Rare Presentations of CNS Melioidosis - An Institutional Experience
Received: 09-Jun-2018 / Accepted Date: 05-Jul-2018 / Published Date: 16-Jul-2018 DOI: 10.4172/2314-7326.1000278
Keywords: Cerebral melioidosis; Burkholderia pseudomallei; Cerebral abscess
Introduction
Melioidosis is a disease caused by Burkholderia pseudomallei, a motile, gram-negative bacillus. It is endemic to eastern Asia and northern Australia. Commonly seen in diabetics, these infections can be seen in other immuno-compromised patients as well and is most often associated with pneumonia. CNS manifestations are rare and fewer than 70 cases have been published worldwide. Among these fewer than 10 cases have presented with primary cerebral abscess [1]. We present two cases of cerebral melioidosis, with the primary focus on strong clinical suspicion, early diagnosis and prompt medical management ascertaining good outcome.
Case Presentation
Case 1
A 39-year-old gentleman presented to our clinic with a non-tender, fluctuant 3 × 4 cm boggy swelling over the anterior region of the scalp with occasional “purulent like” spontaneous discharge of 3 weeks duration. Past history revealed that he was a diabetic on oral hypoglycemic drugs and was treated for pathological fracture of the radius, secondary to melioidosis. He had no symptoms suggestive of raised intracranial pressure and his preliminary neurological examination was unremarkable. The patient underwent incision and drainage of the scalp swelling 6 days back, at a local hospital, following which he noticed an increase in the discharge.
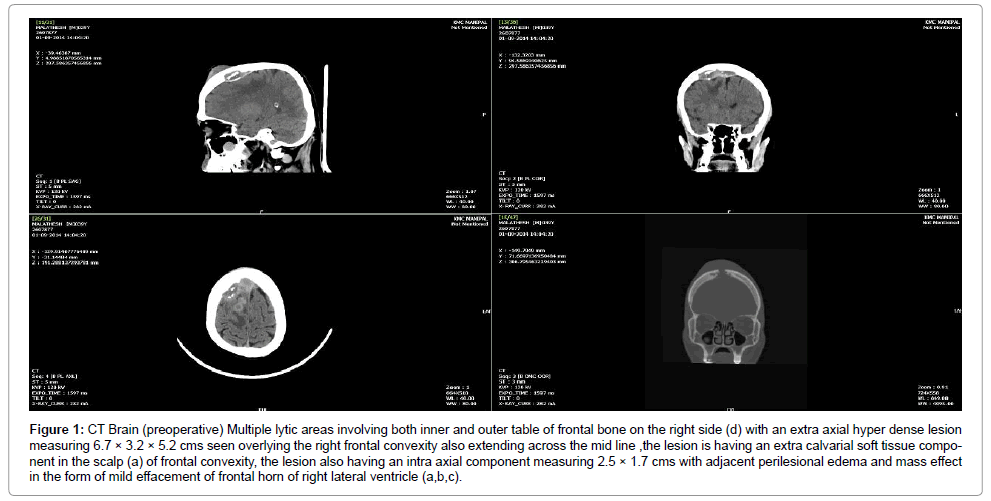
A plain computed tomography (CT) of the brain showed a bony erosion in the right frontal bone extending across the midline, to the left, with involvement of both inner and outer table with an extra-axial hyper dense lesion measuring 6.7 × 3.2 × 5.2 cm overlying the right frontal convexity and extending along the midline with mild effacement of the frontal horn of the right lateral ventricle. There was a 2 × 2.5 cm ring enhancing iso-hypodense lesion in the right frontal region suggestive of an intra-parenchymal abscess (Figure 1).
Figure 1: MRI Brain (preoperative) Peripherally enhancing multiple intracranial collection in the right frontal lobe, largest measuring 3.1 × 2.0 × 1.6 cm, which appears heterogeneously hyper intense on T1W and FLAIR (a,b), showing patchy restriction on DWI (d).An associated extradural component, measuring 7.8 × 1.2 × 1.0 cm (AP × TR × CC) seen overlying the right frontal convexity abutting the superior sagittal sinus, which shows maintained flow void. The collection extends posteriorly behind the coronal suture for about 4 cm with subgaleal extension as well (c).
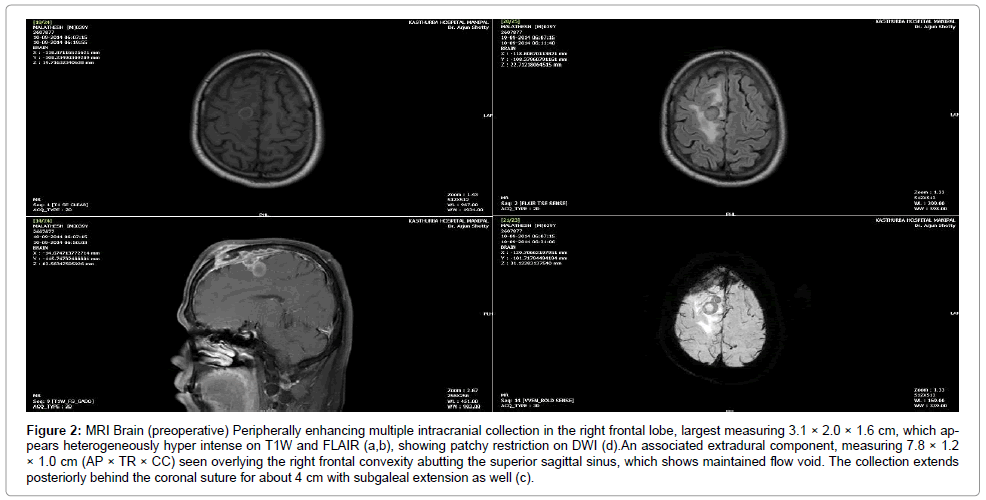
A clinico-radiologic diagnosis of cerebral abscess was made. A Magnetic Resonance Imaging (MRI) of the brain (Figure 2) showed a T2 hyper intense, diffusion restricted collection in the right frontal sub galeal plane and mid-sagittal region with extension into the extra-axial region through a cortical breach (1.5 cm) in the right frontal bone. The collection appeared to track along the coronal suture with an intraaxial ring enhancing conglomerate lesion, largest measuring 2.5 × 1.7 cm in the right superior frontal gyrus, which appeared hyper intense on T2WI and FLAIR with significant peri lesional oedema. There was also irregular pachymeningeal enhancement, suggestive of meningitis.
Figure 2: MRI Brain (preoperative) Peripherally enhancing multiple intracranial collection in the right frontal lobe, largest measuring 3.1 × 2.0 × 1.6 cm, which appears heterogeneously hyper intense on T1W and FLAIR (a,b), showing patchy restriction on DWI (d).An associated extradural component, measuring 7.8 × 1.2 × 1.0 cm (AP × TR × CC) seen overlying the right frontal convexity abutting the superior sagittal sinus, which shows maintained flow void. The collection extends posteriorly behind the coronal suture for about 4 cm with subgaleal extension as well (c).
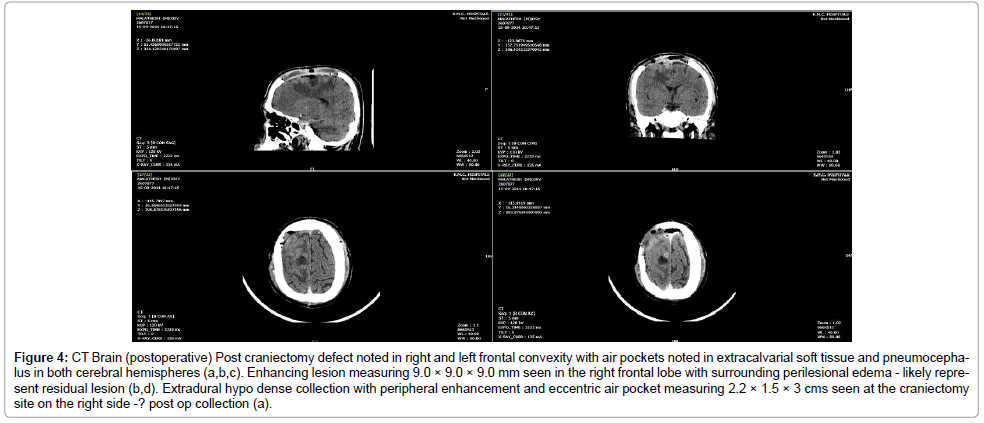
Operative procedure: After starting preoperative oral Doxycycline, the patient was treated with craniectomy and decompression. A bicoronal flap and bifrontal craniectomy with thorough wash of the extradural collection was performed. The bone edge appeared healthy and the extra-axial tissue was sent for aerobic culture and histopathological examination. Culture was sterile however histopathology revealed florid melioidosis (Figure 3). He was continued on oral Doxycycline for 3 months and IV Ceftazidime for 14 days and advised to come for follow-up after 3 weeks. Anticonvulsants were started and continued during his hospital stay and on discharge. He presented to the emergency triage after 1 week, following discharge, with history of left facial twitching and involuntary abnormal movement in the left upper limb and dysaesthesia in the left foot. A repeat plain and contrast CT brain showed resolution of the abscess with postoperative changes (Figure 4). Serum phenytoin level was suboptimal, and he was started on a second anticonvulsant and his symptoms regressed. He was reassured and discharged with advice to follow-up after 2 weeks. On follow-up, patient was symptom free and the scalp wound had healed well with no evidence of any sinus or discharge. He was advised to continue anticonvulsants as prescribed previously and antibiotics course had been completed.
Figure 4: CT Brain (postoperative) Post craniectomy defect noted in right and left frontal convexity with air pockets noted in extracalvarial soft tissue and pneumocephalus in both cerebral hemispheres (a,b,c). Enhancing lesion measuring 9.0 × 9.0 × 9.0 mm seen in the right frontal lobe with surrounding perilesional edema - likely represent residual lesion (b,d). Extradural hypo dense collection with peripheral enhancement and eccentric air pocket measuring 2.2 × 1.5 × 3 cms seen at the craniectomy site on the right side -? post op collection (a).
Case 2
A 41-year-old gentleman, diagnosed case of retroviral disease, presented to our clinic with fever and a fluctuant swelling over the left side of his scalp. He was on maintenance phase of treatment with doxycycline and co-amoxyclav since 2.5 months, for florid burkholderia sepsis. Clinical examination showed him to be febrile, a preliminary neuro exam was unremarkable, local examination of the scalp showed a soft, tender, fluctuant swelling on left side of scalp measuring 6 × 7 cm with an underlying palpable bony defect. CECT brain was suggestive of osteomyelitic erosion of the left parietal bone with subgaleal (6.4 × 6 × 2.2 cm) and subdural collection (max thickness 1 cm). Drainage of the collection with excision of osteomyelitic bone was done. The patient was empirically started on meropenem. The pus culture isolated Burkholderia pseudomallei and antibiotics were changed to Intravenous Ceftazidime. In view of low CD 4 count and recurrence of Burkholderia pseudomallei, a clinical decision was taken to prolong parenteral (IV Ceftazidime) antibiotics for a minimum of 8 weeks, since the organism was resistant to Doxycycline. Repeat CT Brain showed a complete evacuation of collection with no recurrence of collection or fresh lesions. Patient was afebrile and symptomatically better on discharge and maintained on regular follow up.
Operative Procedure: An inverted U-shaped flap was marked, based on the left superficial temporal artery overlying the parietal region. The elevated skin and subgaleal flap showed a localized collection with involvement of the temporal fascia and subgaleal tissue. The un derlying bone was involved and had become brittle with involvement of the outer and inner table. A large craniectomy with thorough debridement of the subcutaneous, subgaleal tissue and temporal fascia was done. Thorough saline wash was given and the abnormal tissue adherent to the Dura was scooped and sent for histopathological examination. Dura was opened in a cruciate incision however no subdural collection was seen. Duroplasty was done and after hemostasis was achieved a thorough closure was done with a suction drain in situ. Final histopathological examination revealed it to be Burkholderia pseudomallei.
Histopathology
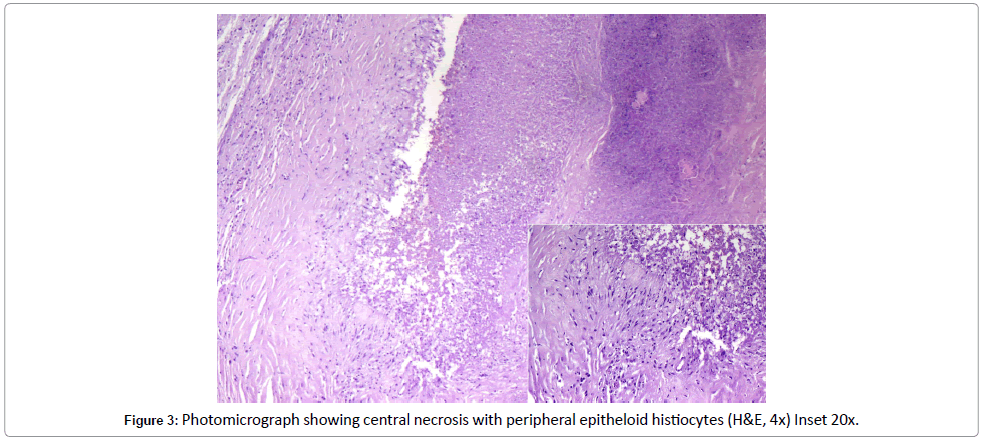
Histopathological examination of the lesion showed features ranging from acute necrotizing to chronic granulomatous inflammation, irrespective of its location. Most often, an abscess with central stellate coagulative necrosis with peripheral granulomas, giant cells, acute and chronic inflammatory cells are seen. Similar morphology, with central necrosis surrounded by pallisading epithelioid histiocytes, was demonstrated in the cerebral lesional biopsy of the present case [2,3].
Discussion
Burkholderia pseudomallei are non-acid fast, non-spore bearing (safety pin appearance) motile saprophytes that are found in the soil and surface water of paddy fields. It is usually transmitted through inoculation, inhalation or direct ingestion. B. pseudomallei can be easily isolated and grown on blood agar and MacConkey agar and have a high prevalence in tropics yet remain underreported in many parts of the world including India [4].
These are hardy organisms and may remain dormant in the host with prolonged latent periods. Based on the route of entry, these organisms have various clinical manifestations as enumerated in (Table 1) [5-8].
| Route of entry | Clinical manifestation | Acute infection | Chronic infection |
|---|---|---|---|
| Inoculation | Subcutaneous abscesses and involvement of deeper muscles and osteomyelitis and discharging sinuses | Acute localized suppurative skin infection | Lymphadenitis |
| Inhalation | Pneumonia | Asymptomatic pulmonary infection | Acute pulmonary infection |
| Blood | Abscesses in the liver, spleen, brain | Acute septicemia with abscesses in multiple organs | Chronic suppurative meliodosis |
Table 1: Clinical manifestation of meliodosis based on route of entry.
Though cranial manifestations are rare (3-10%) [9,10], they include, multiple scalp abscesses, osteomyelitis [11], meningitis [11], subdural empyema [11], encephalitis [12,13], sagittal sinus thrombosis, intra-parenchymal abscesses and meningitis. Encephalomyelitis secondary to melioidosis mimics Guillain-Barre syndrome and has a fatal outcome [14]. CSF analysis shows mononuclear pleocytosis in a background of leukocytosis, with high protein levels and normal glucose [15,16], similar to tuberculosis and can cause false-positive Widal test [17]. On HPE, melioidosis shows chronic abscess with focal granulomatous reaction mimicking tuberculosis, but the AFB staining and bipolar staining of melioidosis (non-AFB, non-spore bearing, bipolar staining - safety pin appearance) helps to differentiate it from tuberculosis, which is a close differential.
The medical management protocol consists of an “intensive phase” with parenteral antibiotics for two weeks followed by a “maintenance phase” with oral antibiotics for 3-6 months. Ceftazidime crosses the blood brain barrier and is the drug of choice for cranial melioidosis. In strains resistant to ceftazidime, carbapenems have shown to have good efficacy. Ceftazidime (40 mg/kg) or imipenem (20 mg/kg) every 8-hourly is advised for a minimum duration of 10 days [18]. For the “eradication phase”, Currie et al. [15] recommend trimethoprim/ sulfamethoxazole (TMP-SMX) at 8/40 mg/kg (up to 320/1,600 mg) every 12 h for 3-6 months. Several randomized trials have emerged over the last 2 decades that mandates the use of IV antibiotics (Ceftazidime and Carbapenems) [19] for 10 days followed by oral antibiotics for 3 months [18], especially in cases of melioid septicemia. It also dictates that the step over to oral antibiotics should be initiated only once the fever subsides or the patient shows clinical improvement, which usually takes 9-10 days. In the first case mentioned above, the primary presentation was that of an cerebral abscess, which later progressed into an empyema, involved the cranial bone to cause osteomyelitis eventually surfacing as a subcutaneous subgaleal collection. The myriad presentations all pointed to a single pathogenic organism. The final removal of the primary focus along with debulking of the bacterial load, lead to alleviation of symptoms. In the second case, diagnosis wasn’t an issue as the patient was immunosuppressed with documented septicemia. The presence of pus in the cranial compartment as a result of septicemia, presented an unusual yet expected complication. Thorough debridement, with lavage and antibiotic therapy lead to a control of the septicemia.
Conclusion
While uncommon, CNS melioidosis remains a differential diagnosis that needs to be kept in mind, especially in the era of AIDS and immunodeficiency. A thorough debridement when possible coupled with appropriate antibiotic therapy usually gives good results. Early diagnosis and prompt treatment assist in a recovery devoid of serious morbidity.
Acknowledgments
We would like to acknowledge the assistance of the department of Microbiology, Kasturba Hospital, Manipal for their invaluable support. We would like to thank the department of Infectious Diseases, Kasturba Hospital, Manipal for their input in treating the patients as well as their care and knowledge that has led to this paper.
References
- Currie BJ, Ward L, Cheng AC (2010) The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20-year Darwin prospective study. PLoS Negl Trop Dis 4: e900.
- Wong KT, Puthucheary SD, Vadivelu J (1995) The histopathology of human melioidosis. Histopathology 26: 51-55.
- Piggott JA, Hochholzer L (1970) Human melioidosis: A histopathologic study of acute and chronic melioidosis. Arch Pathol 90: 101-111.
- John TJ, Mary VJ, Lalitha MK (1990) Melioidosis in India: The tip of the iceberg? Indian J Med Res 103: 62-65.
- Howe C, Sampath A, Spotnitz M (1971) The pseudomallei group: A review. J Infect Dis 124: 598-606
- Leelarasamee A, Bovornkitti S (1989) Melioidosis: Review and update. Rev Infect Dis 11: 413-425.
- Manson-Bahr PEC, Bell DR (1987): Manson’s Tropical Diseases, ed 19. London: Bailliere Tindall pp: 586-606.
- Currie BJ, Fisher DA, Howard DM, Burrow JN, Lo D, et al. (2000) Endemic melioidosis in tropical northern Australia: A 10- year prospective study and review of the literature. Clin Infect Dis 31: 981-986.
- Bergin P, Boyes L, Sage M (2005) Cerebral melioidosis. Australas Radiol 49: 79-83.
- Chadwick DR, Ang B, Sitoh YY, Lee CC (2002) Cerebral melioidosis in Singapore: A review of five cases. Trans R Soc Trop Med Hyg 96: 72-76.
- Baumann BB, Morita ET (1967) Systemic melioidosis presenting as myocardial infarct. Ann Intern Med 67: 836-842.
- Beck RW, Janssen RS, Smiley ML, Schatz NJ, Savino PJ, et al. (1984) Melioidosis and bilateral third-nerve palsies. Neurology 34: 105.
- Howe PW, Holland HM, Burrow JC, Currie BJ (1997) Neurological melioidosis (Burkholderia pseudomallei) mimicking Guillain-Barre syndrome. Anaesth Intensive Care 25: 166-167.
- Currie BJ, Fisher DA, Howard DM, Burrow JN (2000) Neurological melioidosis. Acta Trop 74: 145-151.
- Padiglione A, Ferris N, Fuller A, Spelman D (1998) Brain abscesses caused by Burkholderia pseudomallei. J Infect 36: 335-337.
- Valsalan R, Shubha S, Mukhopadhyay C, Saravu K, Maneesh M, et al. (2009) False-positive widal in melioidosis. Indian J Med Sci 63: 464-467.
- White NJ, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V, et al. (1989) Halving of mortality of severe melioidosis by ceftazidime. Lancet 334: 697-701.
Citation: Ganapathy S, Nair R, Kumar V (2018) Rare Presentations of CNS Melioidosis - An Institutional Experience. J Neuroinfect Dis 9: 278. DOI: 10.4172/2314-7326.1000278
Copyright: © 2018 Ganapathy S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6072
- [From(publication date): 0-2018 - Aug 09, 2025]
- Breakdown by view type
- HTML page views: 5108
- PDF downloads: 964