Review Article Open Access
Quartz Crystal Microbalance in Cell Biology Studies
| Jun Xi*, Jennifer Y. Chen, Marcela P. Garcia and Lynn S. Penn | |
| Department of Chemistry, College of Arts and Sciences, Drexel University, 3141 Chestnut Street, Philadelphia, PA 19104, USA | |
| Corresponding Author : | Jun Xi, PhD Department of Chemistry College of Arts and Sciences Drexel University, 3141 Chestnut Street Philadelphia, PA 19104, USA Tel: 215-895-2648 Fax: 215-895-1265 E-mail: jx35@drexel.edu |
| Received December 06, 2012; Accepted January 11, 2013; Published January 22, 2013 | |
| Citation: Xi J, Chen JY, Garcia MP, Penn LS (2013) Quartz Crystal Microbalance in Cell Biology Studies. J Biochip Tissue chip S5:001. doi:10.4172/2153-0777.S5-001 | |
| Copyright: © 2013 Xi J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
Abstract
In the past two decades, quartz crystal microbalance (QCM) has evolved from a simple mass sensor to
a powerful bioanalytical tool that is capable of assessing the properties of complex biological materials
including cells. This evolution has led to the emergence of applications of the QCM in cell research that are
potentially relevant to fundamental cell biology, pharmaceutical development, medical diagnosis and prognosis, environmental analysis, etc. This review highlights some of the major advancements of QCM-based cell research and summarizes some of the technical advantages of the QCM that have impacted these advancements.
| Keywords |
| Focal adhesions; Actin filaments; Cell motility; Mechanical sensor; Label free; Real-time detection; Quartz crystal microbalance; Energy dissipation; Resonant frequency; Cell adhesion; Cell-substrate adhesion; Cell-cell adhesion; Cell signaling; Epidermal growth factor receptor; G protein coupled receptors; EGFR overexpression; Highaffinity EGFR; Pathway modulator; Drug screening; Cytochalasin D; Cancer prognosis |
| Introduction |
| Sensor systems are devices that convert extremely small chemical, mechanical and electrical changes taking place on the “sensing element” into measurable electrical signals and that display these signals in a useful form [1,2]. Acoustic sensor systems, such as the Quartz Crystal Microbalance (QCM) (Figure 1), have shown versatility [3-5] in chemical [6], physical [7], biological [7,8] and biomedical research [9]. The QCM is label-free, non-invasive, and highly sensitive. It follows, in real time, changes in mass and energy dissipation of the material that is coupled to the surface of the sensor crystal. In recent years, this unique capability has allowed the QCM to be applied to cell biology studies with a special focus on the interaction between cells and the surface to which they are attached [10-16]. |
| This review is intended to provide readers with a brief, up-to-date summary of applications of QCM in cell biology studies. The article will begin with a brief introduction to some of basic principles of the QCM, with an emphasis on those that are critical to cell biology studies. The article will then highlight recent progress in the areas of attachment to surfaces, cell-cell adhesion, modulation of cell adhesion and cell morphology, cell mechanics, cell signaling, and biomarker analysis. Additional examples of QCM-based applications can be found in the articles cited and references therein. |
| Background of the Quartz Crystal Microbalance |
| The core component of the QCM is a thin AT-cut quartz disk, made part of a circuit by the presence of two attached metal electrodes. Due to the piezoelectric nature of the quartz crystal, an oscillating current applied by the electrodes results in oscillating in-plane shear of the quartz crystal [17]. The resonant frequency of this oscillating crystal is sensitive to nanogram-scale changes in mass coupled to the surface. As Sauerbrey demonstrated in 1959, the change in resonant frequency is linearly proportional to the change in mass coupled to the surface of the sensor crystal [18], |
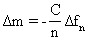 (1) (1) |
| where Δfn is the change in resonant frequency at the vibrational mode number n, Δm is the mass deposited per unit area of crystal surface, and C is the mass sensitivity constant of the instrument. For a 5-MHz crystal, C is 17.7 ng/Hz cm2. This relationship, known as the Sauerbrey equation, is valid when the mass coupled to the surface of the sensor is smaller than the mass of the quartz crystal, which is rigid and elastic, and is evenly distributed on the face of the crystal. However, the sensitivity of the disk-shaped crystal to coupled mass is not uniform but follows approximately a Gaussian distribution, showing a maximum at the center of the disk and diminishing towards the periphery [17,19]. |
| The early applications of QCM consisted mostly of characterizing ultra-thin, compact layers of elastic material (negligible viscous character) in vacuum or a gaseous environment [20]. Once the circuitry was developed to allow the QCM to operate reliably and sensitively in liquids [21,22], it became much more widely used in bioscience and biotechnology [7,8,13,14,23]. However, use of the QCM to characterize soft films of biomolecules infused with and surmounted by aqueous media introduces complications compared with characterization of thin elastic layers. The viscous character of these biological films allows dissipation of vibrational energy and causes deviations from the Sauerbrey equation that invalidates the use of the Sauerbrey equation to determine mass [24]. However, dissipation provides valuable information in its own right. Fortunately, a way to measure the dissipation simultaneously with the frequency has been developed [25]. Determination of dissipation can be based on either impedance analysis of the vibrating system [26] or on analysis of decay in vibrational amplitude with time [17,25]. Both of these approaches provide equivalent information [27], and the change in dissipation is expressed as: |
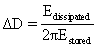 (2) (2) |
| where Edissipated is the energy dissipated during vibration and Estored is the energy stored in the vibrational system. One commercial instrument (Q-Sense Gothenburg, Sweden), termed Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D), allows the simultaneous measurement of changes in both frequency (Δf) and dissipation (ΔD) at various vibrational modes of the crystal (n=1, 3, …). |
| These advancements have provided the QCM with the versatility for various bioanalytical applications such as the study of the kinetics of protein adsorption and desorption in real time [28-31], the determination of the thickness and hydration level of protein films [28], and the characterization of the affinity between surface-bound receptors and soluble ligands [32,33]. The QCM has also been used to monitor the formation of supported lipid bilayers as model cell membranes [34] and the interaction of membrane binding proteins [35-37], DNA [38], and peptides [39] with these bilayers. In addition, the QCM has been widely used in materials science to study the effects of solvents and gaseous environments on neutral polymer films and polyelectrolyte films [40-42], to study the incorporation and binding of proteins to polymer films [34,43,44], and to study the cross-linking of polymeric films [45,46]. |
| Recently, the application of the QCM to the study of whole cells has begun to attract attention. The QCM is able to directly monitor the attachment and spreading of cells on the surface of the quartz sensor crystal [10,47-51], and provide the assessment of the kinetics of these processes [11,52,53]. The QCM technique is considered to be noninvasive to mammalian cells, because the lateral displacement of the surface of the sensor crystal during oscillation rarely exceeds 1 nm [54]. This non-invasive character has prompted the emergence of additional applications, such as the study of functional responses of cells present as a monolayer on the surface of the sensor crystal to external chemical and biochemical stimulation [55-59]. |
| The amplitude of the vibrational shear wave of the QCM diminishes exponentially with distance from the surface of the sensor crystal. This is relevant to a monolayer of cells adhering to the surface of the crystal, because the portion of the cell layer that is sensed is limited to a region close to the bottom of the cell layer. For example, the penetration depth (δ) of the shear wave into a liquid couple to the sensor crystal is given by: |
 (3) (3) |
| where η is the viscosity of the liquid, ρ is the density of the liquid, and fn is the frequency of the nth vibrational mode [17]. An estimate of δ can be made for cells if they are assumed to have the properties of water. For mode n=3, the shear wave has a penetration depth of approximately 100 to 150 nm from the surface of a 5-MHz sensor disk [60]. This depth coincides with the basal region of the cell monolayer, and thus the QCM captures changes not only in the structure of the basal region but also in the interactions between the basal region and the surface of the sensor. By contrast, the QCM is insensitive to changes in the apical regions of the adhered cells [61]. Overall, the QCM measurement provides information about cell-substrate adhesion. |
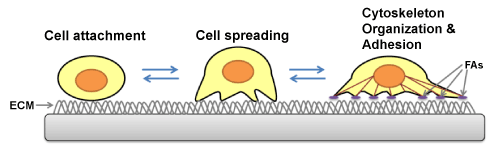
| Cell-substrate adhesion, a phenomenon requiring the synchronization of many molecular interactions, both internal and external to the cell, is an essential process for survival, differentiation, and migration of many types of cells. During the cell-substrate adhesion process, a cell comes into contact with the substrate, attaches loosely, flattens, and begins to spread its membrane over the substrate surface (Figure 2). Simultaneously, the cell forms adhesion complexes that connect the Extracellular Matrix (ECM) with intracellular actin filaments (stress fibers) through membrane receptors (integrins) [62]. These adhesion complexes lead to the establishment of focal adhesions, which anchor the cell securely on the surface of the substrate. In addition to their role in cell-substrate adhesion, focal adhesions, by means of restructuring, play an essential role in regulating migration, proliferation, and differentiation of cells [63]. Thus, by providing information about cell adhesion, QCM-based cell assays can offer a unique, in vitro perspective of behavior of both normal and diseased cells. |
| Cell Attachment to Surfaces |
| During the attachment and spreading of cells on the sensor crystal of the QCM, the interfacial interaction between the cell membrane and substrate surface [10,64,65] was found to produce changes in resonant frequency that are related not only to changes in mass [48,66,67] but also to changes in the fractional surface coverage by the cells [51,61,68,69]. The QCM response often exhibits a sigmoidal profile consisting of a lag phase, a log phase, and a final phase [11,70], corresponding to different stages of the cell attachment process. The response varies with cell type [48,61,66,71] and surface coating [68,69,72,73]. |
| In addition to producing changes in frequency, the process of attachment of cells to the substrate, i.e., the surface of QCM sensor crystal, was shown to produce changes in energy dissipation [52,61,69,74-77]. However, the link between the energy dissipation and the details of cell attachment has not been fully understood. Rodahl et al. [74] speculated that the energy dissipation arises primarily from processes in the liquid trapped between the cell and the surface, in the cell membrane, and in the interior of each cell. Marx and coworkers ascribed the dissipation to the remodeling of actin filaments [75]. Wegner et al. [61] suggested that the dissipation is related to changes in the extracellular matrix, the actin cytoskeleton, the cell-substrate separation distance, and the details of the narrow cleft between cell and substrate. |
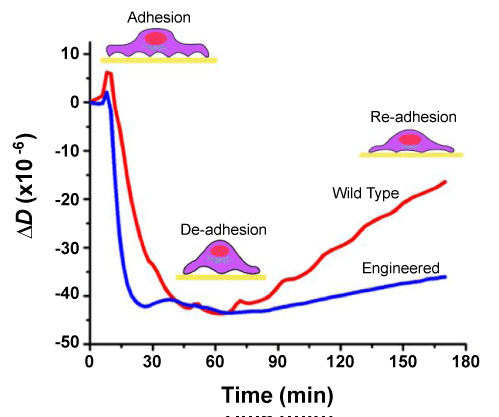
| Because of the uncertainty about the source of the energy dissipation of adhered cells, the energy dissipation of the cell layer has been interpreted only qualitatively and therefore has not been regarded as a mainstream, stand-alone approach for cell assays. Fredriksson et al. [52] attempted to make it more quantitative by introducing a plot of dissipation change, ΔD, versus the simultaneous frequency change, Δf. They used the unique profiles of ΔD/Δf-plots to compare the effects of cell types and surface coatings on the kinetics of cell-substrate adhesion. Some recent studies have shown that the energy dissipation and the level of focal adhesions of MCF-10A cells exhibit a direct, linear relationship [56,78]. This relationship allowed Garcia et al. [79] to probe the kinetics of ligand-induced change in cell-substrate adhesion. They found that cells overexpressing Epidermal Growth Factor Receptor (EGFR) reached a weakly adhered state more quickly and remained in that state for a longer period of time than cells expressing a normal level of EGFR (Figure 3). They suggested that such weakening of cellsubstrate adhesion may be favorable for the initiation and maintenance of EGFR-mediated cell migration. Findings such as these may help propel dissipation measurement to the forefront of the application of QCM in future cell biology studies. |
| Cell-cell Adhesion |
| Cell-cell adhesion refers to the contact formed between neighboring cells, primarily through adherens junctions, tight junctions, and gap junctions. These junctions are mediated by cadherins, occludins, and connexins, respectively [80]. Cell-cell adhesion plays an indispensable role in regulation of such diverse cellular processes as cell morphogenesis, migration, growth and differentiation, transformation to malignancy, and wound healing [81]. Because most cell-cell adhesion junctions are located away from the surface of the substrate, they do not interact directly with the substrate. This creates difficulties in the assessment of the state of cell-cell adhesion with the QCM, because the detection distance of the instrument is limited to the close vicinity of the sensor surface. However, cell-cell adhesion has been found to be influenced by cell-substrate adhesion and vice versa [82], and this makes it possible for information about cell-cell adhesion to be deduced from data obtained from the basal region of the cells with the QCM. For example, Marx et al. [83] used the QCM to probe the initial phase of cell-cell adhesion by examining the cell-substrate adhesion. They observed sigmoidally shaped curves for both frequency and energy dissipation during the process of attachment of cells to the surface of the substrate, similar to the curves observed by others [11]. They attributed the sigmoidal shape to the cooperative effect of cell-cell adhesion on the process of cell-substrate attachment. In a later study, they fit the sigmoidally shaped curves to the Hill equation for cooperative binding and used the derived slope to quantify the cooperativity as described by Zhou et al. [84]. Overall, this indirect approach has enabled an assessment of the role of cell-cell contact in cell-substrate adhesion and spreading. A future challenge will be to adapt the QCM-based approach to the assessment of cell-cell adhesion in a more direct manner. This may be possible with carefully-designed systems that allow manipulation of the cell-cell adhesion while the cell-substrate adhesion remains relatively constant. |
| Modulation of Cell Adhesion and Cell Morphology |
| As integral elements of the adhesion complex, actin filaments undergo constant remodeling to mediate cell adhesion to surfaces in response to environmental cues. Modulation of this remodeling process through chemical inhibition of actin polymerization alters cell-substrate adhesion. This remodeling can be detected with the use of the QCM [62,66,71,84,85-88]. The most popular inhibitor used in such studies is cytochalasin D, which at nanomolar concentrations, is capable of inhibiting polymerization of actin filaments [88,89]. Jasplakinolide which stabilizes rather than inhibits actin filaments [90,91] has also been used in studies of cell-substrate adhesion. |
| The effect of known anti-adhesive peptides, such as GRGDS, RGDS, or RGD, which compete with the binding of ECM to its receptor, integrin, have also been examined with the QCM [71,73,92]. Wegener et al. [71] have shown that anti-adhesive peptides inhibit integrin-mediated attachment and spreading of mammalian cells and that the inhibition is dependent on both peptide concentrations in the medium and amino acid sequence of the peptide. The dependence of cell adhesion in mammalian cells to the specific amino acid sequence in the anti-adhesive peptides was demonstrated independently by Li et al. [72]. |
| In addition to being integral to cell adhesion, actin filaments, along with microtubules and intermediate filaments, constitute the cytoskeleton of the cell, which provides the cell with mechanical strength, structure, and shape [62]. Modulation of the polymeric state of any of these cytoskeletal components with cell-permeable, chemical modulators deforms the cytoskeletal structure, which often results in changes in cell morphology. The QCM has been shown capable of providing a real-time assessment of these morphological changes. Tymchenko et al. [85] used the QCM to examine the effect of disruption of actin filaments with cytochalasin D on cell attachment and spreading and on cell morphology. The observed inhibitory effect correlated well with the results from light microscope studies and could be reversed once cytochalasin D was removed from the system. he effects of modulation of the microtubule network with drugs such as nocodazole and taxol [59,93]. Nocodazole disrupts the microtubule network by causing depolymerization of microtubules [94], whereas taxol stabilizes the microtubule network [95]. A significant decrease in frequency was observed when the cells were treated with nocodazole, but frequency remained unchanged when cells were treated with taxol [59]. The changes in cell morphology and microtubule structure determined from QCM measurements were consistent with the changes determined with fluorescence microscope imaging. All of these examples show that the QCM is a valuable technique for studying the modulation of cell adhesion and cell morphology. |
| Cell Mechanics |
| Many cellular functions such as cell migration, differentiation, division, and signal transduction, either involve or trigger mechanical changes in cells [96,97]. Therefore, the characterization of cell mechanics has become an important aspect of cell biology studies. Because it is noninvasive to mammalian cells [54] and also capable of real-time monitoring, the QCM-based approach has certain advantages for characterization of cell mechanics over techniques such as atomic force microscopy [98,99], optical traps [100], and magnetic force application [101]. |
| Over the years, QCM has shown success in characterization of mechanical properties of cells [57,70,74,102,103]. For example, QCM was used to qualitatively assess changes in viscoelastic properties of the cells during cell attachment to the surface of the QCM sensor crystal [70,103]. Their approach was based on an indirect assessment of the relationship between mechanical properties (e.g., viscous character) of the cells and multiple parameters simultaneously measured from the QCM, including the maximum oscillation amplitude, the decay time constant, and the resonant frequency. Their results revealed that the alteration of cytosolic viscosity through cytoskeleton modulation contributes to the shift in resonant frequency during cell attachment [70]. |
| In a more recent study, combined atomic force microscopy and QCM was used to determine the mechanical behavior of a confluent monolayer of A431 cells in response to exposure to Epidermal Growth Factor (EGF) [57]. The QCM showed a time-dependent decrease in energy dissipation, a result indicative of increased ordering of the basal region in response to exposure to EGF. The atomic force microscope, which probes the tops of the cells, revealed mechanical changes indicative of a reduction in ordering in response to exposure to EGF. The opposite trends observed in the mechanical states of the basal and apical regions of the same cells suggest distinct and regionally specific responses to EGF. This work also demonstrated that the QCM and the atomic force microscope can complement each other to provide a more complete description of the mechanical profile of the cells during cell signaling than either technique alone. The QCM has also been used in combination with other analytical techniques such as surface plasmon resonance [104] and reflectometry [105] to provide more complete descriptions. |
| By far the majority of the QCM-based cell mechanics studies are still qualitative. In an effort to quantify the viscoelasticity of the cell, a continuum mechanics model [106] was applied to frequency data from a confluent cell layer adhered to the surface of the QCM sensor and surmounted with a uniform liquid medium [102]. The viscoelastic properties of the cell layer computed from the model matched reasonably well the viscoelastic properties determined experimentally with other techniques, suggesting that this modeling approach is promising. |
| Cell Signaling |
| Cell-substrate interactions are often mediated by a variety of signaling pathways, such as the Mitogen-Activated Protein Kinase/ Extracellular Signal-Regulated Kinase (MAPK/ERK) pathway, the Phosphoinositide 3-kinase (PI3K) pathway, and the Phospholipase C (PLC) pathway, all of which are known to be responsible for altering cell adhesion when activated with EGF [107-110]. Using the QCM responses of cell-substrate interactions as a real-time, functional readout of these cell signaling pathways were investigated how each of these three pathways mediates the EGF-induced change in cell adhesion [56]. Their approach was to inhibit the activity of a selected signaling protein in the pathway and then to evaluate the effect of pharmacological intervention on the energy dissipation. The dissipation changes suggested that all three pathways were responsible for regulation of the EGF-induced change in cell adhesion in MCF-10A cells. Furthermore, inhibition of each pathway appeared to produce a distinct time-dependent response, which could be an indication that each of these pathways has a distinct role in this process. This suggests that a QCM-based approach could be a useful tool to dissect the role of cell signaling network in regulation of cell adhesion. |
| The QCM has also been used to probe the role of cell signaling in regulation of the adhesion of bacterial cells: Otto et al. [111] assessed time-dependent interactions between E. coli cells and the substrate surface based on the change in resonant frequency per cell and the ratio between the change in dissipation and change in frequency. They showed that the responses exhibited by the cpxR and nlpE mutants were “strikingly similar” to those of wild-type cells in which protein synthesis is inhibited, suggesting that the internal CpxRA signaling pathway is essential for the cell-substrate interaction in stable cellular adhesion. |
| Overall, these examples have demonstrated that the QCM is able to provide a noninvasive, real-time assessment of cell signaling that is critical to the regulation of biological functions. G-Protein-Coupled Receptor (GPCR)-mediated signaling pathways, which are involved in regulation of a variety of essential physiological processes, including sensing, immune response, and homeostasis, can also been studied with the QCM [112]. Since GPCR has been the major target for drug discovery [113], this QCM-based cell assay may have the potential to screen therapeutics that target specific GPCR pathways. Compared to traditional biochemical and biophysical assays, which are often limited to end-point detection or require incorporation of labels that are nonnative to cellular environments [114-116], the QCM-based approach has advantages for the investigation of the signaling pathways of cells adhering to surfaces. |
| Biomarker Analysis |
| Like many other label-free, sensor-based cell assays, QCM-based cell assays are capable of providing the functional readout of cells that are bound to the sensor surface and undergo processes including signaling transduction [55,56,117], growth [118-120], apoptosis [121], exocytoses [122], migration [123], morphological change [84,87], cell cycles [124], etc. Such information is indicative of the physiological state of cells under normal or pathological conditions and has the potential for use in diagnosis and prognosis of human diseases. |
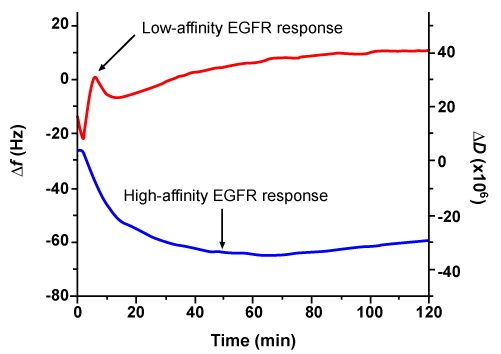
| In a recent study, a QCM-based assay was developed [55] to detect responses of cells to high- and low-affinity EGFR signaling simultaneously [125,126]. High-affinity EGFR, which accounts for less than 10% of the total number of EGFR in a cell, plays an essential role in regulation of cell growth, proliferation, motility, and differentiation [127,128]. On the other hand, low-affinity EGFR, which accounts for more than 90% of the total number of EGFR in a cell, contributes very little to regulation of those important cellular functions. Chen et al. [55] were able to relate the time-dependent change in energy dissipation to the cellular response mediated by high-affinity EGFR; in addition, they related the time-dependent change in frequency to the cellular response mediated by low-affinity EGFR (Figure 4). Together these high- and low-affinity EGFR are highly expressed in a variety of human tumors, and the level of these receptors is a widely used indicator of patient prognosis and of patient response to cancer therapy [129]. However, the correlation between the level of EGFR and patient prognosis and patient response to therapy has been inconsistent. By focusing on the cellular response mediated by high-affinity EGFR, which appears to be more critical to the development of cancer [130-133], a QCM-based cell assay could potentially provide a more accurate assessment of patient prognosis and patient response to therapy. |
| In another study, Zhou et al. [84] employed a QCM-based approach to evaluate dynamic viscoelastic properties of normal cells (HMEC) and malignant cells (MCF-7) during their adhesion and spreading. Their assessment was based on the ratio between the change in energy dissipation and the change in frequency. This ratio has been used previously by others, specifically to assess the properties of cells. Zhou et al. [84] termed this ratio as “Cell Viscoelastic Index” (CVI). They found that MCF-7 cells exhibited a 2.5-fold lower CVI than HMEC, suggesting MCF-7 cells were softer than HMEC during cell adhesion; this finding is consistent with previous reports that malignant cancer cells appear to be softer than normal cells [134,135]. They also used the CVI to evaluate samples from breast cancer patients and found that stage IV malignant cancer cells were 1.8 times softer than MCF-7 cells. This study proved the unique capability of the QCM to differentiate cancerous cells from normal cells [136]. |
| Another property of the cell that has significant implications for cell physiology and disease is cell motility, which refers to the ability of the cell or cells to migrate or invade. For tumors, increase in cell motility has often resulted in a more aggressive phenotype of tumor cells. To explore the capability of the QCM in assessing motility of tumor cells, Tarantola et al. [123] used subtle fluctuations in frequency tracked by the QCM over time as an indicator of micromotility. They attributed the subtle fluctuations in frequency to continuous morphological changes over time in cells attached to the sensor surface. The QCMbased micromotility readings of three different cancer cell lines (HT- 29, HSC-4, FaDu) indicated a decrease in cell motility in the following order: Ht-29>HSC-4>FaDu. These findings correlate with the order of invasiveness of the three cell lines. |
| Overall, QCM offers a non-invasive technique for tracking changes in specific cellular functions or properties-changes that potentially can be used as biomarkers for diagnosis and prognosis of human disease to complement some of existing medical technologies. |
| Concluding Remarks |
| This review has provided a brief account of some of the recent success of the QCM in fundamental and applied cell research. The information yielded by QCM measurements is relevant to chemical, biological, mechanical, medical, environmental, and toxicological aspects of cells. The usefulness of the QCM in various areas of cell research can be attributed to the specific merits of the QCM: |
| Non-invasive and label-free character |
| The minimal vibrational amplitude (lateral motion<1 nm) and the absence of need to incorporate chemical labels (such as fluorescent moieties) eliminates the type of non-native perturbations associated with some of the most popular biochemical and biophysical techniques, such as fluorescence microscopy and atomic force microscopy. |
| High sensitivity and excellent time resolution |
| These attributes allow detailed assessment over time of diverse cellular functions such as cell attachment and spreading, cytoskeleton remodeling, cell growth, ligand-induced de-adhesion, and morphological changes, etc. |
| Simultaneous measurement of more than one property of the cell |
| Frequency changes and dissipation changes monitored together allow determination of changes in both mass and mechanical properties. These data together are capable of revealing many critical aspects of cellular processes and lead to the dissection of a complex system like the cell and its signaling network. |
| Finite sensing depth |
| The sensing depth in liquid medium is less than 250 nm from the sensor surface, thereby localizing detection to the basal a region of the cell layer, a region that cannot be readily isolated for study with techniques used in cell research. This means that QCM-based cell assays can provide a unique perspective on various cellular functions and properties that relate to cell-substrate adhesion. |
| Ease of use |
| The commercial instruments (e.g., QCM-D) are ready to go out of the box. The data are readily viewable in real time while the measurements are been made; this distinguishes the QCM from existing techniques that require extensive calibration, tedious postmeasurement data deconvolution, and/or special set-ups that are not readily available to most research laboratories. |
| Overall, these merits have enabled the transformation of the QCM from a simple detector of mass change into a powerful tool that is currently capable of assessing complex cellular functions and properties under various physiological and environmental conditions. Some of these applications have just begun to emerge in the last few years, such as cell-cell adhesion, cell signaling, and biomarker analysis. They have provided a glimpse of the future directions of the QCMbased cell research, which will be relevant to fundamental cell biology, pharmaceutical development, medical diagnosis and prognosis, environmental analysis, etc. |
| Currently, the QCM is still primarily an assessment tool with a low throughput. Its detection range and sensitivity are not quite as good as some other label-free sensor technologies (e.g. surface plasmon resonance). Overcoming these limitations will rely on further technical advancements, including combining the QCM with other techniques, such as optical or electromechanical techniques, and improving throughput of the QCM by means of arrays of acoustic sensors. Perhaps more importantly, the status of the QCM as a truly useful tool in cell biology requires that progress be made in use of the QCM data to make quantitative interpretations of detailed aspects of cell adhesion. This progress will require an increased understanding of both theoretical and experimental aspects of the relation among changes in frequency, energy dissipation, and cell adhesion. These advancements will have a tremendous impact on the versatility and effectiveness of the QCM in future applications. |
| References |
|
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16392
- [From(publication date):
specialissue-2013 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 11639
- PDF downloads : 4753
