Rare Complication of Acute Necrotizing Encephalopathy in Adults Secondary to Dengue
Received: 02-Jan-2023 / Manuscript No. JNID-23-86400 / Editor assigned: 05-Jan-2023 / PreQC No. JNID-23-86400(PQ) / Reviewed: 20-Jan-2023 / QC No. JNID-23-86400 / Revised: 27-Jan-2023 / Manuscript No. JNID-23-86400(R) / Published Date: 31-Jan-2023
Abstract
Acute necrotizing encephalopathy (ANE) is a rare condition mainly affecting children with a distinct clinicradiologic pattern. Initially thought to be secondary to respiratory viral infections, there have been more insights into the pathogenesis of ANE, including genetics. We present 2 cases of adults who developed this condition with classical clinic-radiologic findings of ANE secondary to severe dengue infection and could not survive. We report these cases intending to raise awareness about this fatal complication of dengue infection, as dengue has become a global healthcare problem.
Keywords
Acute necrotizing encephalopathy; Adults; Dengue
Introduction
Acute Necrotising Encephalopathy (ANE), first proposed by Mizuguchi et al. in 1995 [1], is rare and typically considered to be a para-infectious disease that is triggered mainly by viral infections [2].
It is reported commonly in children in Japan and Taiwan below 5 years of age. However, more cases are being reported in Western countries and adult ones. The common causative agents are Influenza A, mycoplasma, herpes simplex virus (HSV), and human herpes virus‐6 [3,4]. However, other etiologies include Dengue virus, SARS-CoV-2, Varicella-zoster virus, rotavirus, coxsackie virus, and mutations of RNABP2, SCN1A, and CPT2 genes [5,6]. Dengue is one of the most common viral infections in tropical countries. CNS manifestations of dengue include dengue encephalopathy, encephalitis, ADEM, GBS, cranial neuropathies, and peripheral neuropathies. The pathophysiology of ANE in dengue is incompletely understood and is believed to be due to viral invasion into neural supportive tissue and immune‐mediated reactions secondary to viral infections [5]. No specific treatment or preventive method has been identified for this disease. Only 10% of these patients recover completely.
Case Report 1
A 66-year-old male with no prior comorbidities presented with complaints of fever for three days, which is high grade, a/w chills & rigors, and myalgias. He had a history of severe headaches and developed altered sensorium in the form of drowsiness that rapidly worsened to a comatose state. He had no diarrhea, pharyngitis, conjunctivitis, rashes, or bleeding manifestations. There was no history of recent travel, dog bite, immunization, and recent history of COVID-19. His pulse rate was 106 bpm, blood pressure of 70/40 mm Hg, and respiratory rate of 24 breaths/min. Neurological examination revealed a GCS score of E1VTM1 (Eye, Motor, Verbal). Bilateral pupils are 1mm in size and react to light. There was a downward deviation of gaze. The tone was increased with intermittent decerebration. Deep tendon reflexes were brisk with bilateral plantar reflexes extensor. Meningeal signs were absent.
Laboratory workup showed haemoglobin- 13.5 mg/dl, white blood cells - 13000/mm3, platelet count - 60000/mm3. His transaminases were elevated with reversal of alanine transaminase (ALT) to aspartate transaminase (AST) ratio (ALT 275 IU/L, AST 92 IU/L). His urea and creatinine were elevated (urea – 379mg/dl, creatinine – 10.8 mg/dl ). A complete urine examination was normal. CSF analysis showed no cells with increased protein 86mg/dl. Serology tests for mycoplasma, HSV, EBV, and CMV infections were negative. Dengue NS1 was positive. Other infectious results (bacterial blood culture, nasopharyngeal swab for H1N1), metabolic, hematologic, and collagen vascular serologic evaluation were unremarkable. An impression of Expanded Dengue was considered.
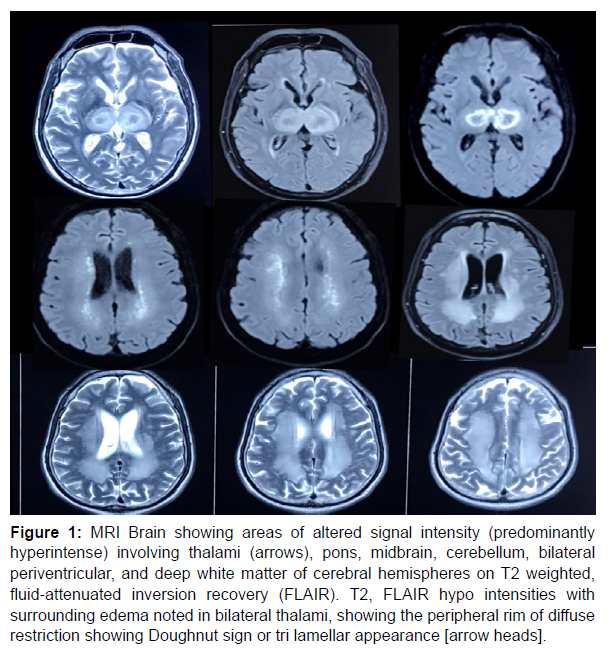
Magnetic resonance imaging (MRI) done (Figure 1) revealed areas of altered signal intensity (predominantly hyperintense) involving thalami, pons, midbrain, cerebellum, bilateral periventricular, and deep white matter of cerebral hemispheres on T2 weighted, fluid‐attenuated inversion recovery (FLAIR). T2, FLAIR hypo intensities with surrounding edema noted in bilateral thalami, showing the peripheral rim of diffuse restriction (Doughnut sign or tri lamellar appearance). Based on clinical examination and MRI findings a diagnosis of Acute Necrotising Encephalopathy (ANE) was made (Figure 2).
Figure 1: MRI Brain showing areas of altered signal intensity (predominantly hyperintense) involving thalami (arrows), pons, midbrain, cerebellum, bilateral periventricular, and deep white matter of cerebral hemispheres on T2 weighted, fluid‐attenuated inversion recovery (FLAIR). T2, FLAIR hypo intensities with surrounding edema noted in bilateral thalami, showing the peripheral rim of diffuse restriction showing Doughnut sign or tri lamellar appearance [arrow heads].
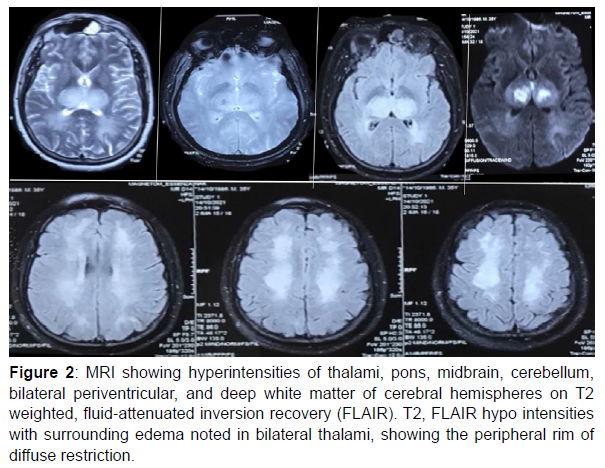
Figure 2: MRI showing hyperintensities of thalami, pons, midbrain, cerebellum, bilateral periventricular, and deep white matter of cerebral hemispheres on T2 weighted, fluid‐attenuated inversion recovery (FLAIR). T2, FLAIR hypo intensities with surrounding edema noted in bilateral thalami, showing the peripheral rim of diffuse restriction.
He was treated with IV fluids, prophylactic antibiotics, oseltamivir, acyclovir, methylprednisolone, ionotropic support, dialysis, and mechanical ventilation. Unfortunately, his condition worsened during three days of hospital stay; he developed multi-organ dysfunction and expired.
Case Report 2
A 36-year-old male with no prior comorbidities presented with complaints of fever for four days which was high grade, a/w chills & rigors, myalgias, vomiting, and loose stools for one day. He developed altered sensorium that rapidly worsened. On examination, he was febrile (1010 F), with a blood pressure of 60/40 mm Hg and a pulse rate of 126 bpm; CNS examination revealed pinpoint pupils, no spontaneous limb movements, no response with deep pain, spasticity in all four limbs, with exaggerated deep tendon reflexes, bilateral plantar reflexes were extensor with neck stiffness. Investigations showed haemoglobin – 13.4 gm/dl, white blood cells – 8700/mm3, and platelet count - 1.4lakh/ mm3. Liver enzymes were elevated (AST – 2448, ALT 1459, ALP -183); complete urine examination was normal. Serology for HIV, HBV, and HCV was negative.
Further evaluation showed Dengue NS1 antigen positive, mycoplasma antigen negative, and a nasopharyngeal swab for the H1N1 virus was negative. A provisional diagnosis of expanded dengue was considered. MRI (figure 2) showed hyperintensities of thalami, pons, midbrain, cerebellum, bilateral periventricular, and deep white matter of cerebral hemispheres on T2 weighted, fluid‐attenuated inversion recovery (FLAIR). T2, FLAIR hypo intensities with surrounding edema noted in bilateral thalami, showing the peripheral rim of diffuse restriction. Based on clinical examination and MRI findings, a diagnosis
Discussion
Annually, there are millions of infections and thousands of deaths related to dengue infection, and the global incidence is increasing [7]. There are four serotypes of Dengue virus: DENV1 to DENV4. Clinical manifestations vary from an asymptomatic state to severe dengue with plasma leakage, bleeding, or organ impairment.
Apart from the typical manifestations of dengue fever, various neurological manifestations, including encephalitis, myelitis, Guillain– Barré syndrome (GBS), and myositis, have been wellreported in dengue infection [8]. Rarer complications include isolated cranial nerve palsies, hypokalaemia paralysis, Posterior reversible encephalopathy syndrome (PRES), and stroke syndromes. These manifestations are mainly associated with DENV-2 and DENV-3 infections [9,13]. Neurological complications were thought to result from the multisystem derangement leading to encephalopathy [9-11]. Although dengue virus initially was considered a non-neurotropic virus [12], neuro-invasion has been demonstrated by the detection of dengue virus antigen in the brain by immunohistochemistry in fatal cases of dengue encephalopathy and also by PCR (polymerase chain reaction) and IgM antibody tests in CSF (cerebrospinal fluid) in patients with dengue encephalitis [13,14].
Recent studies have suggested the role of neuroinflammation in dengue. The non-structural 1 antigen (NS1Ag) is a glycoprotein that helps in viral RNA replication and triggers cytokine release [15]. The NK cells are actively involved in the pathogenesis of neurological manifestations. Early activation of NK cells leads to subsequent activation of T helper (Th) cells. These Th cells transform into Th17 and Th9 cells and promote the release of pro-inflammatory cytokines like interferon-gamma, interleukin (IL) 12, IL-4, and transforming growth factor-beta (TGFB) [16,17]. The cytokines further damage the bloodbrain barrier (BBB) and facilitate the entry of other immune mediators into the brain resulting in neuro inflammation [18].
The neurological manifestations of dengue fever occur more frequently during epidemics than in isolated cases; in DHF/DSS and younger patients. The incidence of encephalopathy and encephalitis, dengue’s most common neurological complications, has been estimated to be between 0.5 and 6.2% [9].
Until now, the pathogenesis of ANE is unclear; autopsy specimens from the patients showed necrosis and petechial haemorrhages in the thalamus and tegmentum of the pons, as well as myelin pallor in the cerebral and cerebellar deep white matter. In addition, vascular endothelial pathology and surrounding vasogenic edema without definite vascular occlusion have also been reported.
Management of ANEC is mainly conservative with antiepileptic drugs and neuroprotective measures. Some studies have shown the beneficial role of antiviral agents such as amantadine, oseltamivir, methylprednisolone pulse doses, plasmapheresis, and intravenous Immunoglobulins [16]. However, the outlook of ANEC is abysmal, with 30% [1] of patients dying during acute illness, and those who survive to have significant neurological impairment. Our patients had brainstem involvement, and it led to brain death, while a report from Korea showed that 40% of their patients with brainstem involvement expired during the acute illness. Another 30% developed severe neurological impairment [17].
References
- Mizuguchi M (1997) Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev 19: 81-92.
- Narra R, Mandapalli A, Kamaraju SK (2015) Acute necrotizing encephalopathy in an adult. J clin l imaging sci 5: 20.
- Wu X, WuW, PanW, WuL, Liu K, et al. (2015) Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm 792578.
- Abbas Q, Jafri S, Ishaque S, Jamil M (2017) Acute necrotizing encephalopathy of childhood secondary to dengue infection: A case report from Pakistan. J Pediatr Neurosci 12:165.
- ItoY, Ichiyama T, Kimura H, Shibata M, Ishiwada N, et al. (1999) Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol 58: 420-425.
- Neilson DE, Adams MD, Orr CM, Schelling DK, Eiben RM, et al. (2009) Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet 84: 44-51.
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization. 2009
- Verma R, SahuR, Holla V (2014) Neurological manifestations of dengue infection: a review. J Neurol Sci 346: 26-34.
- Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, et al. (2001) Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg 65: 848-851.
- Misra UK, Kalita J, Syam UK, Dhole TN (2006) Neurological manifestations of dengue virus infection. Journal of the neurological sciences 244: 117-122.
- Kalita J, Misra UK, Mahadevan A, Shankar SK (2005) Acute pure motor quadriplegia: is it dengue myositis? Electromyography and clinical neurophysiology 45: 357-361.
- Nathanson N, Cole G A (1970) Immunosuppression and experimental virus infection of the nervous system. Adv Virus Res 16: 397-448.
- Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, et al. (2000) Neurological manifestations of dengue infection. Lancet (London, England) 355:1053-1059.
- Lum L C, Lam S K, Choy Y S, George R, Harun F (1996) Dengue encephalitis: a true entity?. Am J Trop Med Hyg54: 256-259.
- García-Rivera EJ, Rigau-Pérez J G (2002) Encephalitis and dengue. Lancet (London, England) 360: 261.
- Okumura A, Mizuguchi M, Kidokoro H, Tanaka M, Abe S, et al. (2009) Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev 31: 221-227.
- Lee C G, Kim J H, Lee , Lee J (2014) Clinical outcome of acute necrotizing encephalopathy in related to involving the brain stem of single institution in Korea. Korean journal of pediatrics 57: 264-270.
- Trivedi S, Chakravarty A (2022) Neurological Complications of Dengue Fever. Current neurology and neuroscience reports 22: 515-529.
Indexed at, Google Scholar, Crossref
Citation: Joel RS, Ashwitha C, Mallikarjuna S (2023) Rare Complication of Acute Necrotizing Encephalopathy in Adults Secondary to Dengue. J Neuroinfect Dis 14: 434.
Copyright: © 2023 Joel RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2358
- [From(publication date): 0-2023 - Dec 15, 2025]
- Breakdown by view type
- HTML page views: 1905
- PDF downloads: 453