Research Article Open Access
Simultaneous Quantification of Propofol and its Non-Conjugated Metabolites in Several Biological Matrices Using Gas Chromatography/Ion Trap ? Mass Spectrometry Method
Sónia Campos1,2*, Joaquim Monteiro3, Luís Antunes1, Paula S Branco4, Luísa M Ferreira4, Luís Félix1 and Paula Guedes de Pinho2*1CITAB - Centro de Investigação e de Tecnologias Agro-Ambientais e Biológicas, University of Trás-os-Montes and Alto Douro, Quinta de Prados, 5001-801 Vila Real, Portugal and LAS - Institute of Molecular and Cell Biology, Porto, Rua do Campo Alegre, nº 823, 4150-180 Porto, Portugal
2REQUIMTE – Toxicological Laboratory, Department of Biological Sciences, Faculty of Pharmacy, University of Porto, Rua Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal
3CESPU, Institute of Research and Advanced Training in Health Sciences and Technologies, Department of Pharmaceutical Sciences, Higher Institute of Health Sciences (ISCS-N), Rua Central de Gandra 1317, 4585-116, Gandra PRD, Portugal
4REQUIMTE - Department of Chemistry, Faculty of Science and Technology, University Nova of Lisbon, Campus de Caparica, 2829-516 Caparica, Portugal
- *Corresponding Author:
- Sónia Campos
REQUIMTE – Toxicological Laboratory, Biological Science Department
Faculty of Pharmacy, University of Porto, Rua Jorge Viterbo Ferreira
228, 4050-313 Porto, Portugal
Tel: +351 22 0428000(ext. 8796)
E-mail: soniapatcampos@gmail.com
Paula Guedes de Pinho
REQUIMTE – Toxicological Laboratory, Biological Science Department
Faculty of Pharmacy, University of Porto, Rua Jorge Viterbo Ferreira
228, 4050-313 Porto, Portugal
Tel: +351 22 0428000 (ext.8796)
E-mail: pguedes@ff.up.pt
Received date: May 27, 2014; Accepted date: June 30, 2014; Published date: July 02, 2014
Citation: Campos S, Monteiro J, Antunes L, Branco PS, Ferreira LM, et al. (2014) Simultaneous Quantification of Propofol and its Non-Conjugated Metabolites in Several Biological Matrices Using Gas Chromatography/Ion Trap – Mass Spectrometry Method. J Anal Bioanal Tech 5: 195. doi: 10.4172/2155-9872.1000195
Copyright: © 2014 Campos S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Propofol is an important compound used for anaesthetic purposes in clinical practice. Nevertheless, in the recent years, the use of propofol has also been reported for recreational, abusive or even for suicidal and criminal purposes. So far, there is a lack of practical techniques validated for simultaneous quantification of propofol and its non-conjugated metabolites (2,6-diispropyl-1,4-quinol and 2,6-diispropyl-1,4-quinone) in plasma and organs, to optimize therapeutics, to prevent undesired effects, and for application in forensic settings.
A simple gas chromatography/ Ion trap – mass spectrometry method was optimized for the detection and quantification of propofol and its non-conjugated metabolites in plasma and organ (liver, heart, kidney and lungs) samples. All compounds were simultaneously extracted from 0.5 mL of plasma and 0.2 g of each organ, following a straightforward and rapid procedure using thymol as internal standard. This method was validated according to international guidelines for analytical methods.
The standard curve ranged from 0.005 to 100 μg/mL for propofol and 0.005 to 50 μg/mL for the non-conjugated metabolites. Intra and inter-assay variability for propofol and its metabolites was less than 15% and the average recovery was greater than 90%. The proof of applicability of this methodology allowed the successful measurement of propofol and its non-conjugated metabolites in plasma and solid tissues from seven New Zealand White rabbits that were submitted to a long-term anaesthesia protocol with a continuous infusion of propofol ranging from 20 to 60 mg/kg/h.
This optimized and validated assay may also be suitable in the monitoring of sedated or anaesthetised animals and humans with continuous infusions of propofol and for use in pharmacokinetic and toxicological studies.
Keywords
Propofol; Propofol non-conjugated metabolites; Propofol method validation
Introduction
The drug, propofol (2,6-diisopropylphenol), is an intravenous anaesthetic which arose from the family of alkylphenols and showed to have sedative-hypnotic properties in animals [1,2] and in humans [3,4], by interacting with GABA receptors [5]. Propofol acquired great acceptation worldwide being extensively used for induction and maintenance of anaesthesia in human and veterinary practice. Moreover, propofol has been given in lower doses to critically ill patients for stabilization and titratable sedation in intensive care units (ICU) [6]. Since it is known to produce mild euphoria and hallucinations, the use of propofol for recreational purposes has been an issue. Furthermore, many deaths have occurred due to its abuse and misuse [7]. Some case reports are available in literature related to suicides by using propofol alone or in mixtures [8,9].
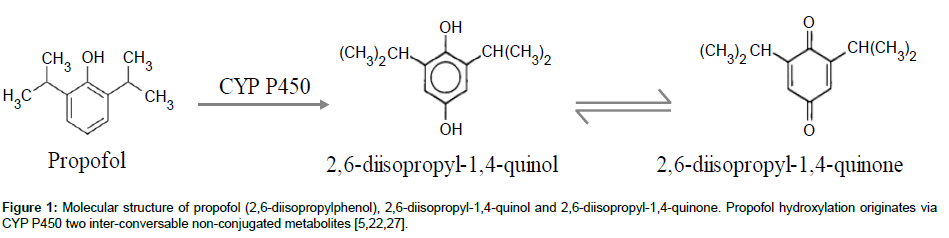
Propofol has a rapid and extensive biotransformation into multiple non-conjugated and conjugated metabolites. The nonconjugated metabolites result from propofol ring hydroxylation (CYP P450) and consist in two inter-conversable isomers: 2,6-diisopropyl- 1,4-quinol and 2,6-diisopropyl-1,4-quinone (Figure 1). As for propofol, they can be found in the blood stream (plasma and serum) and organ tissues and are suspected to have one third of propofol hypnotic activity [10,11]. Furthermore, the infusion of propofol in higher doses and for long periods has been associated with a rare but potentially fatal set of clinical and toxicological features in critically ill patients: Propofol Infusion Syndrome (PRIS). This syndrome has already been described in humans [3,12], rabbits [13] and dogs [14]. The onset mechanism of this syndrome is not well understood but the non-conjugated metabolites of propofol may be implied as trigger compounds [12,13,15]. A huge diversity of clinical signs (asystole, hyperlipidaemia, metabolic acidosis, fatty liver, etc) has been related with this pathology, reinforcing the need of a close monitoring of patients. Nevertheless, there is a lack of practical techniques validated for simultaneous quantifications of propofol and its metabolites, in order to optimize therapeutics, prevent undesired effects and determine the real causes of intoxications or deaths due to propofol use.
Propofol quantification in human blood or plasma was already described using high-performance liquid chromatography (HPLC) [16-21], liquid chromatography with tandem mass spectrometry (LCMS/ MS) [22], gas chromatography-mass spectrometry (GC-MS) [23-25] and gas chromatography quadrupole (EI-MS/MS) [26]. In humans, the quantification of propofol has been performed in blood or plasma, brain, liver and adipose tissue using GC-MS [23,27]. The quantification of non-conjugated metabolites of propofol was previously done in rat liver microssomes [28] using GC-MS and in human blood and urine using HPLC [29]. However, none of these cited methods determine simultaneously propofol and its non-conjugated metabolites in plasma and several organ matrices (liver, lung, heart and kidney).
Hence, the present work aims to develop, optimize and validate the analytical method initially described by Guitton and co-authors [23,28] to allow the simultaneous quantification of propofol and its non-conjugated metabolites (2,6-diisopropyl-1,4-quinol and 2,6-diisopropyl-1,4-quinone), using GC/IT-MS, in plasma and organ tissues, in clinical settings or toxicological studies. The method validation will follow the European Medicine Agency (EMA), the Food and Drug Administration (FDA) and the High Conference Harmonization (ICH) guidelines for analytical methods.
Materials and Methods
Drugs and reagents
Propofol (2,6-diisopropylphenol) and thymol (2-isopropyl- 5-methylphenol) were obtained by Sigma-Aldrich (Portugal). Thymol was used as an internal standard (IS). The non-conjugated metabolite standards (2,6-diisopropyl-1,4-quinol and 2,6-diisopropyl- 1,4-quinone) were synthesised according to an optimized and published procedure [5]. The chloroform and the methanol were provided by Fisher Scientific (Portugal) and ethylacetate was from Fluka (Buchs, Switzerland). Boric acid used in the preparation of the buffer was obtained from Sigma (Sintra, Portugal). Solid NaOH and KCl were acquired from Merck (Darmstadt, Germany). Ultra-pure water was from Milli-Q system (Millipore, Bedford, MA, USA).
Equipment and software
The quantification of propofol and its non-conjugated metabolites in plasma and organ tissues samples was performed using a GC Varian CP-3800 (California, USA) equipped with a selective ion trap mass detector (Varian Saturn 4000, California USA). The chromatographic column was a VF-5 Factor Four model (30 m×0.25 mm×0.25 μm) from Varian. A Combi-PAL autosampler (Varian Pal Autosampler, Switzerland) and Cycle Composer software (CTC Analytics System Software, Switzerland) were used for all experiments. The software used to manage data was the Saturn GC/MS Workstation software 6.8 (California USA). The ionization was performed by electronic impact at 70 eV. The carrier gas was Helium C-60 from Gasin (Porto, Portugal).
The Precellys24® tissue homogenizer (Precellys24® technology, Bertin Technologies, Villeurbanne, France) was used for the homogenization process of organ samples.
Stock and working solutions
Stock solutions of propofol (1 mg/mL), 2,6-diisopropyl-1,4-quinone (1 mg/mL), 2,6-diisopropyl-1,4-quinol (1 mg/mL) and thymol (1 mg/mL) were prepared in methanol. Borate buffer solution (0.1 M) was prepared by dissolution of boric acid in ultra-pure water. It was adjusted to pH=9 with a sodium hydroxide solution (1 M). A 0.2 M potassium chloride (KCl)/ 0.2 M sodium hydroxide (NaOH) solution was prepared by dissolution of KCl and NaOH separately and mixed immediately after preparation, totalling a final solution of 100 mL. The pH was adjusted till 12.4 with NaOH solution (1M). A mixture of chloroform: ethylacetate (70:30 v:v) was used to extract propofol and free-metabolites from the matrix. All solutions were prepared daily. For stability tests, propofol and thymol stock solutions were stored at -80°C for three weeks.
Standard solutions, quality controls (QC) and blank samples
Each analyte prepared from the stock solutions was added to the blank matrix (plasma, liver, kidney, lungs and heart matrix). The final concentrations of the standard solutions were: 0, 0.5, 1, 5, 10, 50 and 100 μg/mL for propofol and 0, 0.1, 0.5, 1, 5, 10 and 50 μg/mL for propofol non-conjugated metabolites. Four quality controls (QC) were selected for each analyte and prepared like standard solutions: propofol – 0.005 (Lower limit of quantification - LLOQ), 1, 10 and 100 μg/ mL; non-conjugates metabolites of propofol – 0.005 (LLOQ), 0.5, 5 and 50 μg/mL. Control samples were prepared by adding 50 μL of IS (0.01 mg/mL) in 0.5 mL of drug-free plasma and organ samples, followed by the extraction procedure. Blank samples were obtained from drug-free plasma and organ samples, followed by the extraction procedure. Daily standard solutions and quality controls were prepared in ice. Some aliquots (containing the organic phase) were stored at 4°C (one week) for stability tests.
Chromatographic conditions
The organic phase obtained from the extraction procedure was injected (2 μL) into the Gas Chromatography/Ion Trap-Mass Spectrometry (GC/IT-MS) injector in split mode 1:10, at 250°C. The setting of column temperature was 100°C (during 1 min), then raised 15°C/min until 300°C (kept for 10 min). In the first 4 min of the analysis the ion source was maintained off to avoid solvent overloading. The temperature of transference line, manifold and ion trap were respectively 280, 50 and 180°C. Only the ions with m/z >50 and <600 were analysed in the ion trap. The emission current was 50 μA and maximum analysis time was 3500 μ. The detection of thymol, propofol and non-conjugated metabolites was conducted in Fullscan mode, and the quantification performed by reprocessing the Fullscan chromatogram with the characteristic m/z fragments of each molecule. For propofol the m/z used were m/z=163 and m/z=178, for thymol the m/z=150 and m/z=135, for 2,6-diisopropyl-1,4-quinol m/z=179 and m/z=194 and for 2,6-diisopropyl-1,4-quinone m/z=149 and m/z=192 [23,25,28].
The identification of each compound in each sample was performed in the same chromatographic conditions, by comparative analysis between the retention times of pure compounds with the compounds in the injected samples.
Extraction procedure in plasma and organ tissues
Arterial blood samples were collected into heparinized tubes and then centrifuged at 4000 rpm for 15 minutes in order to obtain plasma. Plasma and organ samples from liver, lungs, kidneys and heart were preserved at -80°C till analysis.
Extraction procedures were based on previous works [23,27,28] and which were modified, extended and validated. Concerning quantification in organ tissue, to 0.2 g of each tissue sample 0.6 mL of KCl/ NaOH buffer solution was added in a 1.5 mL Precellys24® lysing tube with ceramic beads. Samples were homogenized using the Precellys24®tissue homogenizer by applying 2 cycles at speeds ranging from 4000 rpm to 6800 rpm during 30 seconds. After, the homogenised samples were centrifuged at 1500 rpm for 2 min and 0.5 mL of the supernatant was collected to a 5 mL tube. To a 0.5 mL aliquot of plasma, 0.5 mL organ supernatant or to the propofol/non-conjugated metabolites calibration standards, 50 μL of thymol (0.01 mg/mL) and 1 mL of ultra-pure water were added. To each processed sample 0.5 mL of borate buffer (pH=9) was added and mixed by inversion for 5 min. Then, 300 μL of chloroform: ethylacetate (70:30 v:v) were added and also mixed by inversion for 20 min. After a 10 min centrifugation, 2 μL of the organic phase were injected into the Gas Chromatography/Ion Trap-Mass Spectrometry (GC/IT-MS) [23].
Bioanalytical method validation
GC/IT-MS method validation: The GC/IT-MS method was validated according to international guidelines produced by European Medicine Agency (EMA) [30], Food and Drug Administration (FDA) [31] and International Conference Harmonization (ICH) [32]. Accordingly to these guidelines, to ensure the acceptability of the performance and reliability of the analytical results, the following characteristics were considered: selectivity, carry-over, lower limit of quantification (LLOQ), detection limit (DL), calibration range, accuracy, precision, stability of the analyte in each biological matrix and stability of the analyte and of the internal standard in the stock and working solutions under the entire period of storage and processing conditions. The proof of applicability of the developed methodology, ensuring its simplicity, sensitivity, specificity, accuracy, reproducibility and fastness, was performed to determine propofol and free-metabolites in plasma and tissues organs samples of rabbits submitted to general anaesthesia with propofol.
The selectivity was proved by the analysis of six blank matrices (plasma and organ tissues), with the absence of interfering components with an observable limit of less than 20% of the lower limit of quantification for propofol and its metabolites and 5% for thymol (IS) [30].
The carry-over study was assessed by analysing six blank matrices, after the injection of a high concentration standard solution. Carry over should not be greater than 20% of LLOQ for propofol and its nonconjugated metabolites and 5% for thymol [30]. The matrix effects were investigated using 6 blank matrix from individual donors. For each analyte and IS, the matrix factor and CV (coefficient of variation, %) was calculated as indicated in the EMA guideline and the overall CV calculated for all low and high level of concentration which should be lower than 15%.
The lower limit of quantification (LLOQ) is the lowest concentration of analyte in a sample, which can be reliably quantified, with acceptable accuracy and precision. The LLOQ signal should be at least 5 times the signal of a blank sample [30].
The detection limit (DL) is determined by the analysis of samples with known concentrations of analyte and by establishing the minimum level at which the analyte can be reliably detected [32]. The DL was calculated using the following equation: DL=(3.3 s)/ S, where s is the standard deviation of the mean value resulting from analysis of an appropriate number (N=20) of blank samples and S is the slope of the calibration curve [30].
A six-point calibration curve was constructed for propofol by plotting the ratio of propofol/ IS areas (y) vs propofol concentration (x) (carried out in triplicate). The concentration range for the calibration curve was defined according to expected propofol plasma and organs concentration. Results for blank samples were not used as part of the calibration curve [30]. Slope, intercept and linearity were determined by calculating the regression equation from the plot of peak area ratio vs concentration, for six standard solutions using the linear least squares method [31]. The same procedure was done for each one of the non-conjugated metabolites of propofol. Propofol and non-conjugated metabolites solutions concentrations were also back calculated, using the calibration curve, and mean accuracy values were determined.
The accuracy was tested by calculating the percentage of the nominal value (relative error, %) of the four QC (quality control) of propofol and non-conjugated metabolites concentrations defined. Accuracy was evaluated within a single run (within-run accuracy) and in different runs (between-run accuracy). Within-run accuracy was determined by analysing, in a single run, a minimum of 5 samples per propofol and non-conjugated metabolites concentration level. For the validation of the between-run accuracy, samples, from at least three runs, were analysed on three different days [30].
The precision of the analytical method describes the closeness of repeated individual measures of analyte expressed as the coefficient of variation (CV). For precision determination, samples were analysed within a single run and in different runs, using the same runs and data as for the demonstration of accuracy [30].
Stability was evaluated by using three QC solutions under distinct periods of storage (stock solutions maintained at -80°C for 3 weeks) and processing conditions (organic phase maintained at 4°C during one week) for both propofol and the non-conjugated metabolites.
Extraction procedure validation: Absolute recovery (%) was performed according to Hikiji et al. [27] and calculated by comparing the concentration of extracted propofol and non-conjugated metabolites in organ samples and plasma (n=4 for each tissue) containing 0.5 μg of analyte per gram or mL of tissue, respectively, after the extraction procedure.
Method applicability
The described method was applied to the simultaneous analysis of different analytes in biological samples with different matrices, collected from anaesthetised animals with propofol. An analytical run consisted of a blank sample (processed matrix sample without analyte and without IS), a zero sample (processed matrix with IS), 6 calibration standards, 4 levels of QC samples (in triplicate), plasma samples taken during the anaesthetic period and organ tissues taken at the end of anaesthesia and after animals’ euthanasia. As indicated before, the calibration standards and QC samples have been spiked independently using separately prepared stock solutions [30]. All samples were analysed in triplicate, except when indicated otherwise. The optimized and validated method was used to quantify propofol concentrations in plasma and organs from our study population.
Experimental protocol: Seven healthy male New Zealand White rabbits, average weight 3.67 ± 0.15 Kg, approximately 3 months old, were used. The animals were properly housed, in a room with controlled temperature, ventilation and light/dark cycle. Commercial pellet food and water were provided ad libitum. Animal welfare, behaviour and health were daily evaluated by a specialized technician.
This study was conducted in agreement with the European animal welfare laws for care and use of experimental animals and with the ARRIVE guidelines for animal research, being approved by the national regulatory office: Direcção Geral de Alimentação Veterinária (DGAV), with the protocol No 0420/000/000/2009.
Each animal was prepared for propofol administration and blood samples withdraw (vein and arterial cannulation, respectively). Blank blood samples were taken before anaesthesia in every animal. Induction of anaesthesia started with a propofol bolus of 20 mg/kg [13] followed by an infusion rate of 60 mg.kg-1.h-1. Oxygen support was ensured during the entire anaesthesia. According to the IoC values and reflexes responses, infusion rates (20, 30, 40, 50 and 60 mg/kg/h) were adjusted in order to maintain a similar level of anaesthetic depth along time in all animals. The anaesthesia was kept during twenty consecutive hours with arterial blood samples collected every 3 h for propofol quantification (0, 3, 6, 9, 12, 15, 18 and 20 h). Blood gases measurements were also performed. At the end, the animals were euthanized and organ samples from liver, lungs, kidneys and heart were taken.
Results
Bioanalytical validation
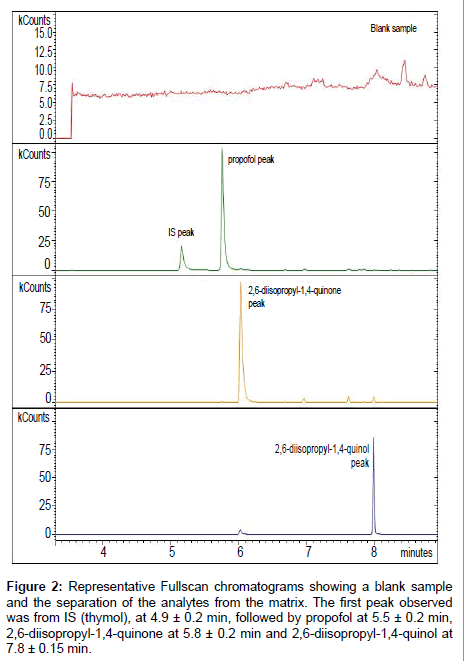
GC/IT-MS method validation: GC/IT-MS acquisition was performed in Fullscan mode. After acquisition chromatograms were processed by using the characteristic m/z of propofol and its non-conjugated metabolites. Four individual and distinctive peaks were observed in every chromatogram (Figure 2). The first peak observed was from thymol, at 4.9 ± 0.2 min, followed by propofol at 5.5 ± 0.2 min, 2,6-diisopropyl-1,4-quinone at 5.8 ± 0.2 min and 2,6-diisopropyl- 1,4-quinol at 7.8 ± 0.15 min. The peak area of each analyte resulted from the abundance of the monitoring ions related to each compound: for propofol the m/z ions used were 163 and 178, for thymol m/z 135 and 150, for 2,6-diisopropyl-quinone m/z 192 and 149 and for 2,6-diisopropyl-quinol the m/z ions 179 and 194 [23]. These nonconjugated metabolites of propofol are described as inter-convertible compounds in tautomeric equilibrium [23]. So as to, the study of the non-conjugated metabolites was performed using the sum of the area obtained for each metabolite. Only for the LLOQ and DL determination they were treated separately.
Figure 2: Representative Fullscan chromatograms showing a blank sample and the separation of the analytes from the matrix. The first peak observed was from IS (thymol), at 4.9 ± 0.2 min, followed by propofol at 5.5 ± 0.2 min, 2,6-diisopropyl-1,4-quinone at 5.8 ± 0.2 min and 2,6-diisopropyl-1,4-quinol at 7.8 ± 0.15 min.
Selectivity: The method selectivity was analysed by comparing the chromatograms of six blank matrices to the chromatogram of the calibrator used to quantify the LLOQ (0.005 μg/mL) of propofol and its non-conjugated metabolites. No other confounding peaks at the retention time of each analyte were observed. A representative chromatogram showing the separation of the analytes from the matrix is shown in Figure 2. As acceptance criteria, the absence of interfering components was validated when signal is lower than 20% of the LLOQ for the analyte and 5% for the IS. The results obtained are represented in Table 1. Generally, obtained data indicate that the method is selective as no potential interfering peaks were detected.
| Analyte (n=6 each matrix) |
Matrix | LLOQ Area(mean) | Blank Area (mean) |
Result (%) |
Acceptance criteria |
|---|---|---|---|---|---|
| Propofol | Plasma | 4422.2 | 311 | 7.03 | <20% |
| Liver | 6637.3 | 290.3 | 4.40 | ||
| Kidney | 3171.8 | 299 | 9.42 | ||
| Lung | 4462.8 | 487.7 | 10.93 | ||
| Heart | 3900.8 | 182.2 | 4.70 | ||
| 2,6-diisopropyl-1,4-quinol | Plasma | 734.5 | 16.2 | 2.20 | <20% |
| Liver | 1664.3 | 17.3 | 1.04 | ||
| Kidney | 2103 | nd | - | ||
| Lung | 2436.2 | 22.7 | 0.93 | ||
| Heart | 1845.2 | 14.5 | 0.79 | ||
| 2,6-diisopropyl-quinone | Plasma | 1363.7 | 41 | 3.0 | <20% |
| Liver | 4807.2 | 32.7 | 0.67 | ||
| Kidney | 3650 | nd | - | ||
| Lung | 3792.5 | 31.7 | 0.83 | ||
| Heart | 3284 | 12 | 0.36 | ||
| IS (Tymol) | Plasma | 109169 | 683.3 | 0.62 | <5% |
| Liver | 119696 | 874.2 | 0.73 | ||
| Kidney | 114529 | 928.8 | 0.81 | ||
| Lung | 121898 | 490.5 | 0.40 | ||
| Heart | 115054 | 753.7 | 0.66 |
IS – internal standard; LLOQ – Lower limit of quantification; nd – not detected
Table 1: Selectivity validation of propofol, 2,6-diisopropyl-1,4-quinone and 2,6-diisopropyl-1,4-quinol in different matrices (plasma, liver, kidney, lung and heart).
Carry-over and matrix effects: The carry-over was not observed in the six blank samples, analysed after the higher concentration standard solution. In agreement with the guidelines, a noise signal of the blank plasma showed to be lower than 20% of the LLOQ and lower than 5% of the IS (Table 2).
| Analyte (n=6 for each) |
Carry-over | Matrix effects | |||||
|---|---|---|---|---|---|---|---|
| LLOQ Area (Mean) |
Blank Area (Mean) | Result (%) |
Acceptance criteria | CV (%) | Acceptance criteria | ||
| LC | HC | ||||||
| Propofol | 4518.98 | 314.04 | 6.95 | <20% | 6.46 | 7.12 | <15% |
| 2,6-diisopropyl-quinol | 1756.64 | 14.14 | 0.80 | <20% | 8.32 | 5.43 | <15% |
| 2,6-diisopropyl-quinone | 3379.48 | 23.48 | 0.69 | <20% | 10.22 | 7.43 | <15% |
| IS | 116069.2 | 746.1 | 0.64 | <5% | 4.82 | 5.19 | <15% |
IS – internal standard; LLOQ – Lower limit of quantification; LC – lower concentration; HC – High concentration
Table 2: Carry-over and matrix effects validation of propofol, 2,6-diisopropyl-1,4-quinone and 2,6-diisopropyl-1,4-quinol.
No matrix effects were found for all matrix and compounds studied with CV (%) lower than 15% (Table 2).
Lower limit of quantification (LLOQ) and detection limit (DL): In the current study, the LLOQ was defined according to bibliography and the expected concentrations in the different matrices. As a favourable criterion for the LLOQ of propofol and its non-conjugated metabolites, the signal should be at least 5 times the signal of a blank sample [31]. The DL was calculated from the standard deviation (SD) of the mean value obtained from the analysis of 20 blank samples and the slope of the calibration curve, as previously described. The LLOQ was defined as 5 ng/mL for all analytes whereas the DL was 2.95 ng/mL for propofol, 0.98 ng/mL for 2,6-diisopropyl-quinol and 0.68 ng/mL for 2,6-diisopropyl-quinone in all biological matrices.
Linearity studies: Linearity was analysed in the concentration range of 0.005-100 μg/mL and 0.005-50 μg/mL for propofol and non-conjugated metabolites, respectively. Firstly, a visual inspection of the calibration curves plot was observed and then, the square correlation coefficient (R2) was calculated by the least squares method. A high correlation coefficient of 0.99 is often used as criterion of good linearity [27]. Moreover, linearity of the regression line was evaluated by a procedure based on the residual sum of squares (RSS): taking the regression line as the mean, a RSS calculated for all data points, revealing values <2.0%, respectively a mean of 0.78% for propofol and 0.68% for nonconjugated metabolites.
The construction of the calibration curves included seven standard solutions. For propofol the set concentrations were: 0.005, 0.5, 1, 5, 10, 50 and 100 μg/mL and for the non-conjugated metabolites were used the following concentrations: 0.005, 0.1, 0.5, 1, 5, 10 and 50 μg/ mL. Four different runs were carried out, in four consecutive days, first for propofol and then, for the non-conjugated metabolites. The linear regression of the ratio of the analyte area to that of the IS versus the propofol or non-conjugated metabolites were used. The mean equations (n=6) of the calibration curve obtained from mean of these seven points were: propofol: Y=1.204 (± 0,010) X + 0.959 (± 0.486), R2=0.9989 and for non-conjugated metabolites: Y=0.916 (± 0.006) X – 0,183 (± 0.130), R2=0.9981, where Y is the ratio of propofol or nonconjugated metabolites peak area/IS peak area and X is the real propofol or non-conjugated metabolites concentration in μg/mL. Mean and SD values for Y and X are indicated inside brackets.
Back-calculated concentrations: Back-calculated concentrations of standard solution are a useful procedure to validate or reject some points of the concentration range used, during the bioanalytical method validation. Table 3 shows, in terms of accuracy, the relative error (RE; %) of the back calculated concentrations from the standard solutions used in the calibration curve. All back calculated concentrations complied with the acceptance criterion defined internationally [30].
| Standard solutions | Mean (n=6) | RE (%) | Acceptance criteria | |
|---|---|---|---|---|
| Propofol | 0.005 | 0.0059 | 18.03 | <20% |
| 0.5 | 0.5118 | 2.36 | <15% | |
| 1 | 1.0099 | 0.99 | ||
| 5 | 5.5973 | 11.9 | ||
| 10 | 10.6835 | 6.84 | ||
| 50 | 50.8894 | 1.78 | ||
| 100 | 100.4091 | 0.41 | ||
| Non-conjugated metabolites | 0.005 | 0.0052 | 4.86 | <20% |
| 0.1 | 0.1095 | 9.53 | <15% | |
| 0.5 | 0.5101 | 2.20 | ||
| 1 | 1.0461 | 4.61 | ||
| 5 | 5.1012 | 2.02 | ||
| 10 | 10.0772 | 0.77 | ||
| 50 | 51.1131 | 2.23 | ||
RE- relative error
Table 3: Back-calculated concentration of standard solution using calibration equation for propofol and non-conjugated metabolites.
Accuracy: The accuracy of an analytical method is described as the closeness of the determined value obtained by the optimized method, by calculating the percentage recoveries of the mean concentration of each analyte at four different concentrations. The four QC were: 0.005 (LLOQ), 1, 10 and 100 μg/mL for propofol and 0.005 (LLOQ), 0.5, 5 and 50 μg/mL for the non-conjugated metabolites. The results for the within-run accuracy (n=5) are expressed in Table 4 whereas Table 5 shows the between-run accuracy (n=3), demonstrating the concordance between experimental and nominal data. Once again, the obtained results fulfil the internationally acceptance criteria: the mean should be within 15% of the nominal values for the QC samples, except for the LLOQ which should be within 20% of the nominal value [30].
| QC (µg/mL) | Mean measured concentrations (µg/mL) (n=5) | RE (%) | CV (%) | Acceptance criteria | |
|---|---|---|---|---|---|
| Propofol | 0.005 | 0.0059 | 18.03 | 3.07 | <20% |
| 1 | 1.009910.6835100.4091 | 0.99 | 0.83 | <15% | |
| 10 | 6.84 | 0.84 | |||
| 100 | 0.41 | 0.54 | |||
| Non-conjugated metabolites | 0.005 | 0.0052 | 4.87 | 7.00 | <20% |
| 0.5 | 0.51105.101351.1131 | 2.20 | 1.07 | <15% | |
| 5 | 2.03 | 2.64 | |||
| 50 | 2.23 | 0.19 | |||
QC- Quality control; RE- Relative error; CV- Coefficient of variation
Table 4: Within-run accuracy and within-run precision data. QC means quality control, RE relative error and CV is the coefficient of variation (n =5).
Precision: Precision ensures the ability of the method to generate reproducible results. Method precision was validated for within-run and between-run, in terms of variation (CV - coefficient of variation, %) as demonstrated in Tables 4 and 5, respectively. The obtained results point that the developed and optimized GC/IT-MS method presents good precision, since it fulfils the acceptance criteria internationally established [30].
| QC (µg/mL) | Mean measured concentrations (µg/mL) (n=5) | RE (%) | CV (%) | Acceptance criteria | |
|---|---|---|---|---|---|
| Propofol | 0.005 | 0.0055 | 9.00 | 8.13 | <20% |
| 1 | 0.920711.0485106.1178 | 3.45 | 4.14 | <15% | |
| 10 | 10.48 | 5.73 | |||
| 100 | 6.12 | 11.67 | |||
| Non-conjugated metabolites | 0.005 | 0.0051 | 2.38 | 6.37 | <20% |
| 0.5 | 0.44885.094350.6116 | 0.68 | 2.58 | <15% | |
| 5 | 1.89 | 6.75 | |||
| 50 | 1.22 | 2.85 | |||
QC- Quality control; RE- Relative error; CV- Coefficient of variation
Table 5: Between-run accuracy and between-run precision data. QC means quality control, RE relative error and CV is the coefficient of variation (n =3).
Stability: The stability of propofol and its non-conjugated metabolites together with IS was evaluated under similar conditions to those that occur in a real time analysis. In agreement with the validation requirements for bioanalytical methods, to validate the storage conditions, we evaluated the stability of frozen stock solutions (three weeks at -80°C) and also the stability of the organic phase stored at 4°C during one week. These tests were performed by preparing in triplicate the lower (0.005 μg/mL) and the higher (100 μg/mL for propofol and 50 μg/mL for non-conjugated metabolites) QC. Accuracy results (Table 6), represented as RE (%), shows that the GC/IT-MS method developed and optimized has good stability and can be used to analyse fresh and frozen samples. Moreover, these results are in agreement with international guidelines (RE<2%) [30].
| Stock solution stability frozen at -80°C for 3 weeks | |||||
|---|---|---|---|---|---|
| QC | Mean (n=3) | RE; % | |||
| Propofol | Q+OH | Propofol | Q+OH | Propofol | Q+OH |
| 0.005 | 0.005 | 0.0051 | 0.0051 | 1.22 | 1.76 |
| 100 | 50 | 100.53 | 50.52 | 0.53 | 1.63 |
| Organic phase stability at 4°C for 1 week | |||||
| QC | Mean (n=3) | RE; % | |||
| Propofol | Q+OH | Propofol | Q+OH | Propofol | Q+OH |
| 0.005 | 0.005 | 0.0050 | 0.0050 | 0.96 | 0.37 |
| 100 | 50 | 101.01 | 50.11 | 1.01 | 0.22 |
QC- Quality control; Q+OH – Non-conjugated metabolites; RE – relative error
Table 6: Results from stability studies of propofol and its non-conjugated metabolites (Q+OH) stock solutions and ethyl acetate/ chloroform organic phase.
Extraction validation: Propofol and its non-conjugated metabolites were recovered from each matrix (plasma, liver, lung, kidney and heart) supplemented at 0.5 μg/mL or 0.5 μg/g. Concerning propofol the recovery (%) was greater than 95% in plasma and heart whereas the higher recuperation of non-conjugated metabolites was observed also in plasma and the lungs (Table 7).
| Matrix (n=4) | Amount added (µg/mL or µg/g ) |
Recovery, % (CV, %) | |
|---|---|---|---|
| Propofol | Non-conjugated metabolites | ||
| Plasma | 0.5 | 116.7 (1.6) | 101.8 (2.5) |
| Liver | 0.5 | 91.5 (1.9) | 91.8 (1.7) |
| Lung | 0.5 | 88.5 (7.7) | 97.8 (3.5) |
| Kidney | 0.5 | 94.4 (1.2) | 93.3 (1.3) |
| Heart | 0.5 | 108.1 (6.1) | 87.9 (0.4) |
Table 7: Propofol and its non-conjugated metabolites were recovered from each matrix (plasma, liver, lung, kidney and heart) supplemented at 0.5 µg/mL or 0.5 µg/g.
Discussion
In this study, we adapted, optimized and validated the methods previously described by Guitton et al. [23,28] and from Hikiji et al. [27]. The hereby proposed method was improved to provide a simple and fast procedure, with reduced time and costs. We simplified some aspects related to sample preparation and simultaneous quantification of different analytes in the same sample and in different matrices in one GC/IT-MS run. In less than one hour, the final results are obtained (mean time need for one sample is approximately 50 min) comprising propofol and non-conjugated metabolites extraction. The chromatographic run is less than 20 min, being the injection an automatic process. Several chromatographic parameters were evaluated, specifically, peak separation, resolution, peak area and retention time of the different analytes.
The GC/IT-MS technique optimized for the simultaneous quantification of propofol and of its two main metabolites has proved to be simple, precise, accurate, linear, reliable, stable and reproducible for a wide range of concentrations in plasma, liver, heart, lung and kidney. Diverse methods have demonstrated the quantification of propofol in biological fluids and solid tissues but not much address to the study of non-conjugated metabolites [23,27,29].
The GC conditions and the steps of the extraction procedure were adjusted to increase selectivity, minimise carry-over and monetize time by analysing different analytes in the same sample. Vree and co-authors [29] have shown the simultaneous quantification and validation method of propofol and its metabolites in plasma and urine, using HPLC with UV detection however this method revealed low sensitivity and was more time consuming compared to ours.
The retention times observed for propofol and IS in our method are less when compared with those already published in other GC-MS methods [23,27,33] with the exception of Stetson’s method [24] that retained propofol around 4.1 min and thymol at 3.5 min, but all failed at quantifying the non-conjugated metabolites. On the contrary, some HPLC methods showed even smaller retention times than ours but, only for propofol and IS [16,17,22]. Concerning the non-conjugated metabolites acquisition retention times, Guitton and colleagues obtained different retention times in two different studies [23,28]. In the first study, 2,6-diisopropyl-quinone was detected at 9.6 min and 2,6-diisopropyl-quinol at 12.0 min, which is consistent with ours [23]. However, in this study by Guitton [23], the non-conjugated metabolites of propofol were not quantified and the extraction procedure was only validated for propofol. In the other study of Guitton [28], 2,6-diisopropyl-2,4-quinol showed a retention time at 8.0 min but, this quantification resulted from the conversion of 2,6-diisopropofol- 1,4-quinol in quinone, in alkaline conditions, and was not defined a conversion ratio of one metabolite into the other, as they are in tautomeric equilibrium. Moreover, these two studies of Guitton were conducted in vitro using rat liver microssomes, which do not include the physiological mechanisms from in vivo animals or humans.
The extraction procedure is simple and repeatable. A battery of samples can be easily prepared and analysed. Furthermore, this method do not demand a large amount of organic solvents or injection volume into the GC column compared with the extraction procedures found on literature [22,24,29,34]. In our study, with a small volume of plasma (500 μL), we were able to provide a low limit of quantification. Nevertheless, we recognize that the volume of plasma used in this method does have the potential to be reduced (250 μL or less), turning it feasibly useful for smaller animals (like exotic animals), paediatric patients or in forensic scenarios with limited samples amount.
The LLOQ value observed with this method for propofol was low and consistent with those observed in the literature using HPLC-UV detection [29,35], HPLC with fluorescence detection [17,21,34,36] and other mass spectrometer methods [7,22,24,26,33,37]. The present method revealed to be 50% more sensitive than the one described by Cohen et al. [22] but showed the same LLOQ as Nolan’s [38] method. With reference to propofol LD, the values obtained in our optimized study are, in most cases, better than the ones already published with HPLC-UV [29], HPLC with fluorescent detection [16,17,21,34] and mass spectrometer methods [24,26,33]. Additionally, this technique also allows the simultaneous quantification of the non-conjugated metabolites in different matrices. Cussonneau et al. [16] set the LD at 3 ng/mL of propofol in plasma using HPLC and Hikiji et al. [27] showed 2.5 ng/mL of propofol in blood and 5 ng/mL in liver using GC-MS. For the non-conjugated metabolites, the LD obtained in the present study was lower than that defined by Loryan et al. [39] that obtained 10 ng/ mL in plasma using HPLC analysis. This value was much lower when compared to 25 ng/mL in blood reported by Guitton and colleagues [28] with the GC-MS assay.
The calibration curves from the current study were fitted by plotting the peak area ratio vs the concentration, in a range between 0.005-100 μg/mL for propofol and 0.005-50 μg/mL for non-conjugated metabolites. Both analytes showed a correlation coefficient greater than 0.99 and no changes in the slope were observed with various samples. The intra-day coefficient of variation oscillated from 0.54 to 3.07% for propofol and 0.19 to 7% for the non-conjugated metabolites whereas the inter-day variation alternated from 5.73 to 11.67% and 6.75 to 2.85% for propofol and its metabolites, respectively. The intra and inter-assay variability was less than 15%. These results are in agreement with the EMA and FDA guidelines for analytical methods validation and indicate that our method is accurate and precise for the simultaneous quantification of propofol and its non-conjugated metabolites in different matrices.
The extraction efficiency of propofol was greater than 100% for plasma and heart, greater than 90% for kidney and liver and above 85% in lung. These mean recovery values are similar or better than other methods [16,17,22,23,27,34]. For the non-conjugated metabolites, the mean absolute recovery was more than 100% for plasma, above 95% for lung and greater than 85% for liver, heart and kidney which is in agreement with previous studies [22,28,29]. However, further recovery studies should be conducted with different concentrations (lower and higher than 0.5 μg/mL or ng/mL) in order to verify the complete validation of the extraction procedure.
Moreover, stability studies indicate that stock solutions are stable for 3 weeks at -80°C and that the organic phase remains reliable one week refrigerated at 4°C. This may be useful if there is a need to reanalyse a sample. So, this optimized method also shows to be reliable and applicable to clinical conditions.
Proof Method Applicability
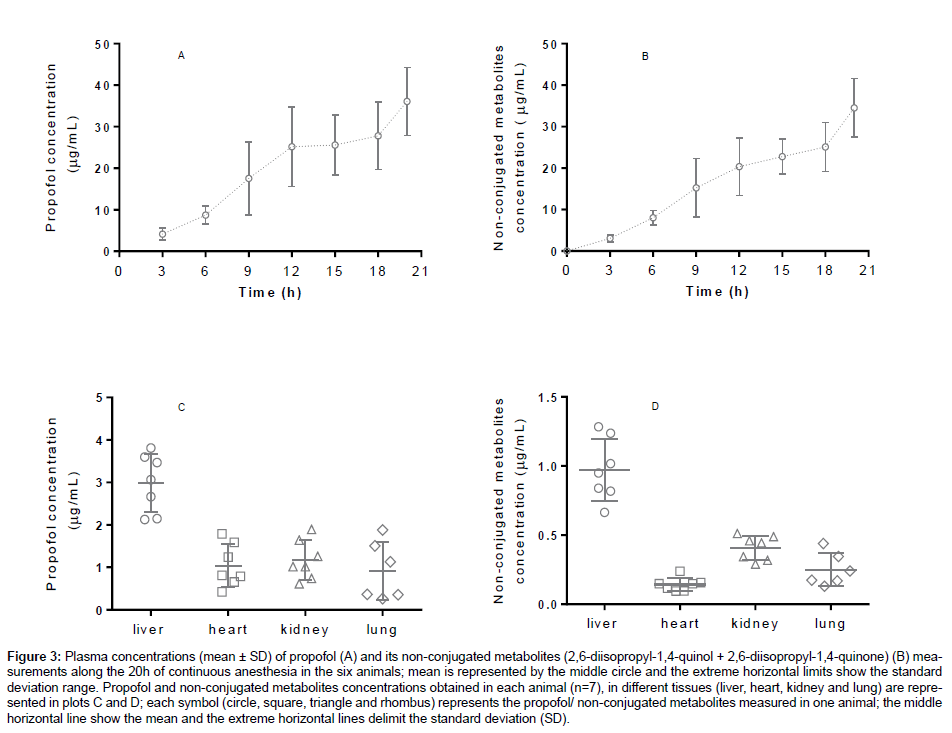
Propofol and its main metabolites were measured in plasma, liver, kidney, heart and lungs of the rabbits. These organs are involved in the metabolism and elimination of propofol and its metabolites [40,41]. The propofol and its non-conjugated metabolites plasma concentrations (μg/ mL) obtained every three hours during the 20 hours of anaesthesia and the concentrations measured in each organ, are shown as mean ± standard deviation (M ± SD) in Figure 3.
A wide range of calibration concentrations was chosen because it was observed in previous works with rabbits plasma that high concentrations of propofol (± 50 μg/mL) could be detect [42]. Our results showed a wide variation of plasma concentrations among animals (Figure 3), which enhances the importance of pharmacokinetic and pharmacodynamic parameters to dose individualization and adjustments in the anaesthetic scheme when using variable infusions or target-controlled infusions of propofol. In addition, measuring propofol and its non-conjugated metabolites in organs is valuable in terms of metabolism, excretion and toxicokinetic studies. According to the obtained results, among the tested organs, liver was the one with higher concentration of propofol, followed by kidney, heart and the lungs. The extrahepatic glucuronidation of propofol to 2,6-diisopropyl- 1,4-quinol in kidney [40,43] and lungs [44] was already reported in humans. As CYP P450 are responsible for propofol glucuronidation and different organs express different CYP’s, it expected that external hepatic metabolism occurs in variable extent among animals and humans [45].
Figure 3: Plasma concentrations (mean ± SD) of propofol (A) and its non-conjugated metabolites (2,6-diisopropyl-1,4-quinol + 2,6-diisopropyl-1,4-quinone) (B) measurements along the 20h of continuous anesthesia in the six animals; mean is represented by the middle circle and the extreme horizontal limits show the standard deviation range. Propofol and non-conjugated metabolites concentrations obtained in each animal (n=7), in different tissues (liver, heart, kidney and lung) are represented in plots C and D; each symbol (circle, square, triangle and rhombus) represents the propofol/ non-conjugated metabolites measured in one animal; the middle horizontal line show the mean and the extreme horizontal lines delimit the standard deviation (SD).
Studies of propofol and non-conjugated metabolites may benefit from the use of animal models, as they provide higher standardization and high quality results, allowing translational knowledge to humans. Animal and also human patients under propofol sedation or anaesthesia may benefit from the knowledge of real propofol concentrations, especially if high doses or prolonged sedations are required, as they can lead to propofol infusion syndrome (PRIS) [12].
Acknowledgements
The authors want to thank the Interdisciplinary Centre of Marine and Environmental Research (CIIMAR), Porto, Portugal; the Laboratory of Toxicology, Department of Biological Sciences, Faculty Pharmacy, University of Porto, Portugal; and the Foundation for Science and Technology (FCT), Lisbon, Portugal, and co-funded by the COMPETE: -01-0124-FEDER-009497 through the project grant: PTDC/CVT/099022/2008, project Pest-C/EQB/LA0006/2013 and FCT personal grant SFRH/BD/72360/2010.
References
- Glen JB, Hunter SC (1984) Pharmacology of an emulsion formulation of ICI 35 868. Br J Anaesth 56: 617-626.
- Cockshott ID, Douglas EJ, Plummer GF, Simons PJ (1992) The pharmacokinetics of propofol in laboratory animals. Xenobiotica 22: 369-375.
- Fudickar A, Bein B, Tonner PH (2006) Propofol infusion syndrome in anaesthesia and intensive care medicine. Curr Opin Anaesthesiol 19: 404-410.
- Vanlersberghe C, Camu F (2008) Propofol. Handb Exp Pharmacol: 227-252.
- Helfenbein J, Lartigue C, Noirault E, Azim E, Legailliard J, et al. (2002) Isotopic effect study of propofol deuteration on the metabolism, activity, and toxicity of the anesthetic. J Med Chem 45: 5806-5808.
- Bailie GR, Cockshott ID, Douglas EJ, Bowles BJ (1992) Pharmacokinetics of propofol during and after long-term continuous infusion for maintenance of sedation in ICU patients. Br J Anaesth 68: 486-491.
- Han E, Jung S, Baeck S, Lee S, Chung H (2013) Deaths from recreational use of propofol in Korea. Forensic Sci Int 233: 333-337.
- Colucci AP, Gagliano-Candela R, Aventaggiato L, De Donno A, Leonardi S, et al. (2013) Suicide by self-administration of a drug mixture (propofol, midazolam, and zolpidem) in an anesthesiologist: the first case report in Italy. J Forensic Sci 58: 837-841.
- Mannocchi G, Napoleoni F, Napoletano S, Pantano F, Santoni M, et al. (2013) Fatal self administration of tramadol and propofol: a case report. J Forensic Leg Med 20: 715-719.
- Simons PJ, Cockshott ID, Douglas EJ, Gordon EA, Knott S, et al. (1991) Species differences in blood profiles, metabolism and excretion of 14C-propofol after intravenous dosing to rat, dog and rabbit. Xenobiotica 21: 1243-1256.
- Guitton J, Buronfosse T, Desage M, Flinois JP, Perdrix JP, et al. (1998) Possible involvement of multiple human cytochrome P450 isoforms in the liver metabolism of propofol. Br J Anaesth 80: 788-795.
- Kam PC, Cardone D (2007) Propofol infusion syndrome. Anaesthesia 62: 690-701.
- Ypsilantis P, Politou M, Mikroulis D, Pitiakoudis M, Lambropoulou M, et al. (2007) Organ toxicity and mortality in propofol-sedated rabbits under prolonged mechanical ventilation. Anesth Analg 105: 155-166.
- Vernooy K, Delhaas T, Cremer OL, Di Diego JM, Oliva A, et al. (2006) Electrocardiographic changes predicting sudden death in propofol-related infusion syndrome. Heart Rhythm 3: 131-137.
- Cray SH, Robinson BH, Cox PN (1998) Lactic acidemia and bradyarrhythmia in a child sedated with propofol. Crit Care Med 26: 2087-2092.
- Cussonneau X, De Smet E, Lantsoght K, Salvi JP, Bolon-Larger M, et al. (2007) A rapid and simple HPLC method for the analysis of propofol in biological fluids. J Pharm Biomed Anal 44: 680-682.
- Knibbe CA, Koster VS, Deneer VH, Stuurman RM, Kuks PF, et al. (1998) Determination of propofol in low-volume samples by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 706: 305-310.
- Dowrie RH, Ebling WF, Mandema JW, Stanski DR (1996) High-performance liquid chromatographic assay of propofol in human and rat plasma and fourteen rat tissues using electrochemical detection. J Chromatogr B Biomed Appl 678: 279-288.
- Mazzi G, Schinella M (1990) Simple and practical high-performance liquid chromatographic assay of propofol in human blood by phenyl column chromatography with electrochemical detection. J Chromatogr 528: 537-541.
- Dawidowicz AL, Fijalkowska A (1995) Determination of propofol in blood by HPLC. Comparison of the extraction and precipitation methods. J Chromatogr Sci 33: 377-382.
- Yeganeh MH, Ramzan I (1997) Determination of propofol in rat whole blood and plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 691: 478-482.
- Cohen S, Lhuillier F, Mouloua Y, Vignal B, Favetta P, et al. (2007) Quantitative measurement of propofol and in main glucuroconjugate metabolites in human plasma using solid phase extraction-liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 854: 165-172.
- Guitton J, Desage M, Lepape A, Degoute CS, Manchon M, et al. (1995) Quantitation of propofol in whole blood by gas chromatography-mass spectrometry. J Chromatogr B Biomed Appl 669: 358-365.
- Stetson PL, Domino EF, Sneyd JR (1993) Determination of plasma propofol levels using gas chromatography-mass spectrometry with selected-ion monitoring. J Chromatogr 620: 260-267.
- Cale R, Aragão I, Martins H, Cardoso G, Ferreira LM, et al. (2009) Propofol and metabolites monitoring in serum of patients with induced sedation. Toxicology Letters 189: S113-S114.
- Crifasi J, Honnold R, Kubas R (2012) Determination of Propofol in Biological Samples. Agilent Technologies guidelines.
- Hikiji W, Kudo K, Usumoto Y, Tsuji A, Ikeda N (2010) A simple and sensitive method for the determination of propofol in human solid tissues by gas chromatography-mass spectrometry. J Anal Toxicol 34: 389-393.
- Guitton J, Burronfosse T, Sanchez M, Désage M (1997) Quantitation of propofol metabolite, 2,6-diisopropyl-1,4-quinol, by gas chromatography-mass spectrometry. Analytical Letters 30: 1369-1378.
- Vree TB, Lagerwerf AJ, Bleeker CP, de Grood PM (1999) Direct high-performance liquid chromatography determination of propofol and its metabolite quinol with their glucuronide conjugates and preliminary pharmacokinetics in plasma and urine of man. J Chromatogr B Biomed Sci Appl 721: 217-228.
- European Medicines Agency (2011) Guideline on bioanalytical method validation.
- FDA (2013) Q2 (R1) Validation of Analytical Procedures: Text and Methodology.
- ICH guideline (2005) Q2 (R1): Validation of analytical procedures: test and methodology.
- Peeters MY, Kuiper H, Greijdanus B, van der Naalt J, Knibbe CA, et al. (2007) Gas chromatography-mass spectrometric assay for propofol in cerebrospinal fluid of traumatic brain patients. J Chromatogr B Analyt Technol Biomed Life Sci 852: 635-639.
- Yarbrough J, Harvey R, Cox S (2012) Determination of propofol using high performance liquid chromatography in whole blood with fluorescence detection. J Chromatogr Sci 50: 162-166.
- Dawidowicz AL, Kalitynski R (2003) HPLC investigation of free and bound propofol in human plasma and cerebrospinal fluid. Biomed Chromatogr 17: 447-452.
- Plummer GF (1987) Improved method for the determination of propofol in blood by high-performance liquid chromatography with fluorescence detection. J Chromatogr 421: 171-176.
- Miekisch W, Fuchs P, Kamysek S, Neumann C, Schubert JK (2008) Assessment of propofol concentrations in human breath and blood by means of HS-SPME-GC-MS. Clin Chim Acta 395: 32-37.
- Nolan A, Reid J (1993) Pharmacokinetics of propofol administered by infusion in dogs undergoing surgery. Br J Anaesth 70: 546-551.
- Loryan I, Lindqvist M, Johansson I, Hiratsuka M, van der Heiden I, et al. (2012) Influence of sex on propofol metabolism, a pilot study: implications for propofol anesthesia. Eur J Clin Pharmacol 68: 397-406.
- Raoof AA, Augustijns PF, Verbeeck RK (1996) In vivo assessment of intestinal, hepatic, and pulmonary first pass metabolism of propofol in the rat. Pharm Res 13: 891-895.
- Wang L, Ko KW, Lucchinetti E, Zhang L, Troxler H, et al. (2010) Metabolic profiling of hearts exposed to sevoflurane and propofol reveals distinct regulation of fatty acid and glucose oxidation: CD36 and pyruvate dehydrogenase as key regulators in anesthetic-induced fuel shift. Anesthesiology 113: 541-551.
- Silva A, Campos S, Monteiro J, Venâncio C, Costa B, et al. (2011) Performance of anesthetic depth indexes in rabbits under propofol anesthesia: prediction probabilities and concentration-effect relations. Anesthesiology 115: 303-314.
- Raoof AA, van Obbergh LJ, de Ville de Goyet J, Verbeeck RK (1996) Extrahepatic glucuronidation of propofol in man: possible contribution of gut wall and kidney. Eur J Clin Pharmacol 50: 91-96.
- Chen YZ, Zhu SM, He HL, Xu JH, Huang SQ, et al. (2006) Do the lungs contribute to propofol elimination in patients during orthotopic liver transplantation without veno-venous bypass? Hepatobiliary Pancreat Dis Int 5: 511-514.
- Favetta P, Degoute CS, Perdrix JP, Dufresne C, Boulieu R, et al. (2002) Propofol metabolites in man following propofol induction and maintenance. Br J Anaesth 88: 653-658.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17495
- [From(publication date):
July-2014 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 12754
- PDF downloads : 4741