUMS group, the total distance of exercise in the XPJY and sertraline group was increased (p<0.05); however, there was still no significant difference in the number of times of entering the central area and the residence time in central area among the groups (p>0.05) (Table 1).
| Variables | Total distance (mm) | Number of times of entering central area | Residence time in central area (s) | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 42 | Day 1 | Day 42 | Day 1 | Day 42 | |
| Control | 39088 ± 3616 | 37694 ± 7886 | 3.3 ± 2.7 | 2.9 ± 2.5 | 11.05 ± 9.86 | 6.01 ± 3.12 |
| CUMS | 38933 ± 4880 | 27887 ± 4209* | 3.2 ± 2.3 | 2.5 ± 1.8 | 11.89 ± 2.63 | 5.89 ± 1.34 |
| CUMS+XPJY | 39564 ± 7001 | 35601 ± 2920△△ | 3.5 ± 1.9 | 3.8 ± 2.3 | 10.01 ± 7.09 | 5.45 ± 4.31 |
| CUMS+ sertraline | 39597 ± 2114 | 34988 ± 6562△ | 3.7 ± 2.0 | 2.8 ± 3.2 | 11.90 ± 5.88 | 6.38 ± 3.54 |
Note: Values were presented as mean ± S.E.M. n=6. *p<0.05 vs. control group; △p<0.05 vs. CUMS group, △△p<0.01 vs. CUMS group.
Table 1: Open field exploratory behavior test.
In this study, after 42 days of unpredictable mild stress intervention, the sucrose preference rate of rats in CUMS group decreased significantly, the immobility time prolonged and the total distance of exercise reduced. The rat model of depression was successfully established.
Effects of XPJY on CK content
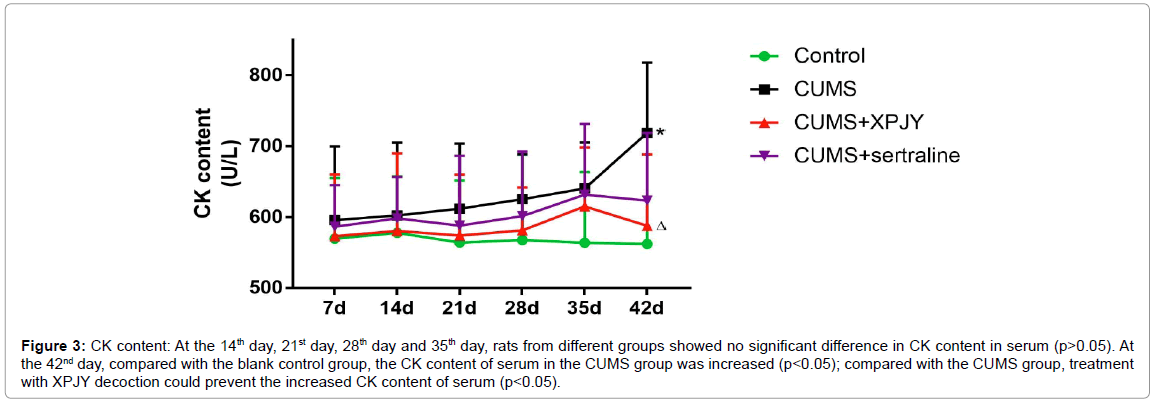
At the 14th day, 21st day, 28th day and 35th day, rats from different groups showed no significant difference in CK content in serum (p>0.05). At the 42nd day, compared with the blank control group, the CK content of serum in the CUMS group was increased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the increased CK content of serum (p<0.05) (Figure 3).
Figure 3: CK content: At the 14th day, 21st day, 28th day and 35th day, rats from different groups showed no significant difference in CK content in serum (p>0.05). At the 42nd day, compared with the blank control group, the CK content of serum in the CUMS group was increased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the increased CK content of serum (p<0.05).
Effects of XPJY on TNF-α content
At the 14th day, compared with the blank control group, the TNF-α content of skeletal muscle in the CUMS group was increased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the increased TNF-α content of skeletal muscle (p<0.05). At the 28th day, compared with the blank control group, the TNF-α content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased TNF-α content of skeletal muscle (p<0.01, p<0.05). At the 42nd day, compared with the blank control group, the TNF-α content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased TNF-α content of skeletal muscle (p<0.01, p<0.01) (Figure 4).
Figure 4: TNF-α content: At the 14th day, compared with the blank control group, the TNF-α content of skeletal muscle in the CUMS group was increased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the increased TNF-α content of skeletal muscle (p<0.05). At the 28th day, compared with the blank control group, the TNF-α content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased TNF-α content of skeletal muscle (p<0.01, p<0.05). At the 42nd day, compared with the blank control group, the TNF-α content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased TNF-α content of skeletal muscle (p<0.01, p<0.01).
Effects of XPJY on IL-6 content
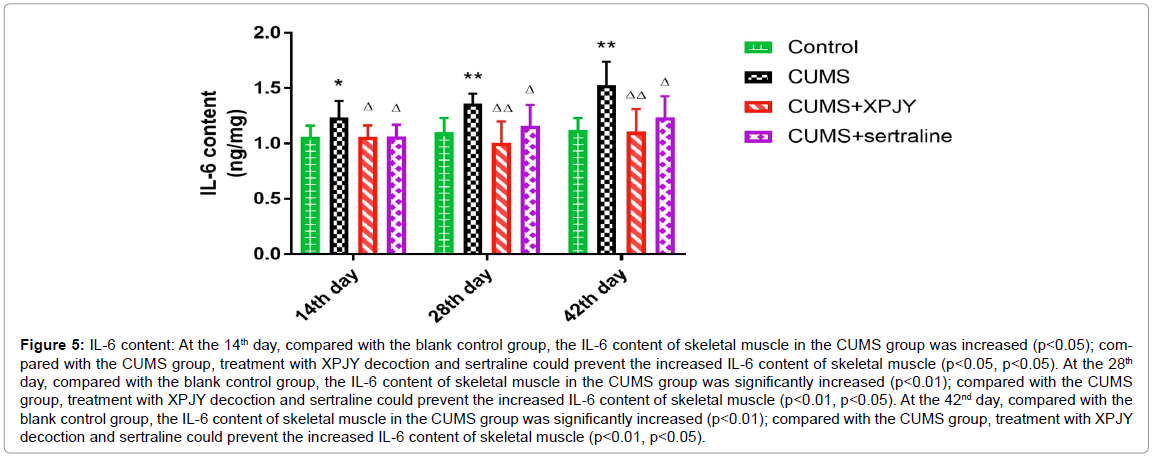
At the 14th day, compared with the blank control group, the IL-6 content of skeletal muscle in the CUMS group was increased (p<0.05); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased IL-6 content of skeletal muscle (p<0.05, p<0.05). At the 28th day, compared with the blank control group, the IL-6 content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased IL-6 content of skeletal muscle (p<0.01, p<0.05). At the 42nd day, compared with the blank control group, the IL-6 content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased IL-6 content of skeletal muscle (p<0.01, p<0.05) (Figure 5).
Figure 5: IL-6 content: At the 14th day, compared with the blank control group, the IL-6 content of skeletal muscle in the CUMS group was increased (p<0.05); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased IL-6 content of skeletal muscle (p<0.05, p<0.05). At the 28th day, compared with the blank control group, the IL-6 content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased IL-6 content of skeletal muscle (p<0.01, p<0.05). At the 42nd day, compared with the blank control group, the IL-6 content of skeletal muscle in the CUMS group was significantly increased (p<0.01); compared with the CUMS group, treatment with XPJY decoction and sertraline could prevent the increased IL-6 content of skeletal muscle (p<0.01, p<0.05).
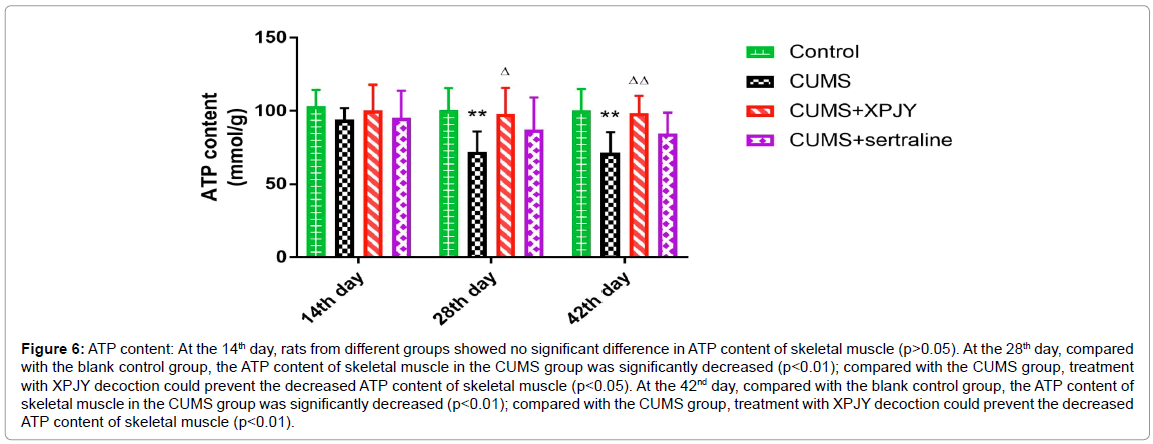
Effects of XPJY on ATP content
At the 14th day, rats from different groups showed no significant difference in ATP content of skeletal muscle (p>0.05). At the 28th day, compared with the blank control group, the ATP content of skeletal muscle in the CUMS group was significantly decreased (p<0.01); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased ATP content of skeletal muscle (p<0.05). At the 42nd day, compared with the blank control group, the ATP content of skeletal muscle in the CUMS group was significantly decreased (p<0.01); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased ATP content of skeletal muscle (p<0.01) (Figure 6).
Figure 6: ATP content: At the 14th day, rats from different groups showed no significant difference in ATP content of skeletal muscle (p>0.05). At the 28th day, compared with the blank control group, the ATP content of skeletal muscle in the CUMS group was significantly decreased (p<0.01); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased ATP content of skeletal muscle (p<0.05). At the 42nd day, compared with the blank control group, the ATP content of skeletal muscle in the CUMS group was significantly decreased (p<0.01); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased ATP content of skeletal muscle (p<0.01).
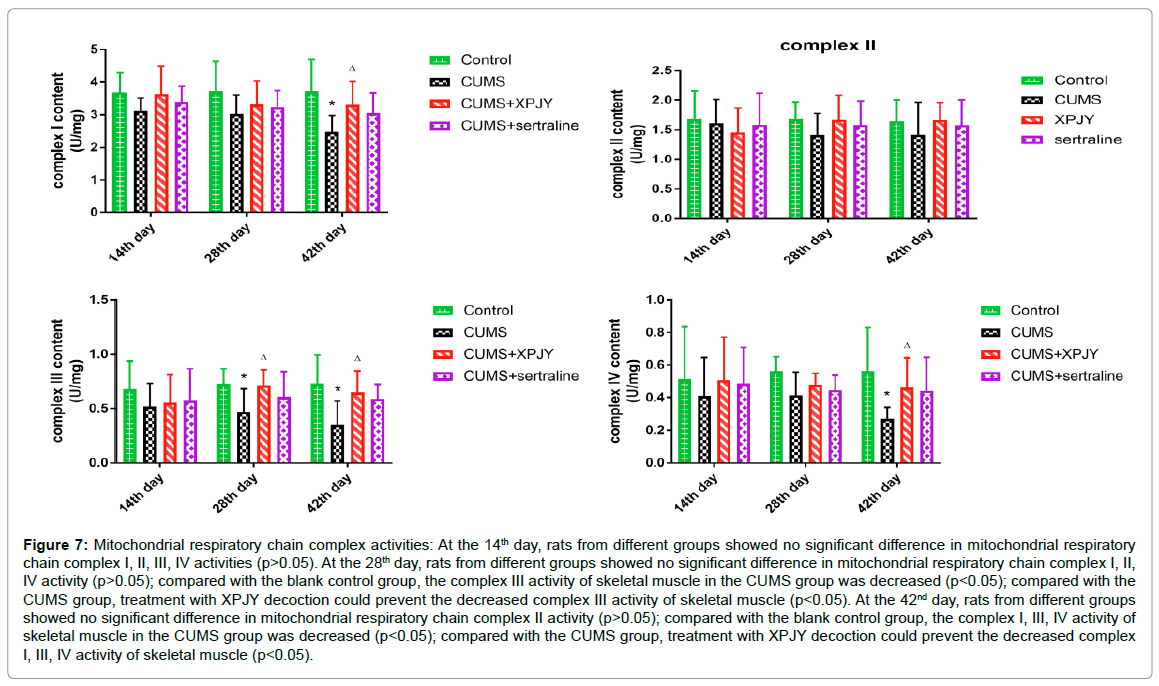
Effects of XPJY on mitochondrial respiratory chain complex activities
At the 14th day, rats from different groups showed no significant difference in mitochondrial respiratory chain complex I, II, III, IV activities (p>0.05). At the 28th day, rats from different groups showed no significant difference in mitochondrial respiratory chain complex I, II, IV activity (p>0.05); compared with the blank control group, the complex III activity of skeletal muscle in the CUMS group was decreased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased complex III activity of skeletal muscle (p<0.05). At the 42nd day, rats from different groups showed no significant difference in mitochondrial respiratory chain complex II activity (p>0.05); compared with the blank control group, the complex I, III, IV activity of skeletal muscle in the CUMS group was decreased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased complex I, III, IV activity of skeletal muscle ( p<0.05) (Figure 7).
Figure 7: Mitochondrial respiratory chain complex activities: At the 14th day, rats from different groups showed no significant difference in mitochondrial respiratory chain complex I, II, III, IV activities (p>0.05). At the 28th day, rats from different groups showed no significant difference in mitochondrial respiratory chain complex I, II, IV activity (p>0.05); compared with the blank control group, the complex III activity of skeletal muscle in the CUMS group was decreased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased complex III activity of skeletal muscle (p<0.05). At the 42nd day, rats from different groups showed no significant difference in mitochondrial respiratory chain complex II activity (p>0.05); compared with the blank control group, the complex I, III, IV activity of skeletal muscle in the CUMS group was decreased (p<0.05); compared with the CUMS group, treatment with XPJY decoction could prevent the decreased complex I, III, IV activity of skeletal muscle (p<0.05).
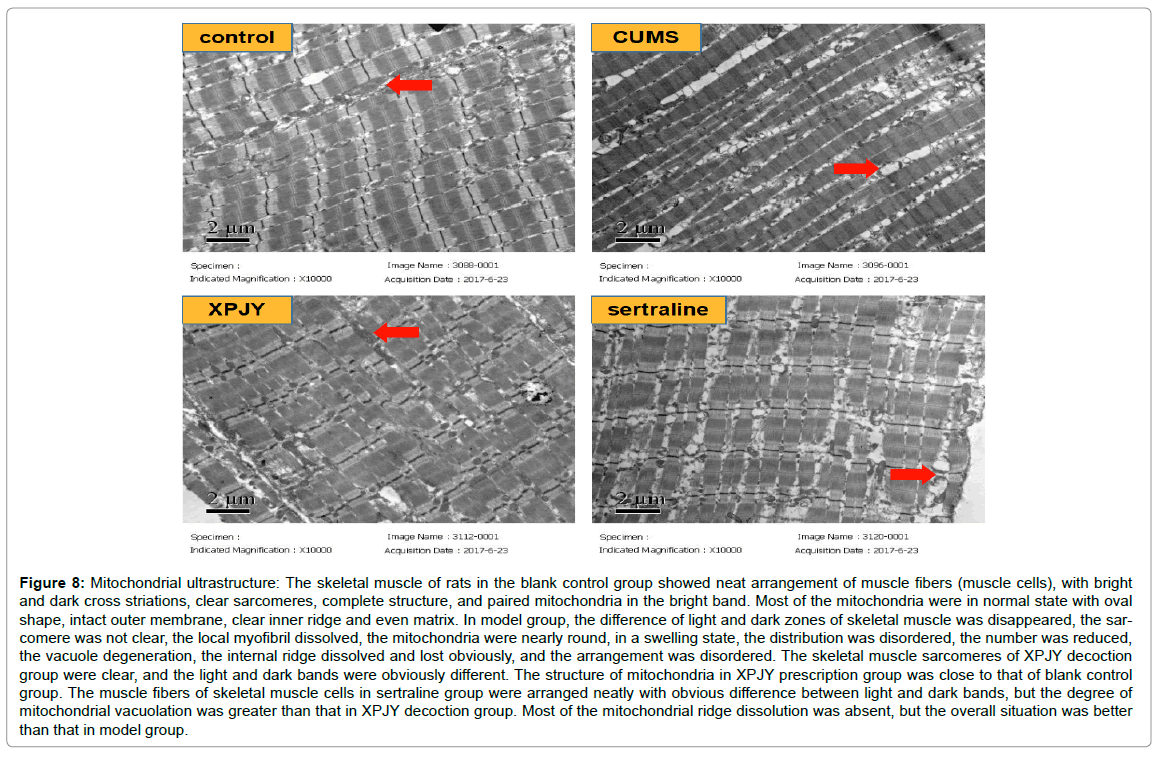
Effects of XPJY on mitochondrial ultrastructure
The skeletal muscle of rats in the blank control group showed neat arrangement of muscle fibers (muscle cells), with bright and dark cross striations, clear sarcomeres, complete structure, and paired mitochondria in the bright band. Most of the mitochondria were in normal state with oval shape, intact outer membrane, clear inner ridge and even matrix. In model group, the difference of light and dark zones of skeletal muscle was disappeared, the sarcomere was not clear, the local myofibril dissolved, the mitochondria were nearly round, in a swelling state, the distribution was disordered, the number was reduced, the vacuole degeneration, the internal ridge dissolved and lost obviously, and the arrangement was disordered. The skeletal muscle sarcomeres of XPJY decoction group were clear, and the light and dark bands were obviously different. The structure of mitochondria in XPJY prescription group was close to that of blank control group. The muscle fibers of skeletal muscle cells in sertraline group were arranged neatly with obvious difference between light and dark bands, but the degree of mitochondrial vacuolation was greater than that in XPJY decoction group. Most of the mitochondrial ridge dissolution was absent, but the overall situation was better than that in model group (Figure 8).
Figure 8: Mitochondrial ultrastructure: The skeletal muscle of rats in the blank control group showed neat arrangement of muscle fibers (muscle cells), with bright and dark cross striations, clear sarcomeres, complete structure, and paired mitochondria in the bright band. Most of the mitochondria were in normal state with oval shape, intact outer membrane, clear inner ridge and even matrix. In model group, the difference of light and dark zones of skeletal muscle was disappeared, the sar-comere was not clear, the local myofibril dissolved, the mitochondria were nearly round, in a swelling state, the distribution was disordered, the number was reduced, the vacuole degeneration, the internal ridge dissolved and lost obviously, and the arrangement was disordered. The skeletal muscle sarcomeres of XPJY decoction group were clear, and the light and dark bands were obviously different. The structure of mitochondria in XPJY prescription group was close to that of blank control group. The muscle fibers of skeletal muscle cells in sertraline group were arranged neatly with obvious difference between light and dark bands, but the degree of mitochondrial vacuolation was greater than that in XPJY decoction group. Most of the mitochondrial ridge dissolution was absent, but the overall situation was better than that in model group.
Discussion
In present study, rats subjected to 42 days of CUMS exhibited decreased sucrose preference ratio, prolonged the immobility time and reduced the total distance of exercise. Moreover, at 14 days of stress, the TNF-α and IL-6 content in skeletal muscle increased. At 28 days of stress, the TNF-α and IL-6 content in skeletal muscle increased more obvious; the ATP and complex III content in skeletal muscle decreased. At 42 days stress, on the basis of the above changes, the CK content in serum increased, the complex I, IV in skeletal muscle decrease and the destruction of skeletal muscle mitochondrial structure appeared. Rats in group treatment with XPJY decoction, the efficacy trend of which was better than the other drug groups, could decrease CK, TNF-α and IL-6 content, increase ATP and complex I, III, IV content, prevent abnormal changes in mitochondrial morphology of the above tissues. However, although sertraline can reduce the content of inflammatory factors, it has no significant effect on the function and structure of mitochondria.
In recent years, some scholars have proposed the IN-PRO (inflammatory- neuroprogressive) hypothesis of depression, which holds that “stress can activate the immune system, increase the release of cytokines, further affect the synthesis, release and metabolism of neurotransmitters, change neuroplasticity and neuroendocrine function, and ultimately trigger depression” [11-13]. Clinical studies showed that the levels of IL-1, TNF-α and IL-6 in peripheral blood and cerebro-spinal fluid of patients with depression were significantly higher than those of normal subjects. Animal experimental studies have found that peripheral or central administration of IL-1β, IL-6 and LPS can induce depression-like behavior in animals [14-16]. Cytokines are the important material basis of immune system acting on nerve and endocrine system in neuroendocrine-immune network. According to their different roles in inflammatory reaction, inflammatory factors can be divided into proinflammatory factors and anti-inflammatory factors. TNF-alpha and IL-6 are important proinflammatory factors.
As early as 1975, Carswell et al. discovered that serum contains a factor that can kill some tumor cells or cause hemorrhagic necrosis in some tumor tissues and named it tumor necrosis factor (TNF). In 1985, Shalaby named TNF produced by activated macrophages as TNF-α (TNF-α) [17]. TNF-α can not only induce inflammation by activating the NF-kappa B signaling pathway, but also bind with endothelial cells to increase the production of peroxide anions, stimulate cell degranulation and myeloperoxidase, which increase the secretion of inflammatory factors such as IL-8, IL-1 and GM-CSF by endothelial cells, and promote neutrophils on endothelial cells. Adhesion, which stimulates local inflammation, is an important inflammatory mediator. At the same time, TNF-α stimulates the production and release of IL-1 and IL-8 by monocytes and macrophages, leading to further expansion of inflammation. Studies have shown that TNF-α can increase NO synthesis by activating inducible nitric oxide synthase (iNOS), and then stimulate peripheral blood monocytes to release IL-8, which further aggravates inflammation [18,19]. TNF-α and its corresponding receptors are expressed in microglia of the brain, so inflammation induced by TNF-α is closely related to depression. TNF-alpha is involved in inflammation of peripheral and central nervous systems. IL-6 is produced by monocytes and macrophages, which can promote T cell proliferation and stimulate cytotoxic T cell response; stimulate B cell activation and proliferation, and differentiate into plasma cells to increase the synthesis of immunoglobulin; induce hepatocytes to synthesize acute phase proteins; promote hematopoietic stem cells from G0 to G1 phase, and make macrophages, monocytes and cells [20,21]. In this study, we found that the levels of inflammatory factors (TNF-a, IL-6) in gastrocnemius muscle of depressive rats were also increased outside the brain, and there was a significant change on the 14th day of stress, suggesting that the inflammatory response induced by chronic stress may be systemic, and this change may be one of the mechanisms of muscle pain in depressive rats.
Mitochondria are mostly distributed in organs and tissues with high energy requirements, such as brain and muscle, so the function of brain and muscle is more susceptible to mitochondrial abnormalities. In recent years, more and more scholars have linked the pathogenesis of depression with mitochondria. First of all, depressive symptoms are common in patients with mitochondrial diseases. Fattal [22] investigated 36 patients with mitochondrial disorders. The results showed that the lifelong prevalence of depression in patients with mitochondrial disorders was as high as 54%, which was significantly higher than that in the general population. Secondly, patients with depression have mitochondrial abnormalities. Shao [23] found that 6 of the 13 mtDNA- encoded transcripts in the prefrontal cortex of depressive patients were significantly reduced. Thirdly, there are abnormal mitochondrial function in the animal model of depression. Chronic mild stress animal model is a commonly used animal model of depression. Using this model to study the mitochondrial function of depressed animals has achieved some results. Yu Gong [24] studied the function and ultra-structure of mitochondria in depressed animals. The results showed that chronic mild stress for 6 weeks could inhibit the mitochondrial respiratory rate in hippocampus, cortex and hypothalamus, decrease the mitochondrial membrane potential and damage the ultrastructure of mitochondria.
A large number of clinical and basic studies have shown that mitochondrial abnormalities are closely related to depression. Studies have confirmed that the mechanism of mitochondrial abnormalities in depression is also related to many aspects. First, inflammation associated with depression can lead to mitochondrial damage. Studies have shown that proinflammatory cytokines can alter ETC complexes and complex related enzymes [25]. Stressors could also lead to increased ROS and oxidative stress through various channels, such as BDNF, HPA axis, inflammation channels, ROS could induce lipid peroxidation damage in mitochondrial inner membrane and nerve cell membrane, which can lead to depression [26,27], in addition, ROS could also directly damage mtDNA [28]. Mitochondria are the center of substance metabolism and energy transformation in the body. They exist in almost all aerobic eukaryotic cells and are the main energy supplying sites for cell activities. Mitochondria produce ATP through oxidative phosphorylation during muscle contraction, which provides energy for muscle contraction. Mitochondria, as a buffer of cellular calcium, have the ability to absorb, release and regulate the concentration of cytosolic Ca2+ in order to maintain the function of cells and keep muscles in normal state. Moreover, mitochondria can make muscle more use of lipids rather than carbohydrates for metabolism, thereby reducing lactic acid production and delaying fatigue. Therefore, mitochondrial function directly determines the physiological function of skeletal muscle cells. Mitochondrial damage can lead to muscle fatigue and soreness. Thus, mitochondrial function is significantly correlated with somatization symptoms. Ann [29] investigated 21 depressive patients with obvious somatization symptoms with an average age of 49 +9 years and compared with 10 healthy people with sedentary habits. The results showed that depressive patients with obvious somatization symptoms had decreased mitochondrial ATP production in muscle tissue, suggesting that the decrease of mitochondrial energy production constituted at least a companion. Somatization symptoms are part of the genetic susceptibility to depression. This study found that serum CK content, skeletal muscle ATP content, respiratory chain complex content and mitochondrial structure in depressive rats changed significantly after inflammation (average 28-42 days), suggesting that chronic stress can lead to abnormal function and structure of skeletal muscle mitochondria, which is related to muscle fatigue in depressive rats and other symptoms.
This research showed that early application of sertraline inhibited the increase of TNF-α and IL-6 in skeletal muscle but did not prevent the damage of mitochondrial function and structure. This may be one of the reasons why SSRIs did not improve somatic symptoms of depression. XPJY decoction, a Chinese herbal formula, have been reported to have an antidepressant effect in rats exposed to chronic stress through improving depressive behavior, increasing serum 5-HT, decreasing serum corticosterone, reducing the inflammatory factors in serum and hippocampus, increasing the expression of cAMP, PKA, CREB and BDNF in hippocampus [30-33]. This study found that XPJY decoction could prevent the increase of TNF-ɑ, IL-6 and CK in skeletal muscle, as well as prevent the decrease of ATP and respiratory chain complex I, III, IV in skeletal muscle, and avoid the damage of mitochondrial morphology and structure caused by stress in CUMS depressive rats. Therefore, XPJY decoction has anti-inflammatory effect on skeletal muscle and improves the function and structure of skeletal muscle’s mitochondria in CUMS depressive rats, which shows that XPJY decoction may become an important drug to improve the somatic symptoms of depression.
Conclusion
CUMS can increase the levels of TNF-α, IL-6 and CK in skeletal muscle of rats, decrease the levels of ATP, respiratory chain complex I, III, IV and damage the structure of mitochondria, Inflammation appeared on the 14th day of chronic stress, but abnormal mitochondrial function appeared on the 28th to 42nd day of chronic stress. Electron microscopy showed that the skeletal muscle fibers and connective tissue structure of CUMS depressive rats were normal, but the morphological structure of mitochondria was damaged. It is suggested that the somatic symptoms of muscle soreness and fatigue in depression may be more related to myocyte inflammation and mitochondrial damage. Early application of sertraline could prevent the increase of pro-inflammatory factors in skeletal muscle tissue, but it couldn’t improve the function and structure of mitochondria. Early application of XPJY decoction could significantly reduce the content of pro-inflammatory factors in skeletal muscle tissue and serum CK, prevent the damage of mitochondrial function and structure of skeletal muscle, and may have a good effect on somatic symptoms such as muscle fatigue and soreness in depression. It is highly recommended to be combined with SSRIs in the treatment of patients with depression. Clinical experience has found that the Chinese medicine prescription has remarkable curative effect, and no adverse reactions occur when used alone or in combination.
Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 81273624, 81573843).
Funding
Funding is by The National Natural Science Foundation of China (No. 81273624, 81573843).
Availability of Data and Materials
We have presented all our main data in the form of figures and tables. The datasets supporting the conclusions of this article are included within the article.
Authors’ Contribution
Conceived and designed the experiments: Rongjuan Guo, Yang Li, Shengli Zhang, Yao Yu. Performed the experiments: Yang Li, Shengli Zhang, Yao Yu, Kaihang Guo. Analyzed the data: Yang Li, Shengli Zhang, Rongjuan Guo. Contributed reagents/materials/ analysis tools: Rongjuan Guo, Yang Li, Yao yu, Kaihang Guo. Wrote the paper: Yang Li, Shengli Zhang, Rongjuan Guo. General ideas and conception: Rongjuan Guo, Yang Li, Yao Yu.
Competing Interests
The authors declare that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Consent for Publication
My article excludes human participants and clinical data.
Ethics Approval and Consent to Participate
Procedures involving animals and their care were conducted in conformity with NIH guidelines (NIH Pub. No. 85-23, revised 1996) and was approved by Animal Care and Use Committee of Beijing University of Chinese Medicine.
References
- Tao Y, Zhang L (2018) Efficacy and safety of fluoxetine combined with quetiapine in the treatment of refractory depression. Practical Medicine in China 03: 129-130.
- Ligthart L, Gerrits MM, Boomsma DI, Penninx BW (2013) Anxiety and depression are associated with migraine and pain in general: an investigation of the interrelationships. J Pain 14: 363-370.
- Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J (1999) An international study of the relation between somatic symptoms and depression. N Engl J Med 341: 1329-1335.
- Means-Christensen AJ, Roy-Byrne PP, Sherbourne CD, Craske MG, Stein MB (2008) Relationships among pain, anxiety, and depression in primary care. Depress Anxiety 25: 593-600.
- Björnsdóttir SV, Jónsson SH, Valdimarsdóttir UA (2014) Mental health indicators and quality of life among individuals with musculoskeletal chronic pain: a nationwide study in Iceland. Scand J Rheumatol 43: 419-423.
- Shiri R, Kaila-Kangas L, Ahola K, Kivekäs T, Viikari-Juntura E, et al. (2013) The relation of co-occurring musculoskeletal pain and depressive symptoms with work ability. J Occup Environ Med 55: 1281-1285.
- Karp JF, Scott J, Houck P, Reynolds CF, Kupfer DJ, et al. (2005) Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry 66: 591-597.
- Ferguson M, Dennehy EB, Marangell LB, Martinez J, Wisniewski SR (2014) Impact of fatigue on outcome of selective serotonin reuptake inhibitor treatment: secondary analysis of STAR*D. Curr Med Res Opin 30: 2109-2118.
- Louca-Jounger S, Christidis N, Svensson P, List T, Ernberg M (2017) Increased levels of intramuscular cytokines in patients with jaw muscle pain. J Headache Pain 18: 30.
- Vyatkina G, Bhatia V, Gerstner A, Papaconstantinou J, Garg N (2004) Impaired mitochondrial respiratory chain and bioenergetics during chagasic cardiomyopathy development. Biochimica et biophysica acta 1689: 162-173.
- Maes M (2011) Depression is an inflammatory disease.but cell-mediated immune activation is the key component of depression.Prog Neuropsychopharmacol Biol Psychiatry 35: 664-675.
- Song C,Wang H (2011) Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry 35: 760-768.
- Berk M,Kapczinski F,Andreazza AC (2011) Pathways underlying neuroprogression in bipolar disorder: focus oninflammation,oxidative stress and neurotrophic factors.Neurosci Biobehav Rev 35: 804-817.
- Patel A (2013) The role of inflammation in depression. Psychiatr Danub Suppl 2: S216-S223.
- Noto C, Rizzo LB, Mansur RB (2014) Targeting the inflammatory pathway as a therapeutic tool for major depression. Nueroimmunomodulation 21: 131-139.
- Martin C, Tansey KE, Schalkwyk LC (2015) The inflammatory cytokines: molecular biomarkers for major depressive disorder?. Biomark Med 9: 169-180.
- Park KM, Bowers WJ (2010) Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal 22: 977-983.
- Li X, Huang Q, Ong CN (2010) Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett 293: 109-116.
- Usha S, Ajit K, Rajesh S (2010) Calreticulin transacetylase catalyzed modification of the TNF-alpha mediated pathway in the human peripheral blood mononuclear cells by polyphenolic acetates. Chemico Biological Interactions 185: 263-270.
- Hovhannisyan Z, Treatman J, Littman DR (2011) Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterolog 140: 957-965.
- Rose-John S, Waetzig GH, Scheller J (2007) The IL-6/slL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets 11: 613-624.
- Fattal O, Link J, Quinn K (2007) Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS spectrums 12: 429-438.
- Shao L, Martin MV, Watson SJ (2008) Mitochondrial involvement in psychiatric disorders. Ann Med 40: 281-295.
- Gong Y, Chai Y, Ding JH (2011) Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neuroscience letters 488: 76-80.
- Samavati, L, Lee I, Mathes I, Lottspeich, F, Huttemann M (2008) Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem 283: 21134-21144.
- Che Y, Zhou Z, Shu Y (2015) Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neuroscience letters 584: 208-213.
- Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39: 73-82.
- Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, Kalynchuk LE (2018) Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Frontiers in Neuroscience. 2018; 12: 1-13.
- Gardner A, Boles RG (2008) Symptoms of somatization as a rapid screening tool for mitochondrial dysfunction in depression. Bio Psycho Social Medicine 2: 7.
- Wang C, Guo J, Guo R (2017) Effect of Xingpi Jieyu decoction on spatial learning and memory and cAMP-PKA-CREB-BDNF pathway in rat model of depression through chronic unpredictable stress. BMC Complement Altern Med 17: 73.
- Chunye W, Rongjuan G, Xiaochen Z (2014) Effect of Xingpi Jieyu decoction on learning-memory behavior and inflammatory factors level in depression rats. Beijing Journal of Traditional Chinese Medicine 2014: 503-506.
- Chunye W, Rongjuan G (2014) The effect of Xing pi jie yu decoction on depressive behavior and serum 5-HT as well as corticosterone of depression rats from chronic stress. World chinese medicine 2014: 1633-1639.
- Qingjie Y, Jianyou G, Jianwei W, Yang L, Xiaofei D, et al. (2017) Study on syndrome of stagnation of liver qi and spleen deficiency in depression based on corticosterone-inflammation-mitochondrial network and effects of Xing Pi Jie Yu Decoction. China Journal of Traditional Chinese Medicine and Pharmacy 2017: 2241-2245.
Citation: Li Y, Zhang S, Yu Y, Guo K, Guo R (2019) Protective Effect of XPJY Decoction on Inflammation and Mitochondrial Function/Structure of Skeletal Muscle in Depressive Rats. J Neuroinfect Dis 10: 284. DOI: 10.4172/2314-7326.1000284
Copyright: © 2019 Li Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4171
- [From(publication date): 0-2019 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 3229
- PDF downloads: 942