Removal of Chromium and Some Selected Nutrients from Tannery Waste Water by Adsorption Methods of Sugar Bagasse Activated Carbon: Case Study on Addis Ababa Tannery Share Company
Received: 24-May-2021 / Accepted Date: 23-Jun-2021 / Published Date: 30-Jun-2021 DOI: 10.4172/2573-458X.1000223
Abstract
Leather tanning has been ranked as one of the most polluting activities in Ethiopia because most of them do not treat or partially treat wastewater and release to the environment which pose many environmental and health problems. As a result cost effective and efficient method of wastewater treatment was undertaken by the use of sugar bagasse activated carbon. In this paper, study was performed at Addis Ababa tannery share Company to remove their wastewater constituents of chromium, ammonium, nitrate and sulfide which has concentrations of 2300.4 mg/l, 163 mg/l, and 250 mg /l and 120 mg/l, respectively in which all are above the permissible limit. The prepared activated carbon has particle size of 300µm, moisture content of 8.1%, bulk density of 0.423 g/cm3 and porosity of 67.21%. The effects of pH and adsorbent dose were also studied on the removal of these pollutants and the result shows that the adsorptions of ammonium nitrate and sulfide were 80.56%, 71.86% and 81.79%, respectively for 2 g of adsorbent and for chromium it was 100% for 1.5 g adsorbent. On the other hand, the adsorptions of ammonia, nitrate and sulfide were 87.63%, 77.95% and 100%, respectively at pH 5. For chromium it was 100% at pH 7. The activated carbon has different adsorption capacity for different nutrient and metal ion studied in which chromium100% > sulfide 81.79% > ammonia 80.56% > nitrate 71.86%. At pH 5 removal of nutrients were in the order of sulfide 100% > ammonia 87.63% > nitrate 77.95%
Keywords: Sugar bagasse activated carbon; Adsorption of chromium and nutrients; Tannery wastewater
Introduction
Background and justification
In Ethiopia industrial wastewater has become one of the most serious problems because of the increase in discharge of untreated effluent to the environment. High level of pollutants which are discharged into rivers pose severe problems for plant, animal and human health due to their toxicity and persistence in the environment [1].
Leather tanning has been ranked as one of the most polluting activities in Ethiopia due to the high growth rate and availability of livestock skins and hides (EEPA, 2003). There are more than 20 tanning industries of which 90% of them directly discharge wastewater into nearby surface water or onto open land without treatment [2].
The transformation of raw or semi-pickled skins into commercial products requires high water consumption, roughly 50-150 liters and about 300 kg chemicals are added per ton of hides [3,4]. The major chemicals used in the various processing stages include chromium salts, sulfate, sodium sulfide, lime powder, ammonium sulfate, sodium chloride, sulfuric acid, sulphonated and sulfated oils, formaldehyde, pigments, dyes and anti-fungal agents. These chemicals in tannery effluents cause the highest toxic intensity per unit of output [5]. There are two ways of tanning: chrome tanning and vegetable tanning. Chrome tanning is favored by the majority of leather industry than vegetable tanning because of the speed of processing, low cost, flexibility and greater stability of the leather [6].
Tannery wastewater is characterized by a strong color and is heavily polluted with high organic (chemical oxygen demand (COD) and biochemical oxygen demand (BOD) and inorganic impurities (ammonia, sulfide, nitrate and chloride), dissolved and suspended solids, and other specific pollutants such as vegetable and/or synthetic tannins, sulfonated oils, chromium, arsenic, and surfactants [7,8].
Chromium is one of the most toxic heavy metals to both plants and aquatic organisms. Uptake of the chromium into the leather is not complete and relatively large amounts are found in the effluent; estimated range, from 2,000 – 3,000 mg dm-3 [9].
Chromium ion in liquid tanning wastes occurs in two forms; trivalent Cr3+ and hexavalent Cr6+. The hexavalent form is 500 times more toxic than the trivalent [10].
In addition to chromium, tannery wastewater also contains high amounts of ammonia, nitrate and sulfide which come from the chemicals used in the process.
Treatment of tannery wastewaters is expensive; so many low income countries only employ a primary treatment which may be biological and physico-chemical processes; such as, ion exchange resins, everse osmosis and electrolysis system and chemical removal systems such as precipitation and coagulation [11-13]. These methods however, are either expensive or/and produce secondary pollution and are often not considered cost effective for small sized tannery industries [14]. Adsorption has been found to be superior to other techniques for water reuse in terms of the initial cost, simplicity of design, and easiness of operation.
Currently, the most common processes for elimination of chromium are adsorption, reverse osmosis and chemical reactions that involve reduction and precipitation. Among them adsorption has been shown as a feasible alternative method for removing traces of chromium from waste water [15].
Cost-effective alternative technologies or adsorbents for the treatment of metal-containing wastewaters are needed. Activated carbons are more effective in the removal of heavy metals and nutrients due to some specific characteristics that enhance the removal efficiency of the activated carbon for the removal of contaminants from water and wastewater [16]. Natural materials that are available in large quantities or certain waste products from industrialoperationsagricultural by products may have potential as inexpensive adsorbents [17,18]. Generally, adsorbents can be assumed as low cost if they require little processing, are abundant in nature, or are a by-product or waste material from another industry [19]. Adsorption by activated carbon is an attractive method because of its cost and possible regeneration of the carbon and useful chemical.
The present investigation is devoted to study the removal of chromium and nutrients from tannery wastewater by using low cost activated carbon as waste from sugar industry. This industrial by-product is available in large amount in sugar refinery factories in Ethiopia and also in many other countries in the world. To our knowledge, this sugar waste does not find any economic application and represents a solid pollutant to the environment.
In this study, removal of chromium, ammonia, nitrate and sulfide from tannery wastewater with activated carbon prepared from sugarcane bagasse were investigated. The effects of pH, adsorbent dose and characteristics of activated carbons on the adsorption isotherm were studied. Therefore, our approach is to study the chromium and some selected nutrients such as: Nitrate, Ammonia and sulfide removal efficiency by using low-cost adsorbent materials under variable pH and adsorbent dose in order to remove the hazardous chromium and nutrients from tanning wastewater.
Statement of the problem
The high tannery industrial growth coupled with lack of technology for efficient effluent treatment and unimplemented or/and lack of effluent discharge standards/ policies/ lead to serious consequences for rivers receiving tannery effluents from Addis Ababa Tannery share company. These causes increased pressure on ecology, health and environmental resources.
The concentrations of tannery effluents in Ethiopia contain large amount of chromium and nutrients above the permissible standards [20]. The chromium is one of the most toxic metals to plants, animals and microorganisms. Even in low concentrations, it has a toxic effect upon aquatic biota such as fish and disrupting the food chain, causes soil salinity in irrigated farmland and possibly affecting aquatic organisms. On the other hand ammonia, nitrate and sulfide pose series health and environmental problems even at very low concentrations. These adverse effects require effluent treatment of chromium and nutrient-rich wastewater before discharging into the rivers. On the other hand there is no treatment plant for Addis Ababa tannery Share Company and they directly release their wastewater to the nearby water.
Objectives of the Study
General objective
Production of activated carbon from sugarcane bagasse and to treat wastewater produced by Addis Ababa tannery Share Company for its constituent particularly: chromium (Cr) and selected nutrients.
Specific objectives
1. To produce activated carbon from sugar bagasse.
2. To study the concentration of chromium, ammonia, nitrate and sulfide in the wastewater.
3. To evaluate the efficiency of activated carbon for Chromium removal.
4. To evaluate the efficiency of activated carbon for Nitrate removal.
5. To evaluate the efficiency of activated carbon for Ammonia removal.
6. To evaluate the efficiency of activated carbon Sulfide removal.
7. To study the effects of pH and adsorbent dose on the adsorption of Chromium, Ammonia, Nitrate and Sulfide.
Literature Review
Tanning and Characteristics of Tannery Wastewater
Tanning is a process by which put refable biological material, such as leather, is converted into a stable material which is resistant to microbial attack and has enhanced to wet and dry heat. During the tanning process, about 300 kg chemicals are added per ton of hides. Based on the tanning agents, tanning operations are further divided into vegetable tanning and chrome tanning. Vegetable tanning is usually done in series of process by using natural organic substances [21].
The manufacturing of leather can be divided into two parts; (1) beam house operations and (2) tanning process. In beam house operations, the removal of dirt and blood by washing is the first step after which the hides are then soaked in water for softening and removal of salts. After the removal of salts, fatty tissue is removed by fleshing. Liming is done to swell the hides for the better penetration of tanning agents and hair removal. Chemical dissolution of the hair and epidermis with an alkaline medium of sulfide and lime takes place. Hides are then neutralized with acids and ammonium salts and treated with enzymes to remove the hair remnants and to degrade proteins. Pickling is usually done to prepare the hides for tanning. Salts are added to prevent the hides from swelling [21].
The production process in a tannery can be further split into four main categories, namely, beam house, tanning (tan-yard), post-tanning and finishing operation. The beam-house is a source of an important pollutant charge, not only with respect to the organic matter but also with respect to solid materials and sulphide [22]. The characteristics of the wastewater vary considerably from tannery to tannery depending upon the size of the size of the tannery, chemicals used for the specific process, amount of water used, upon the size of the tannery, chemicals used for the specific process, amount of water used, type of final product produced by a tannery and technology advancement. According to a composite tannery wastewater has ammonia (261 mg/L), nitrate (567mg/l), sulfide (148mg/l) and total chromium (12 - 64 mg/l) [23].
Chemistry of Chromium
Understanding of chemistry and geochemistry of chromium is important in developing remediation systems that can deal with industrial pollution. Chromium is a steel-gray, lustrous, hard metal. Chromium is a heavy metal, symbolically represented by Cr, with atomic number of 24, and mass number of 51.9961. It belongs to the first series of transition metals. It is the 17thmost abundant element in the earth's crust. Chromium occurs naturally in ultramafic igneous rocks, soil, plants, animals, and in volcanic dust and gases. It may occur in nine different forms of oxidation state ranging from Cr (-II) up to Cr (+VI), but the two common valence states are trivalent Cr (III), the most stable, and hexavalent Cr (VI) which is not thermodynamically stable. Solubility of chromium (III) depends on pH; decreases dramatically at pH value greater than 4.5. Cr (III) can form stable complexes with organic legends at pH value as high as 8.5 [24]. Chromium can be combined with various nonmetals (oxygen, fluorine, chlorine, etc.) and polyatomic anions (such as nitrate, sulfate, etc.), forming relatively stable, soluble and insoluble compounds.
Uses of Chromium
Since its discovery, Cr has become a very important industrial metal because of its many applications in ferrous and nonferrous alloy metal fabrication, and in the chemical industry [25]. Uses of different forms of chromium are summarized in Table 1.
Table 1:Uses of Different Forms of Chromium.
| Form | Uses |
|---|---|
| Cr(O) | -Stainless steel production -Alloy production |
| Cr(III) | - Alloy manufacturing -Brick lining -Chrome plating - Leather tanning -Textiles -Copying machine toner |
| Cr(VI) | -Chrome plating -Leather tanning -Textiles -Copying machine toner |
Environmental Impacts of Chromium
Chromium contamination of soil and groundwater is one of the significant environmental problems today. Chromium is believed to be the second common inorganic contaminant after lead. The toxicity of chromium does not reside solely with the elemental form but varies greatly among a wide variety of chromium compounds. Oxidation state and solubility are crucial factors in this regard [26].
Chromium occurs in the environment primarily in two valence states, the oxidized hexavalent chromium, Cr (VI), and the less oxidized trivalent chromium, Cr (III). On the other hand, Cr (VI) is much more toxic and mobile in groundwater than the relatively immobile Cr (III) and possesses mutagenic and carcinogenic activity [27]. The maximum levels permitted in drinking water are 5 mg/L for trivalent and 0.05 mg/L for hexavalent chromium [28]. Cr (VI) can be transported great distances in groundwater owing in part to its high solubility. Cr (III) can be transformed to the more soluble Cr (VI) if the redox conditions along the transport pathway change from reducing to oxidizing. Under natural conditions, Cr (III) has been found to be oxidized to Cr (VI) by Mn.
Toxicity of Chromium
Chromium has a ‘chronic’ toxic effect upon aquatic life is toxic to fish life since they rapidly penetrate cell walls. They are mainly absorbed through the gills and the effect is accumulative [29]. Average daily intake of 50-200μg/day of Cr (III) is safe and adequate for adults (USPHS, 1997). However, the consumption of contaminated fish, other foodstuffs and drinking water could increase the daily intake levels far beyond those recommended levels. Adverse health effect of Cr are gastro-intestinal irritation, stomach ulcers, heart burning, respiratory tract infection, sever cough, fever and loss of eyesight. Lung cancer and kidney failure were the reported causes of death in many cases [14,3,24].
Skin contact may result in systemic poisoning, damage or even severe burns, interference with the healing of cut or scrapes which, if not treated properly, may lead to ulceration and severe chronic allergies. Eye exposure may cause permanent damage. In general, Cr (VI) is more toxic, more soluble, more mobile and hence absorbed into cells more readily than Cr (III) [30]. This chromium can replace other metals in biological systems with toxic effects and its accumulation throughout the food chain leads to serious ecological and health problems (UNIDO, 2000).
Investigations have been performed on fish under conditions of exposure insufficient to cause severe toxicity, yet sufficient to cause visible changes in behavior at a dosages of 0.2 mg/L. Chromium is also toxic to agronomic plants at about 5 to 100μg/g of available Cr in the soil [24]. Plants absorb Cr (VI) better than Cr (III); whereas Cr (VI) is more toxic. Plant growth studies of solution cultures with low levels of Cr have indicated that Cr is not an essential component of plant nutrition [31]. Although some crops are not affected by low concentration of Cr, it is toxic at high concentration or may reduce yield. Skiffington, et al., observed that barely crops could tolerate a Cr levels up to 5 mg/L but a 75 % yield reduction was observed [32]. Higher application of chromium to the plant soil growing media brought poor quality and poor growth of the seedlings [33].
Sources of Chromium in Tannery Wastewater
Chrome tanning
Chrome tanning is the most common type of tanning in the world. Chrome tanned leathers are characterized by top handling quality, high hydrothermal stability and excellent user properties [34]. Chrometanned leather tends to be softer and more pliable than vegetabletanned leather, has higher thermal stability, is very stable in water, and takes less time to produce than vegetable-tanned leather [35].
By this technique, around 8% of the leather weight is added as chromium salt. However, a significant share of the pollution resulting from tannery wastewater comes from this stage, due to the chrome that is not fixed to leather amounting 15% of the total chrome added to the tanning bath [36].
Sources of Ammonia, Nitrate and Sulfide In Tannery Wastewater
Sources of ammonia in tannery wastewater
These compounds are mostly the outcome of the deliming process, with comparatively small volumes being produced from liming and unhairing. After unhairing the hides are thoroughly washed and refloated with water at 25°C. Depending on the type of hides processed and the degree of deliming desired 2-4% ammonium sulfate is applied. Running will also vary depending on the degree of deliming desired. Ammonium salt, high purity and very low iron content. Usage efficient removal of lime after unhairing [37].
Un haired hide are strongly alkali due to the content of sodium sulfide and lime as a result of employing these chemicals in the un hairing process. The alkalinity of raw hide has to be decreased because the following processes proceed in acid conditions. Hides are then neutralized with acid ammonium salts and treated with enzymes to remove the hair remnants and to degrade proteins. This results in a major part of the ammonium load in the effluent [21].
Sources of nitrate in tannery wastewater
The principal forms of nitrogen in tannery wastewater are organic substances mainly proteins and amines from hides and skins, a volatile irritating, offensive odorous ammonia gas (NH3) and a highly pollutant and carcinogenic compound of nitrate (NO3-). Nitrification is the biological oxidation of ammonium nitrogen in to nitrite or nitrate through the action of group of bacteria called nitrifies [37].
Sources of sulfide in tannery wastewater
The sulfide content in tannery effluent results from the use of sodium sulfide and sodium hydro sulphide, and the breakdown of hair in the unhairing process. When the pH of the effluent drops below 9.5, hydrogen sulfide evolves from the effluent: the lower the pH, the higher the rate of evolution. Characterized by a smell of rotten eggs, a severe odour problem occurs [21].
Environmental and Health Impacts of Ammonia, Nitrate and Sulfide
Toxicity of ammonia
Ammonia has a very strong odor that is irritating and that you can smell when it is in the air at a level higher than 50ppm. Low levels of ammonia may harm some people with asthma and other sensitive individuals (ATSDR, 2004). You can taste ammonia in water at levels of about 35ppm (ATSDR, 2004). Lower levels than this occur naturally in food and water.
Major health effects ammonia is skin, eyes, throat, or lungs may be severely burned at high concentrations. These burns might be serious enough to cause permanent blindness, lung disease, or death. Likewise, if you accidentally ate or drank concentrated ammonia, you might experience burns in your mouth, throat, and stomach [38]. Ammonia’s aquatic toxicity is principally due to the un-ionized form, NH30 [39]. Ammonia is a toxic compound that can adversely affect fish health. Ammonia can be acutely toxic to fish mainly due to its effect on the central nervous system, because it causes “acute ammonia intoxication” - which includes convulsions and death. Concentrations of ammonia that are acutely toxic to fish may cause loss of equilibrium, hyper excitability, increased breathing, cardiac output, and oxygen uptake, and, in extreme cases, convulsions, coma, and death. Lower concentrations of ammonia can cause a reduction in hatching success, reduction in growth rate and morphological development, and pathologic changes in tissues of gills, livers, and kidneys. It also put the fish at greater risk of predation from predators such as birds and mammals that would not accumulate or be similarly impaired by ammonia exposure [40].
Toxicity of nitrate: The toxicity of nitrate to humans is mainly attributable to its reduction to nitrite. The major biological effect of nitrite in humans is its involvement in the oxidation of normal Hb to metHb, which is unable to transport oxygen to the tissues (WHO, 2011).
Nitrite was shown to react with nitrosatable compounds in the human stomach to form N-nitroso compounds. Many of these N-nitroso compounds have been found to be carcinogenic. Thus, a link between cancer risk and endogenous nitrosation as a result of high intake of nitrate and/or nitrite and nitrosatable compounds is possible [41,42].
The United States National Research Council found some suggestion of an association between high nitrate intake and gastric and/or oesophageal cancer [43].
Possible relationships between nitrate intake and effects on the thyroid have also been studied. It is known that nitrate can competitively inhibit iodine uptake, as with similar anions. The nitrate effect on thyroid function may be strong if a nutritional iodine deficiency exists simultaneously [44,45].
The main toxic action of nitrate on aquatic animals is due to the conversion of oxygen-carrying pigments (e.g., hemoglobin, hemocyanin) to forms that are incapable of carrying oxygen (e.g., methemoglobin) [46].
Toxicity of sulphide: Sulfide is one of the major components of the tannery effluent. It causes an irritating, rotten-egg smell above 1 ppm and at concentrations above 10 ppm, the toxicological exposure limits are exceeded. It is highly toxic to human beings. It can cause headaches, nausea and affect central nervous system even at low levels of exposure [21].
At the physiological level, sulfide has two major effects on mammals: local inflammatory and irritative effects on moist membranes including the eye and respiratory tract, and cessation of respiratory function, specifically cardiac arrest due to paralysis of the respiratory centers of the brainstem [47]. Adverse effects of sulfide on the nervous system include nervousness, headache, lightheadedness, sleep disturbances, insomnia, drowsiness, and fatigue, weakness of extremities, spasms, disturbed equilibrium, vertigo, convulsions, and agitation delirium and in severe cases, deep coma, nerve paralysis, unconsciousness and death.
Documented effects of sulfide on freshwater fishes include: enhanced survival and growth at low sulfide concentrations between 0.02 and 0.4ppm; reduced survival and growth at sulfide concentrations greater than 0.45 ppm HS; lower swimming endurance; tissue irritation and necrosis; lower food consumption and conversion: inhibited spawning behavior and reduced egg production; and lower survival of eggs, and smaller size and higher incidence of deformities in newlyhatched larvae [47].
Worlds Water Quality Standards
Water is essential to sustain life and a satisfactory (adequate, safe and accessible) Supply must be available to all. Improving access to safe drinking-water can result in tangible benefits to health. Every effort should be made to achieve a drinking-water quality as safe as practicable. (Table 2) [48].
Table 2: World Drinking Water Quality Guideline For Selected Chemicals.
| Parameters of concern | Guideline value (mg/l) |
|---|---|
| Chromium | 0.05 |
| Nitrate (as NO3-) | 50 |
| Ammonia | 1.5 |
| Sulfide as Hydrogen sulfide | 0.05 |
| Sulfides (as S2-) | 1 |
Environmental Principles, Policy and Standards in Ethiopia
Water policy of Ethiopia
Development activities carried out so far in the water sector in totality or individually reveal a very low level of performance. The cause for this poor achievement and the dilemma for the failure of the country's water resources to significantly contribute to the overall socio-economic development of the Ethiopian people’s lie mainly in the absence of a well-defined coherent policy and the lack of the required huge investment.
Water resources protection
1. Create appropriate mechanisms to protect the water resources of the country from pollution and depletion so as to maintain sustainable development and utilization of water resources.
2. Establish standards and classification for various uses of water in terms of quality and quantity for Different scenarios including limits and ranges for desirable and permissible levels.
3. Establish procedures and mechanisms for all actions that are detrimental to water resources including waste discharges, source development, catchment management etc.
Water quality management: 1. Develop water quality criteria, guidelines and standards for all recognized uses of water and ensure their implementation.
2. Formulate receiving water quality standards and legal limits for pollutants for the control and protection of indiscriminate discharges of effluents into natural water courses.
3. Develop appropriate water pollution prevention and control strategies pertinent to the Ethiopian context.
Ethiopian Standards for selected chemicals (Table 3).
Table 3: EEPA Standards For Some Selected Chemicals For Receiving Water Bodies.
| Parameters of concern | Guideline value (mg/l) |
|---|---|
| Chromium (as total Cr) | 2 |
| Chromium (as Cr VI) | 0.1 |
| Total ammonia as(N) | 30 |
| Sulfides(as S2- | 1 |
Treatment Methods of Tannery Wastewater
Treatment of tannery effluent is a challenge because it is a mixture of biogenic matter of hides, inorganic chemicals and a large variety of organic pollutant. Chromium, inorganic and organic matter removal methods are physicochemical and Biological.
Physico-Chemical methods
Physico-chemical methods include precipitation, coagulation/ flocculation, adsorption, membrane filtration, ion exchange, advanced oxidation etc. Mostly, chemical precipitation methods are practiced for the removal of chromium and organic matter, but it has the disadvantage of producing secondary by products [49].
Using precipitation method there is 99% Cr removal, 85 - 90% BOD removal and 60 - 70% removal of COD can be achieved [50].
Biological treatment methods
Biological methods have been recognized as a viable possibility for the degradation of the wastewaters [51]. In biological treatment, microorganisms convert metals, inorganic and organic wastes into stabilized compounds. Typical biological treatment processes includes trickling filters, activated sludge, Sequencing Batch Reactor (SBR) and Phytoremediation.
Trickling filter
The trickling or biological filter system involves a bed, which is formed by a layer of filter medium held within a containing tank or vessel, often cast from concrete, and equipped with a rotating dosing device over which the wastewater is gently sprayed by a rotating arm. Water needs to be trickled several times over the rock before it is sufficiently cleaned. Using a trickling filter, a removal of 85 - 90% for BOD and 60 - 70% for COD can be achieved [52].
Activated sludge
Activated sludge process is a continuous or semi-continuous flow system containing a mass of activated microorganisms that are capable of stabilizing organic matter [53].
The unsettle able suspended solid and other constituents are adsorbed on or entrapped by the activated sludge floc (sluge + microbial cells) [54]. Metals like Cr can also precipitate and removed together with the sludge. According to a BOD and COD removal of 90% and 80% respectively can be achieved using activated sludge for tannery wastewater treatment [55].
Phytoextraction
The remediation of chromium and nutrients typically makes use of wetlands through the natural abilities of certain plant species to remove or stabilize these chemicals by means of bioaccumulation, Phytoextraction, rhizofiltration or phytostabilisation. High removal efficiency for total Cr (98%) can be achieved using wetlands for tannery wastewater treatment [56].
Constructed wetland technology
Constructed wetland is an artificial marsh or swamp, created for anthropogenic discharge such as industrial wastewater, storm water runoff or sewage treatment, and as habitat for wildlife, or for land reclamation after mining or other disturbance. Natural wetlands act as bio-filter or “Kidney’s of Landscape”, removing sediments and pollutants such as heavy metals and nutrients from the wastewater, and constructed wetlands can be designed to emulate these features. In Ethiopia, some researchers have investigated the wide use of constructed wetland for different type of wastewater, including domestic and industrial (Tannery wastewater) and have shown significant improvements in water quality in these systems [56].
However, the above mentioned wastewater treatment method have the following limitations:
They generally require larger land areas, long time, and high effort and higher cost than that of other wastewater treatment system particularly adsorptions.
They are economical relative to other options only where land is available and affordable especially for biological treatment methods.
Since tannery wastewater is composed of different chemicals in which some of them are toxic to microorganisms which affect their degrading capability, it is not efficient like that of activated carbon.
Chemical processes to treat waste water either pose secondary pollutant or economically coasty. However, activated carbon is economically cheep, not produce secondary pollutant, reused and used also for metal recovery.
Removal of Chromium, Ammonia, Nitrate, and Sulfide by Activated Carbon
There are a number of different techniques for the removal of chromium and nutrients from waste water. Adsorption by activated carbon is a cost-effective and very important method of chromium and nutrient removal from water and wastewater. Percentage removal of nitrate by activated carbon was about 69.5 % for chromium it was 98.86% for ammonia 80.43% [57-59].
Adsorbents
Adsorption is the process by which molecules of a substance, such as a gas or a liquid, collect on the surface of another substance, such as a solid. Due to increasing environmental awareness and legal constraints imposed on discharge of effluents, the need for cost effective alternative technologies is essential for removal of heavy metals and nutrients from industrial wastewater. An innovative technique that is both efficient and economical is biosorption. This has evolved as the frontline of defense especially for metals that could not be removed by other techniques [60].
Activated carbon
Activated carbon is a material used to filter harmful chemicals from contaminated water and air. It is composed of black granules of coal, wood, nutshells or other carbon-rich materials. As contaminated water or air flows through activated carbon, the contaminants sorbs (stick) to the surface of the granules and are removed from the water or air. Used in many industries to purify, decolorize, deodorize, dechlorinate, detoxicate, filter, recover salts, treat wastewater and used as catalysts and catalysts supports [61].
Sugar bagasse activated carbon
Sugar cane bagasse is a byproduct of sugarcane industries obtained after the extraction of juice for production of sugar. About 54 million dry tones of bagasse are produced annually throughout the world [62].
Bagasse must be modified physically and chemically to enhance its adsorptive properties towards,organic molecules, inorganic and metal ions, routinely found in water and wastewater. This is effectively accomplished by converting bagasse to an activated carbon. Bagasse is reported as a suitable resource for preparation of activated carbon [62].
Factors Affecting Adsorption
Effects of particle size
Smaller particle size reduce internal diffusion and mass transfer limitation to the penetration of the adsorbate inside the adsorbent (i.e., equilibrium is more easily achieved and nearly full adsorption capability can be attained) [63].
Effects of Ph
The degree of ionization of a species is affected by the pH (e.g., a weak acid or a weak basis). This, in turn, affects adsorption [63].
Effects of Dose of The Adsorbent
The adsorption process is primarily associated with surface of the adsorbent which is related to the amounts of the adsorbent and hence adsorption increases with the increase in surface or amounts of the adsorbent [63].
Materials and Methods
Chemicals and reagents
Chemicals used throughout the experiments were: Sulfuric acid for the treatments of sugar bagasse, Nitric acid for preservation of tannery wastewater, hydrochloric acid and sodium hydroxide for pH adjustment, Sugar bagasse used to prepare activated carbon. Distilled water was used in all experiments.
Instruments
Magnetic stirrer model GL-3250A: was used to agitate mixture of wastewater sample and activated carbon.
Digital pH meter: was used to measure pH of the solutions.
Filter paper (Whatman, 0.45μm): to filter the wastewater sample.
Electronic balance model No.: JD210-4: to weigh the Sugar bagasse and activated carbon.
Perkin Elmer model 3110 Flame Atomic Adsorption spectrometer (FAAS) operating with air acetylene flame was used to measure concentrations of metal ions in the solutions.
The detail of all instrument and chemicals were listed at the annexes.
Experimental procedure
Sample collection and analysis
Wastewater samples were collected in plastic bottles from main drains of the Addis Ababa tannery industry. The plastic bottles were thoroughly cleaned by repeated washing with distilled water and then dried over night. Effluent wastewater samples from five composite cells were collected and used for analysis of Chromium ion and selected nutrient concentrations.
Wastewater characterization was carried out for the following physico-chemical water quality parameters; pH, temperature, Ammonia nitrogen, Nitrate, Sulfide and Cr. The parameters; Nitrate, ammonia nitrogen and Sulfide were estimated using spectrophotometer (DR/2010, HACH, USA) according to HACH instructions [64]. The pH and temperature of the effluent of the system was measured using pH meter. Wastewater samples were taken to EEPA for analysis of chromium metals using Flame Atomic Absorption Spectrophotometer (AAS) (model AAS NOUA-400, Germany).
Sample preparations and preservations: Tannery wastewater samples were filtered through 0.45μm filter paper prior to preservation. Filter paper was acid washed and dried before use. After filtration the filtrate were acidified for preservation purpose and taken as experimental samples for dissolved metal and nutrient analysis.
Loss of metals by absorption or precipitation in sample containers was avoided by acidifying the sample properly using HNO3. Sample preservation was made by adding 1.5ml of concentrated HNO3 per liter of sample as. To assist in maintaining the natural chemistry of the samples, preservation method such as pH control, refrigeration and protecting from light were performed [65].
Preparations of adsorbents:
Production of activated carbon
Sugarcane bagasse was collected in plastic bags from wonji sugar factory Ethiopia. The samples were initially washed using distilled water and sun dried. The dried samples were then cut into small size approximately 1 cm and washed several times with distilled water. After repeated washing of the samples, the samples were oven dried at 105°C for 24 hours [66].
The activated carbon will be prepared by treating one part of raw bagasse (cut into small pieces) with two parts (by weight) of concentrated sulfuric acid and kept in an oven maintained at 150– 165ºC for a period of 24 hours. The acid treatment can be carried out by weighing a known amount of bagasse and treat it with 0.1M H2SO4 (3ml H2SO4+497ml) of distilled water. The carbonized material will be washed well with double distilled water to remove the free acid and dried at 105–110ºC for 24 h. The dried material will be subjected to thermal activation at 700ºC for 30 min. For batch studies the adsorbent material will be powdered and sieved to the desired particle size (300μm). Activated carbon production method was according to [65].
Study of the adsorbent
Particle size: Method for the determination of particle size was done according to (Dagmawi Mulgeta) [64].
A known amount of sugarcane bagasse was weighed, and put on the sieve shaker with different sieve size.
After shaking or vibrating the sieve, the powder was collected with the required size.
After the required sizes of the bagasse was obtained, carbonization and activation process were carried out.
Ash Content (%)
Ash determination method was according to (Moore) [66]. Ash a content of the activated carbon was measured by weighing a known amount of activated carbon and heating it to 115oC for 15 hours. The carbon was then heated in a muffle furnace at 7000C for 30 h. The crucible was then removed and placed in desiccators and weighed after cooling. The ash content was calculated as:
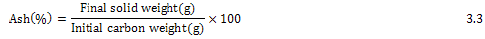
The determination of ash content was done in triplicates and the average was reported.
Moisture Content
One gram of the adsorbent was placed in an oven of 1050C for 24 hours. The difference between initial mass (mi) and the final mass of the activated carbon was calculated and percentage moisture content was deterrrmined. The methods were according to (Dagmawi Mulgeta) [64].

The experiment was carried out in triplicate and the average was reported.
Bulk Densities
The methods were according to (Dagmawi Mulgeta) [64].

![]() Mass of oven dry bagasse sample) &
Mass of oven dry bagasse sample) &  = Total volume (cm3)
= Total volume (cm3)
Porosity
100 ml of water were poured into cup and a line was drawled where the water comes up to. 100 ml in the total volume column were written on data sheet. The water was dumped out. The cup was filled with the first bagasse sample up to the line drawled. Using graduated cylinder, water were poured into the cup slowly and carefully until the water reaches the top of the sample. The volume of water remaining in the graduated cylinder was corded on data sheet. The volume remaining was subtracted from the total volume. This is the amount of water added to sample. The volumes of water added to the sample were recorded on data sheet – this is the pore space. To determine the porosity of the sample, the pore space volume was divided by the total volume and multiplied by 100 [67].
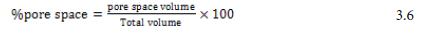
The experiment was carried out in triplicate and the average was reported.
Determination of chromium
50ml test tube was taken and filled with 25ml of wastewater. The solutions are filtered by whatman filter paper. After filtrations, the wastewaters were diluted to 10%. After dilution wastewater were analyzed for its metal content by Atomic Adsorption Spectroscopy.
Factors Influencing Adsorption
Effects of pH
The experimental procedure was carried out as described by (Okieimen C.O and Okieimen FE) [68]. The effect of pH on the adsorption of Cr was studied by adjusting pH at pH values of 2, 5, 7 and 9. Test tubes were taken and filled with 25ml of wastewater sample and adjusted to their respective pH. Before adjusting the pH, pH of each samples were measured by using digital pH meter. The samples were libeled as: sample I; sample II; sample III and sample IV. For adjustment of pH 10ml of 1N NaOH and 0.1MHCl were used as required.
After the pH adjustment to respective pH, 1.5g of the adsorbent was added to each sample and stirred by magnetic stirrer for about 5 minutes and stored at room temperatures for about 24 hrs. After this time the solutions were filtered and analyzed for it’s for its metal content left in the solutions. The experiments have been done four times for about 4 months and the repeated average results of each experiment were recorded.
Effects of adsorbent dose
To study the effects of the amounts of adsorbent on Cr adsorption, a series of 50ml test tubes were taken and filled with 25ml of filtered wastewater sample for each test tube. Test tubes were labeled as: test-tube 1, test-tube 2, test-tube 3.and test-tube 4. Adsorbents of 0.8g, 1g, 1.5g and 2g were added to respective test-tube. The samples were agitated for 5 minutes by magnetic stirrer and stored at room temperatures for about 24 hours. After this time, the samples were filtered and analyzed for the concentrations of metals retained in the solution [66]. The experiments have been done four times for about 4 months and the repeated average results of each experiment were recorded.
Determinations of Ammonia, Nitrate and Sulfide
All parameters were analyzed according to America Public Health Association [64]. Determinations of Nutrients were made by HACH method of nutrient analysis. Samples were analyzed for parameters ammonia, nitrate and Sulfides by Nessler, Cadmium reduction HR and Methylene blue method respectively.
Studies of factors affecting adsorptions of nutrients
Effects of pH on the adsorptions of nutrients: The effects of pH on the adsorption of Ammonia, Nitrate and sulfide on to activated carbon were studied by evaluating the adsorption at pH values of 2, 5, 7 and 10. Test tubes were taken and filled with 25 ml of wastewater sample and adjusted to their respective pH. Before adjusting pH, the pH of each samples were measured by using digital pH meter. The samples were libeled as: sample E; sample F; sample G and sample H. For adjustment of pH 10ml of 1N NaOH and 0.1MHCl were used as required. After the samples pH were adjusted to respective pH, 1g of the adsorbent was added to each sample and stirred by magnetic stirrer for about 5 minutes and stored at room temperatures for about 24 hrs. After this time the solutions were filtered and analyzed by for its Ammonia, Nitrate and Sulfide content left in the solutions. The experiments have been done three times for about 3 months and the repeated average results of each experiment were recorded.
Effects of adsorbent dose on the adsorptions of nutrients: To study the effects of the amounts of adsorbent on the adsorption of Ammonia, Nitrate and sulfide a series of 50 ml test tubes were taken and filled with 25 ml of filtered wastewater sample for each test tube. Test tubes were labeled as: test-tube A, test-tube B, test-tube C and test-tube D. Adsorbents of 0.8g, 1g, 1.5g and 2g were added to respective test tubes. The samples were agitated for 5 minutes by magnetic stirrer and stored at room temperatures for about 24 hours. After this time, the samples were filtered and analyzed for its Ammonia, Nitrate and Sulfide content left in the solutions. The experiments have been done three times for about 3 months and the repeated average results of each experiment were recorded.
New things in the method
There were two things new in the paper: the first one is that the adsorption process we have been reviewed was taken place by placing the mixture if the adsorbent and adsorbate on the shaker through all the contact time or adsorption process. However, since the use of shaker is for mixing the mixture, we placed for 5 minutes on the magnetic stirrer to mix the solutions and stored at room temperature for contact time of 24 hours and analyze the concentrations left after this time. The second thing is that except chromium, we haven’t seen the literatures for the adsorption of the ammonium, nitrate and sulfide by sugar bagasse activated carbon, however there were adsorptions of these nutrients by activated carbon from other sources.
Results and Discussion
Studies of removal efficiency of activated carbon
Effluent wastewater characteristics: Effluent of Addis Ababa Tannery Share Company contains large amounts of chromium, and nutrients. The composite wastewater from this industry contains the following amounts of chromium and some selected nutrients.
Activated carbon characterization (Table 4).
Table 4: Effluent Wastewater Characteristics.
| Parameters | Unit in mg/l |
|---|---|
| Chromium(Cr) | 2304 |
| Ammonia(NH3-N) | 163 |
| Nitrate((NO3-) | 250 |
| Sulfide(S2-) | 12 |
Ash content : The ash content is a measure of the mineral content and other inorganic matter in biomass and is used in conjunction with other procedures to determine to total composition of biomass samples [67]. The percent ash content of the prepared activated carbon was (53%) which is comparable with that of commercial activated carbon (52%) [66].
Bulk density: The bed or bulk density is the mass of adsorbent in a specific volume. The bulk density of the prepared activated carbon was (0.423g/cm3) which is comparable with that of commercial activated carbon (0.387g/cm3) [66].
Moisture content: Adsorption capacities of the activated carbon are affected by its moisture (water content). In this regard, concluded that the moisture contents of activated carbon had a more marked influence on the adsorption capacity of the activated carbons. As result low moisture contents of the activated carbon were preferable [69]. The percent moisture contents of the activated carbon prepared from sugarcane bagasse were about (8.1%) and that of commercial activated carbon was (7.8%).
Particle size: We can define a particle as being a discrete sub portion of a substance. That increase in particle size decreases the percent removal. At a fixed adsorbent dosage, the decrease in particle size increases the metal uptake. The increase in the uptake by smaller particles was due to the greater accessibility to pores and to the greater surface area for bulk adsorption per unit mass of the adsorbent. The particle size of prepared activated carbon was (300μm) size. Such an effect is probably due to the small particle size increase the total surface area and therefore the ability of Cr to penetrate all the internal pore structure of carbon is very high (viz. reduces the external mass transfer resistance).
Porosity: The porosity of a material is a measurement of how much of its volume is open space (also called pore space). Porosity is usually expressed as a percentage of the material’s total volume. The porosity of the activated carbon also shows the active surface area of the powder.
Depending on the raw materials, activated carbons would have different surface characteristics, including surface functional groups, surface area, porosity, and pore size distribution. It is well known that the extent of activation affects the Pore structure of the resulting carbons [70]. Since adsorption process was directly proportional to pore space of the activated carbon, large percentage of pore space was preferable and also the reason why we activate at high temperature was to increase the number of pore space. The porosity in percent of the prepared activated carbon was about (67.21%) and that of commercial activated carbon was (68.09%).
Studies of factors affecting Cr adsorption
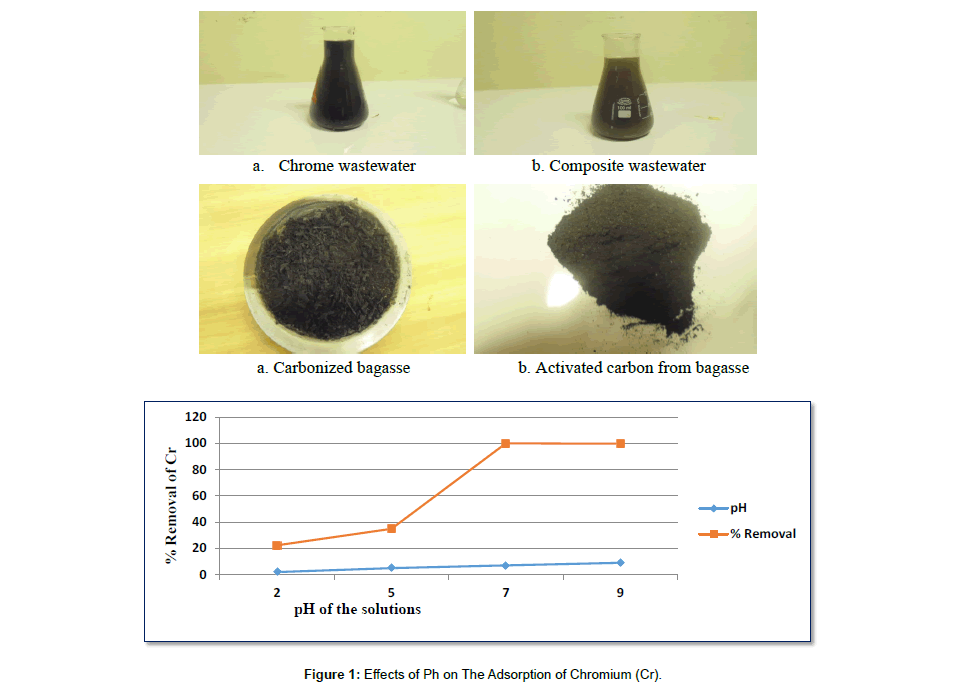
Effect of pH: The effect of pH was determined by studying adsorption of Cr at initial Cr concentration of 230.4 mg/l with adsorbent doses of 1.5g of activated carbon over a pH range of 2 to 9 pH. (Table 5).
Table 5: Results for the Effects of Ph on the Removal of Cr.
| pH | Adsorbent dose in gram | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
Average Q in mg of metal/gram of adsorbent |
|---|---|---|---|---|---|
| 2 | 1.5 | 230.4 | 178.93 | 22.34 | 3.43 |
| 5 | 1.5 | 230.4 | 149.9 | 34.92 | 5.35 |
| 7 | 1.5 | 230.4 | 0 | 100 | 15.36 |
| 9 | 1.5 | 230.4 | 0.70505 | 99.69 | 15.31 |
The effect of pH on the adsorption of Cr by activated carbon was shown in Figure 1 which shows that maximum adsorption was observed at pH 7 (100%) with decreasing values on either side of this pH range. pH dependence of metal adsorption can largely be related to type and ionic state of the functional group present in the adsorbent and also to the metal chemistry in the solution.
Under acidic conditions, there was a competition between chromium ion and hydrogen ion which decreases adsorption. However, at pH around 7 or neutral there is less competition of both hydrogen ion and hydroxide ion.
For pH above 8 furthermore, as pH increases there is competition between hydroxide ion and chromate ions. The former being the dominant species at higher pH values. The net positive surface potential of sorbent decreases, resulting in the weakening of electrostatic forces between sorbent and sorbet, which ultimately leads to reduced sorption capacity.
The high pH value causes a reduction in the electrostatic attraction between negatively charged Cr ions and positively charged adsorbent surfaces, therefore reduced percent removal was observed at high pH.
Adsorption of Cr at different pH can be explained by the species distribution of chromium in water and the nature of adsorbent surface. In acidic pH, the predominant species of Cr(III) cations are: Cr3+, CrOH2+ and CrOH+2 and under acidic conditions, the surface of the adsorbent becomes protonated and hence there is a decrease in the electrostatic attraction between the Cr(III) species and the adsorbent surface, with a consequent decrease in percentage adsorption. But as pH increases, the adsorbent surface becomes less protonated and will have strong attraction for cationic species of Cr. Similar results has been obtained with maximum adsorption of 98.86% at pH 6.6. [71].
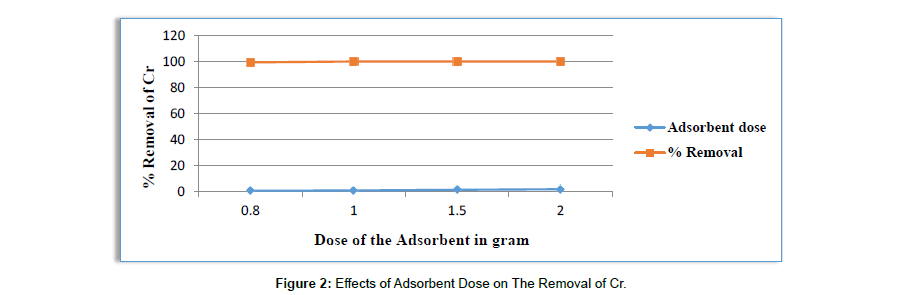
Effect of Dose of the adsorbent on the removal of Cr: To optimize the adsorbent dose and time for the removal of Cr from the solution, adsorption studies were carried out at initial Cr concentration of 230.4mg/l with different adsorbent doses of (0.8g, 1g, 1.5g and 2g) of adsorbent (Table 6). As shown from (Figure 2) the graph percentage Cr adsorption at constant time of (24hrs) and at different doses indicate that sorption of Cr increased with increasing dose of activated carbon. The reason is an increase in adsorption with dose can be attributed to increased surface area and the availability of more binding sites for adsorption. The removal of Cr by Activated carbon ranged from 99.39% to 100%. After addition of 2g of adsorbent percentage removal was become constant. Therefore, it can be concluded that the rate of Cr binding with adsorbent was increases as adsorbent dose increases and become constant after addition of 1.5g/25ml of wastewater. The increase in the percentage adsorption with increase in the adsorbent dosage is due to the increase in the number of adsorption sites (Agaje Bedemo, 2007).
Table 6:Results for the Effects of Adsorbent Dose on The Removal of Cr.
| pH | Adsorbent dose in gram | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
Average Q in mg of metal/gram of adsorbent |
|---|---|---|---|---|---|
| 4 | 0.8 | 230.4 | 1.3953 | 99.39 | 28.62 |
| 4 | 1 | 230.4 | 0.02456 | 99.985 | 23.03 |
| 4 | 1.5 | 230.4 | 0 | 100 | 15.36 |
| 4 | 2 | 230.4 | 0 | 100 | 15.36 |
Adsorption of Ammonia, Nitrate and Sulfide
Adsorption of Ammonia (NH3-N)
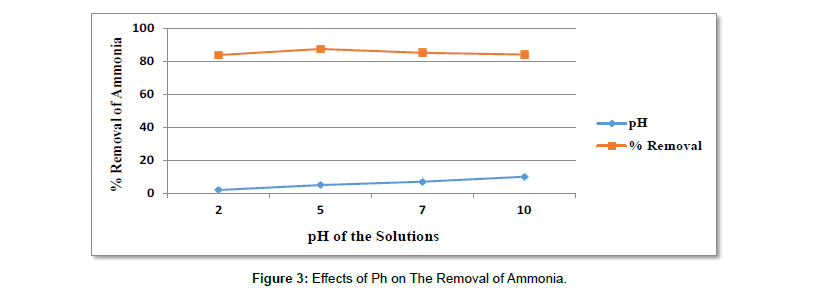
Effects of pH on the adsorption of ammonia: For ammonia, the properties of ammoniacal nitrogen in aqueous solution explain the result; the existence of two types of elements, ammonia, NH3 (alkaline) and ammonium ions, NH4+ (acidic) (Table 7).
Table 7: Results For The Effects of Ph on The Removal of Ammonia.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 163 | 26.19 | 83.93 |
| 5 | 1 | 24 | 163 | 20.17 | 87.63 |
| 7 | 1 | 24 | 163 | 23.92 | 85.32 |
As shown in Figure 3 the percent removal of ammonia at pH 2 was about (83.93%), at pH 5 (87.63%), at pH 7 (85.32%) and at pH 10 it was about (84.22%). From the experimental result obtained, the percent removal of ammonia was increased from pH 2 to 5. After pH 5, the adsorption was decreased with maximum adsorption at pH 5 of (87.63%) removal. The reason is probably, at pH < 5 there was H+ competition and at basic pH there was (OH-) competition.
Therefore, the removal of ammonia is supposed to be higher at low pH, and increases up to pH 5, due to the cationic exchange mechanism in aqueous solution [72]. The adsorption data for the uptake of ammonia versus pH is presented in Figure 4 the optimum pH for ammonia removal was at pH 5 (87.63%); as shown in Figure 4. The research by (Azharit et al.) also showed that the optimum pH for ammonia adsorption at pH 5–6 [58,73].
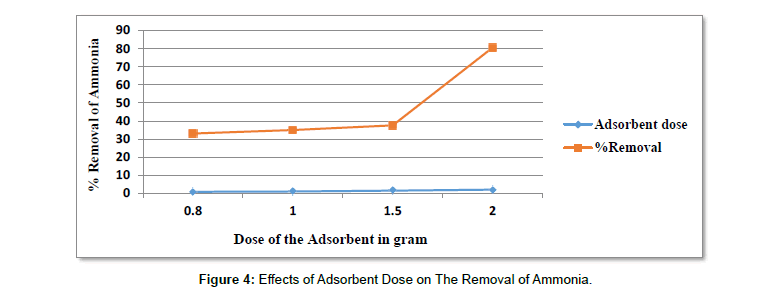
Effects of adsorbent dose on the adsorption of ammonia: Figure 5 represents the influence of activated carbon dose in (g) of 0.8, 1.0, 1.5 and 2 g on the removal of ammonia. At adsorbent dose of 0.8g, it was about (33.11%), at 1g (34.96%), at 1.5 (37.62%) and at adsorbent dose of 2g it was about (80.56%), with maximum adsorption at 2g of adsorbent and with percent removal of (80.56%). It can be seen from the results that percent removal of ammonia increases with increasing amount of the adsorbent. This is due to the greater availability of the active sites or surface area at the adsorbent dose is increased. The study by (Moinuddin G. et al.,) also forwarded the same conclusion (Table 8) [74].
Table 8:Results For The Effects of Adsorbent Dose on The Removal of Ammonia.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 163 | 109 | 33.11 |
| 9 | 1 | 24 | 163 | 106 | 34.96 |
| 9 | 1.5 | 24 | 163 | 101.67 | 37.62 |
| 9 | 2 | 24 | 163 | 31.67 | 80.56 |
Adsorption of Nitrate (NO3-)
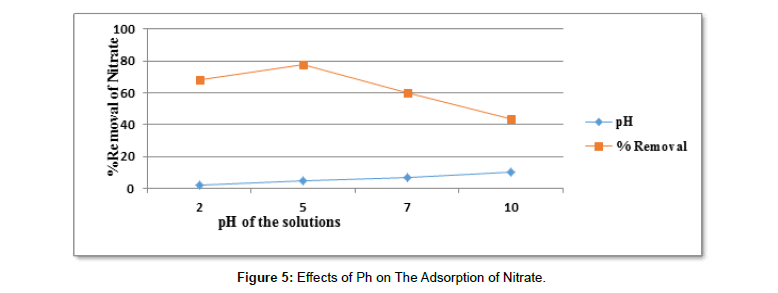
Effects of pH on the adsorption of nitrate: As shown from the Figure 6 at pH 2 percent adsorption was about (68.22%) and at pH 5 it was (77.95%). At pH 7 it was (59.92%) and at pH 10, the adsorption was about (43.88%). From the result obtained, we deduce that the adsorption capacity of activated carbon increased with increasing pH, reaching a maximum (77.95%) at pH 5. Further increase in pH above 5 led to a decrease in adsorption capacity. Other studies have reported maximum NO-3 adsorption at this pH values [75]. This might be due to the hindrance caused by ions (added externally to adjust the pH of the adsorbate solution) to occupy the adsorbent surface [76].
Lower NO-3adsorption at pH>5 was due to excess competing H+ ions on the surface, causing an electrostatic repulsion between the surface and NO-3 ions [77]. The study by (Moonis et al.,) on the investigation of adsorption of nitrate using granular activated carbon showed the maximum adsorption of nitrate at pH 5 of 71.45% removal (Table 9) [77].
Table 9: Results For The Effects of Ph on The Removal of Nitrate.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 250 | 79.45 | 68.22 |
| 5 | 1 | 24 | 250 | 55.11 | 77.95 |
| 7 | 1 | 24 | 250 | 100.25 | 59.92 |
| 10 | 1 | 24 | 250 | 140.29 | 43.88 |
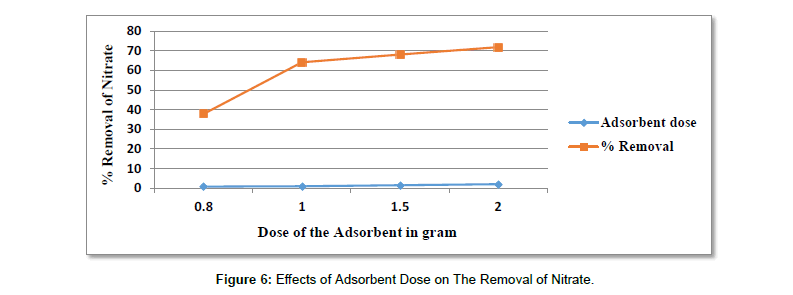
Effects of Adsorbent dose on The Adsorption of Nitrate (Figure 7 and Table 10).
Table 10: Results For The Effects of Adsorbent Dose on The Removal of Nitrate.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 250 | 155.33 | 37.86 |
| 9 | 1 | 24 | 250 | 89.67 | 64.13 |
| 9 | 1.5 | 24 | 250 | 79.67 | 68.13 |
| 9 | 2 | 24 | 250 | 70.33 | 71.86 |
As shown from (Figure 8) the influence of activated carbon dose in (g) of 0.8, 1.0, 1.5 and 2 g on the removal of nitrate increases as the increase in the gram of the adsorbent. At adsorbent dose of 0.8g, it was about (37.86%), at 1g (64.13%), at 1.5 (68.13. %) and at adsorbent dose of 2g it was about (71.86. %), with maximum adsorption at 2g of adsorbent and with percent removal of (71.86%). It can be seen from the results that percent removal of nitrate increases with increasing amount of the adsorbent. This is due to the greater availability of the active sites or surface area, when the adsorbent quantity is increased. It also showed that the uptake of nitrate increases as the adsorbent dose increases.
Adsorption of Sulfide (S2-)
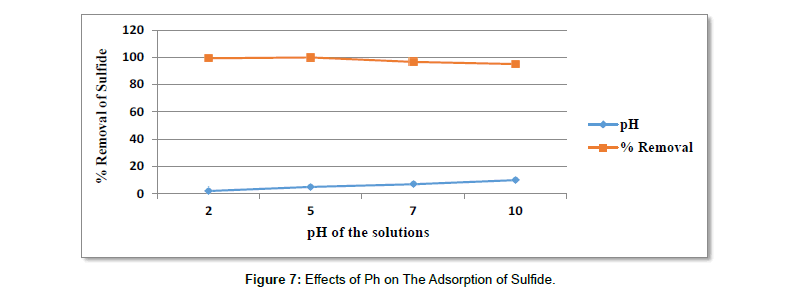
Effects of pH on The adsorption of sulphide: The effect of pH on adsorption of sulfide by sugar bagasse activated carbon was observed by varying the pH from 2 to 10. At pH 2 percent adsorption of sulfide was (99.42%) and at pH 5 it was (100%). At pH 7 it was (96.72%) and at pH 10 the adsorption was about (95.16%) [78-80]. From the result obtained the adsorption capacity of activated carbon for sulfide increased with increasing pH up to pH 5, reaching maximum adsorptions of (100%). Further increases in pH above 5 led to decrease in adsorption capacity. The reason why the adsorption of sulfide at low pH decrease is that, there was a competition between sulfide ion, hydrogen ion and solution ion that were actually added to adjust pH of the solution [80- 85]. At pH above 5, there was a decrease in the adsorption of sulfide. The reason is there were a competition between sulfide ion, hydroxide ion and ion of the solution that were added during pH adjustment.
In both cases there is less un occupied pore space for sulfide ion to be adsorbed. However, the adsorption of sulfide ion is maximum at pH 5 with percent adsorption of (100%). The reason is probably the less number of both hydrogen and hydroxide ion (Table 11) [85-90].
Table 11:Results For The Effects of Ph On The Removal of Sulfide.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 12 | 0.07 | 99.42 |
| 5 | 1 | 24 | 12 | 0 | 100 |
| 7 | 1 | 24 | 12 | 0.39 | 96.72 |
| 10 | 1 | 24 | 12 | 0.58 | 95.16 |
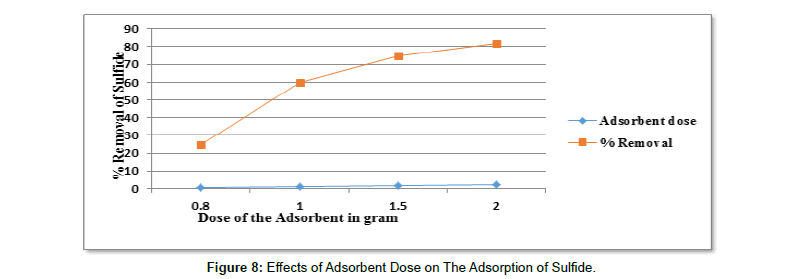
Effects of Adsorbent Dose on The Adsorption of Sulfide (Table 12).
Table 12:Results For The Effects of Adsorbent Dose on The Removal of Sulfide.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 12 | 9 | 25 |
| 9 | 1 | 24 | 12 | 4.82 | 59.83 |
| 9 | 1.5 | 24 | 12 | 3.036 | 74.69 |
| 9 | 2 | 24 | 12 | 2.18 | 81.79 |
To investigate the effects of dose of the adsorbent adsorption studies were carried out with different adsorbent doses of activated carbon (0.8g, 1g, 1.5g and 2g) [91-96]. As shown from the (Figure 8) percentage s2- adsorption increase with the increase dose of activated carbon [97-100]. The reason is an increase in adsorption with dose can be attributed to increased surface area and the availability of more binding sites for adsorption [111-114]. The adsorption of sulfide with Activated carbon of (0.8g, 1g, 1.5g and 2g) was (25%, 59.83%, 74.69% and 81.79%) respectively. As shown from the Figure 9 the adsorption increases with the increase in dose of the adsorbent and reached maximum adsorption at adsorbent dose of 2g and percent adsorption of (81.79%). Therefore, it can be concluded that the rate of sulfide binding with adsorbent was increases as adsorbent dose increases [115,116].
The treated wastewater at pH 5 has concentrations of ammonia 20.17mg/l even if it is above the guide line this amount can also found naturally in the water and food (ATSDR, 2004), nitrate 55.11mg/l which is comparable with 50mg/l of guide lines, sulfide 0mg/l which is below the guide line of 1mg/l and for chromium at pH 7 it was about 0mg/l which is comparable to guide line (Figures 10 and 11) [117].
Conclusions and Recommendations
The activated carbon prepared from bagasse, was used as an adsorbent to remove: chromium, ammonia, nitrate and sulfide from tannery wastewater. Form the study the following conclusions were drawn:
The activated carbon has moisture content of (8.1%), particle size of (300μm), porosity of (67.21%) and bulk density of (0.423g/ cm3).
The adsorptions of ammonia, nitrate and sulfide were (80.56%), (71.86%) and (81.79%) respectively for 2g of adsorbent and for chromium it was (100%) at 1.5g adsorbent. At pH5, the adsorption of ammonia, nitrate and sulfide were (87.63%), (77.95%) and (100%) respectively. For chromium at pH 7 the percent removal was 100%. From the results obtained we conclude that the activated carbon has different adsorption efficiency for different nutrients and metal ion in which chromium (100%) > sulfide (81.79%) > ammonia (80.56%) > nitrate (71.86%). At pH 5 removal of nutrients were in order of sulfide (100%) > ammonia (87.63%) > nitrate (77.95%).
Recommendation
Base on the study made the following Recommendations were given.
➢ The federal and city environmental protection authorities have to formulate an incentive scheme to motivate the tannery to manage their wastewater.
➢ Awareness creations are appropriate for the farmer around this tannery factory to not use the wastewater without treatment.
➢ Further study should be undertaken to study the efficiency of this activated carbon in broader manner.
➢ This study was restricted to limited parameters during the treatments of tannery wastewater as a result of chemicals, equipments and finance, for future it should be better if it will be funded and supported well.
Annexes
Annexe 1: List of Apparatus
A. Analytical Instruments
Sample bottles, Separatory funnels, Pipettes, Measuring cylinder, Digital pH meter, Spoons, Sieve analyzer, Oven, Cruciales, Dedicators, Magnetic stirrer (Figures 12-14).
Annexe 2: List of Chemicals and Materials
➢ De-ionized water
➢ Nitric acid (concentrated).
➢ Sodium hydroxide (diluted)
➢ Hydrochloric acid (diluted)
➢ Sugar bagasse activated carbon.
Annexe 3. Laboratory procedures
B. Sample Preparations and Preservations
➢ Tannery wastewater from Addis Ababa tannery Share Company was filtered through a o.45μm filter paper to remove solid or gross solid waste that may interfere the analysis process. After filtrations the samples were preservated using concentrated Nitric acid per liter of wastewater samples and stored in refegirator till analysis (Tables 13-18).
Table 13: Results For The Effects of Varying Adsorbent Dose on The Adsorption of Cr of The Tannery Wastewater at Constant: Temperature (20±2ºc), Ph (4), Contact Time of (24hrs) and Adsorbent Dose of (O.8g, 1g, 1.5g, 2g).
| pH | Adsorbent dose in gram | Initial concentration in mg/l | Final con. In mg/l | % removal | Q in mg of metal/gram of adsorbent |
|---|---|---|---|---|---|
| 4 | 0.8 | 230.4 | 1.567 | 99.32 | 28.6 |
| 4 | 1 | 230.4 | 0.02486 | 99.98 | 23.04 |
| 4 | 1.5 | 230.4 | 0 | 100 | 15.36 |
| 4 | 2 | 230.4 | 0 | 100 | 11.52 |
| 4 | 0.8 | 230.4 | 1.3454 | 99.42 | 28.63 |
| 4 | 1 | 230.4 | 0.01237 | 99.9946 | 23.03 |
| 4 | 1.5 | 230.4 | 0.0001 | 99.9956 | 15.36 |
| 4 | 2 | 230.4 | 0.0011 | 99.99 | 11.52 |
| 4 | 0.8 | 230.4 | 1.412 | 99.387 | 28.62 |
| 4 | 1 | 230.4 | 0.04891 | 99.97 | 23.03 |
| 4 | 1.5 | 230.4 | 0 | 100 | 15.36 |
| 4 | 2 | 230.4 | 0.0001 | 99.9956 | 11.52 |
| 4 | 0.8 | 230.4 | 1.2567 | 99.4545 | 28.64 |
| 4 | 1 | 230.4 | 0.01213 | 99.9947 | 23.03 |
| 4 | 1.5 | 230.4 | 0.00001 | 99.99997 | 15.36 |
| 4 | 2 | 230.4 | 0 | 100 | 15.36 |
Table 14: Average Results For The Effects of Adsorbent Dose on The Removal Of Cr.
| pH | Adsorbent dose in gram | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
Average Q in mg of metal/gram of adsorbent |
|---|---|---|---|---|---|
| 4 | 0.8 | 230.4 | 1.3953 | 99.39 | 28.62 |
| 4 | 1 | 230.4 | 0.02456 | 99.985 | 23.03 |
| 4 | 1.5 | 230.4 | 0 | 100 | 15.36 |
| 4 | 2 | 230.4 | 0 | 100 | 15.36 |
Table 15: Results For The Effects of Varying Ph of The Tannery Wastewater on The Adsorption of Cr At Constant: Temperature (20±2ºc), Adsorbent Dose of (1.5g) and Contact Time of (24hrs).
| pH | Contact time in hours | Initial concentration in mg/l | Final con. In mg/l | % removal | Q in mg of metal/gram of adsorbent |
|---|---|---|---|---|---|
| 2 | 24 | 230.4 | 180.8 | 21.53 | 3.3 |
| 5 | 24 | 230.4 | 151.5 | 34.24 | 5.26 |
| 7 | 24 | 230.4 | 0 | 100 | 15.36 |
| 9 | 24 | 230.4 | 0.7255 | 99.68 | 15.31 |
| 2 | 24 | 230.4 | 179.5 | 22.09 | 3.39 |
| 5 | 24 | 230.4 | 153.2 | 33.50 | 5.1 |
| 7 | 24 | 230.4 | 0.0001 | 99.99 | 15.35 |
| 9 | 24 | 230.4 | 0.6873 | 99.70 | 15.31 |
| 2 | 24 | 230.4 | 180 | 21.87 | 3.36 |
| 5 | 24 | 230.4 | 149.6 | 35.06 | 5.38 |
| 7 | 24 | 230.4 | 0.0001 | 99.99 | 15.35 |
| 9 | 24 | 230.4 | 0.5342 | 99.76 | 15.32 |
| 2 | 24 | 230.4 | 175.4 | 23.87 | 3.67 |
| 5 | 24 | 230.4 | 145.3 | 36.94 | 5.67 |
| 7 | 24 | 230.4 | 0 | 100 | 15.36 |
| 9 | 24 | 230.4 | 0.8732 | 99.62 | 15.30 |
Table 16: Average Results For The Effects of Ph on The Removal of Cr.
| pH | Adsorbent dose in gram | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
Average Q in mg of metal/gram of adsorbent |
|---|---|---|---|---|---|
| 2 | 1.5 | 230.4 | 178.93 | 22.34 | 3.43 |
| 5 | 1.5 | 230.4 | 149.9 | 34.92 | 5.35 |
| 7 | 1.5 | 230.4 | 0 | 100 | 15.36 |
| 9 | 1.5 | 230.4 | 0.70505 | 99.69 | 15.31 |
Table 17: Results For The Effects of Varying Adsorbent Dose on The Adsorption of Nitrate (NO3-) For Constant Contact Time of 24 Hours, Temperature (20±2ºc) and Ph of 9 Wastewater.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Final con. In mg/l | % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 250 | 155 | 38 |
| 9 | 1 | 24 | 250 | 90 | 64 |
| 9 | 1.5 | 24 | 250 | 80 | 68 |
| 9 | 2 | 24 | 250 | 70 | 72 |
| 9 | 0.8 | 24 | 250 | 157 | 37.2 |
| 9 | 1 | 24 | 250 | 91 | 63.6 |
| 9 | 1.5 | 24 | 250 | 78 | 68.8 |
| 9 | 2 | 24 | 250 | 69 | 72.4 |
| 9 | 0.8 | 24 | 250 | 154 | 38.4 |
| 9 | 1 | 24 | 250 | 88 | 64.8 |
| 9 | 1.5 | 24 | 250 | 81 | 67.6 |
| 9 | 2 | 24 | 250 | 72 | 71.2 |
Table 18: Average Results For The Effects of Adsorbent Dose on The Removal of Nitrate.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 250 | 155.33 | 37.86 |
| 9 | 1 | 24 | 250 | 89.67 | 64.13 |
| 9 | 1.5 | 24 | 250 | 79.67 | 68.13 |
| 9 | 2 | 24 | 250 | 70.33 | 71.86 |
C. Determinations of Chromium content
➢ Chromium concentrations were determined using atomic Adsorption Spectrophotometer (AAS) with an air-acetylene flame as described by APHA (Tables 20-23) [6].
Table 19: Results For The Effects of Varying Adsorbent Dose on The Adsorption of Sulfide (S2-) For Diluted Wastewater For Constant Contact Time of 24 Hours, Temperature (20±2ºc) and Ph of 9 Wastewater.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Final con. In mg/l | % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 12 | 9 | 25 |
| 9 | 1 | 24 | 12 | 4 | 66.67 |
| 9 | 1.5 | 24 | 12 | 3 | 75 |
| 9 | 2 | 24 | 12 | 2 | 83.3 |
| 9 | 0.8 | 24 | 12 | 10 | 16.67 |
| 9 | 1 | 24 | 12 | 5 | 58.33 |
| 9 | 1.5 | 24 | 12 | 3.11 | 74.08 |
| 9 | 2 | 24 | 12 | 2.43 | 79.75 |
| 9 | 0.8 | 24 | 12 | 8 | 33.33 |
| 9 | 1 | 24 | 12 | 5.46 | 54.5 |
| 9 | 1.5 | 24 | 12 | 3 | 75 |
| 9 | 2 | 24 | 12 | 2.12 | 82.33 |
Table 20: Average Results For The Effects of Adsorbent Dose on The Removal Of Sulfide.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 12 | 9 | 25 |
| 9 | 1 | 24 | 12 | 4.82 | 59.83 |
| 9 | 1.5 | 24 | 12 | 3.036 | 74.69 |
| 9 | 2 | 24 | 12 | 2.18 | 81.79 |
Table 21: Results For The Effects of Varying Adsorbent Dose on The Adsorption of Ammonia (NH3-N) For Diluted Wastewater For Constant Contact Time of 24 Hours, Temperature (20±2ºc) and Ph of 9 Wastewater.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Final con. In mg/l | % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 163 | 109 | 33.13 |
| 9 | 1 | 24 | 163 | 107 | 34.35 |
| 9 | 1.5 | 24 | 163 | 102 | 37.42 |
| 9 | 2 | 24 | 163 | 33 | 79.75 |
| 9 | 0.8 | 24 | 163 | 110 | 32.5 |
| 9 | 1 | 24 | 163 | 106 | 34.96 |
| 9 | 1.5 | 24 | 163 | 103 | 36.8 |
| 9 | 2 | 24 | 163 | 32 | 80.36 |
| 9 | 0.8 | 24 | 163 | 108 | 33.7 |
| 9 | 1 | 24 | 163 | 105 | 35.58 |
| 9 | 1.5 | 24 | 163 | 100 | 38.65 |
| 9 | 2 | 24 | 163 | 30 | 81.59 |
Table 22: Average Results For The Effects of Adsorbent Dose on The Removal of Ammonia.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 9 | 0.8 | 24 | 163 | 109 | 33.11 |
| 9 | 1 | 24 | 163 | 106 | 34.96 |
| 9 | 1.5 | 24 | 163 | 101.67 | 37.62 |
| 9 | 2 | 24 | 163 | 31.67 | 80.56 |
Table 23: Results For The Effects of Varying Ph on The Adsorption of Nitrate (NO3-) For Constant Contact Time of 24 Hours, Temperature (20±2ºc) and Adsorbent Dose of (1g) Wastewater.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Final con. In mg/l | % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 250 | 80 | 68 |
| 5 | 1 | 24 | 250 | 55 | 78 |
| 7 | 1 | 24 | 250 | 100 | 60 |
| 10 | 1 | 24 | 250 | 140 | 44 |
| 2 | 1 | 24 | 250 | 80.34 | 67.86 |
| 5 | 1 | 24 | 250 | 56.1 | 77.56 |
| 7 | 1 | 24 | 250 | 101.2 | 59.52 |
| 10 | 1 | 24 | 250 | 139 | 44.4 |
| 2 | 1 | 24 | 250 | 78 | 68.8 |
| 5 | 1 | 24 | 250 | 54.22 | 78.3 |
| 7 | 1 | 24 | 250 | 99.56 | 60.2 |
| 10 | 1 | 24 | 250 | 141.87 | 43.25 |
D. Determinations of Nutrients
➢ The nutrients Ammonia, Nitrate and Sulfide were determined by HACH methods of nutrient analysis (Tables 24-26).
Table 24: Average Results For The Effects of Ph on The Removal of Nitrate.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 250 | 79.45 | 68.22 |
| 5 | 1 | 24 | 250 | 55.11 | 77.95 |
| 7 | 1 | 24 | 250 | 100.25 | 59.92 |
| 10 | 1 | 24 | 250 | 140.29 | 43.88 |
Table 25: Results For The Effects of Varying Ph of The Solution on The Adsorption of Sulfide (S2-) For Constant Contact Time of 24 Hours, Temperature (20±2ºc) and Adsorbent Dose of (1g) Wastewater.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Final con. In mg/l | % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 12 | 0.08 | 99.33 |
| 5 | 1 | 24 | 12 | 0 | 100 |
| 7 | 1 | 24 | 12 | 0.4 | 96.67 |
| 10 | 1 | 24 | 12 | 0.58 | 95.167 |
| 2 | 1 | 24 | 12 | 0.07 | 99.42 |
| 5 | 1 | 24 | 12 | 0 | 100 |
| 7 | 1 | 24 | 12 | 0.4 | 96.67 |
| 10 | 1 | 24 | 12 | 0.60 | 95 |
| 2 | 1 | 24 | 12 | 0.06 | 99.5 |
| 5 | 1 | 24 | 12 | 0 | 100 |
| 7 | 1 | 24 | 12 | 0.38 | 96.83 |
| 10 | 1 | 24 | 12 | 0.57 | 95.25 |
Table 26: Average Results For The Effects of Ph on The Removal of Sulfide.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 12 | 0.07 | 99.42 |
| 5 | 1 | 24 | 12 | 0 | 100 |
| 7 | 1 | 24 | 12 | 0.39 | 96.72 |
| 10 | 1 | 24 | 12 | 0.58 | 95.16 |
Table 27: Results For The Effects of Varying Ph on The Adsorption of Ammonia (NH3-N) For Constant Contact Time of 24 Hours, Temperature (20±2ºc) and Adsorbent Dose of (1g) Wastewater.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Final con. In mg/l | % removal |
|---|---|---|---|---|---|
| 2 | 1 | 24 | 163 | 26 | 84.05 |
| 5 | 1 | 24 | 163 | 21 | 87.12 |
| 7 | 1 | 24 | 163 | 24 | 85.276 |
| 10 | 1 | 24 | 163 | 26 | 84.05 |
| 2 | 1 | 24 | 163 | 25.57 | 84.31 |
| 5 | 1 | 24 | 163 | 20 | 87.73 |
| 7 | 1 | 24 | 163 | 23.76 | 85.42 |
| 10 | 1 | 24 | 163 | 25 | 84.66 |
| 2 | 1 | 24 | 163 | 27 | 83.44 |
| 5 | 1 | 24 | 163 | 19.51 | 88.03 |
| 7 | 1 | 24 | 163 | 24 | 85.276 |
| 10 | 1 | 24 | 163 | 26.12 | 83.97 |
Table 28: Average Results for the Effects of Ph on the Removal of Ammonia.
| pH | Adsorbent dose in gram | Contact time in hours | Initial concentration in mg/l | Average Final con. In mg/l | Average % removal |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 1 | 24 | 163 | 26.19 | 83.93 | |||||
| 5 | 1 | 24 | 163 | 20.17 | 87.63 | |||||
| 7 | 1 | 24 | 163 | 23.92 | 85.32 | |||||
| 10 | 1 | 24 | 163 | 25.71 | 84.22 | |||||
Acknowledgements
➢ First of all, thanks to the Almighty Allah for giving me the patience and persistence to start and finish this thesis work which is full of great challenge and big obstacle in which passing through it was very difficult and seems to be impossible.
➢ Secondly, I would like to express my heartfelt appreciation and gratitude to my advisor Dr.Gajananda Kuwairkpam for his invaluable advice and hopeful encouragements. The big thing that I never forget from my advisor is that the clarity of his word which is very understandable which enable me to did my work efficiently and effectively.
➢ Thirdly, I would like to thanks Dr. Mekibib Dawit and my friend Nuri Haji who helped me to join this M.Sc course. I would also thanks Dr. Mekibib Dawit and Ato Andualem mokonin to allow me to use chemicals to analyze nutrients in tannery wastewater. My gratitude also extends to wonji sugar factory to give me sugar bagasse; Addis Ababa tannery Share Company to gave me wastewater and some information’s.
➢ The last but not least, I want to thanks my families for giving me an opportunity to learn and my friends who shared their ideas and support me throughout the course of the work.
References
- Zinabu GM, Zerihun D (2002) The Chemical Compostion of of the effluent from Awassa Textile Factory and Its Effects on Aquatic Biota. SINET: Ethiop J Sci 25: 263-74.
- Seyoum L, Fasil A, Gumaelius L, Dalhammar (2004) Biological Nitrogen and Organic Matter Removal from Tannery Wastewater in Pilot Plant Operations in Ethiopia. Appl Microbiol Biotechnology. Springer-Verlag.
- Infogate GTZ (2002) Treatment of Tannery wastewater. Germen Appropriate Technology (GATE) information system.
- Anthony DC (1997) Modern Tanning Chemistry, British School of Leather Technology, Nene College of higher education, Boughton Green Road. Mountain Park Northampton, UK NN2 7AL. Chemical Society Review. 111 - 26.
- Khan AG (2001) Relationships between Chromium Bio Magnification Ratio, Accumulation Factor and Mycorrhizae in Plants Growing on Tannery Effluent-Polluted Soil. Environ Inter 26: 417-23.
- Hafez AI, El-Manharawy MS, Khedr MA (2002) RO Membrane Removal of Unreacted Chromium from Spent Tanning Effluent. A Pilot- Scale Study, Part 2.
- Szpyrkowicz L, Naumczyk J, Zilio-Grandi F (1995) Electrochemical treatment of tannery wastewater using TiPt and Ti/Pt/Ir electrodes. Water Research 29: 517–24.
- Rajalo G, Petrovskaya T (1996) Selective electrochemical oxidation of sulphides in tannery wastewater. Environ Tech 17: 605–12.
- WHO 1996. Guidelines for drinking water quality, 2nd ed. Vol. 2 Health criteria and other supporting information, and WHO, Addendum, Geneva, World Health Organization. Summary tables. 2: 940-9; 281-3.
- Selvaraj KS, Manonmani PS (2003) Removal of hexavalent chromium using distillery sludge, Bioresource Tech 89: 207–11.
- Kocaoba S, Akcin G (2002) Removal and Recovery of Chromium and Chromium Speciation with MINTEQ2A2. Talanta. 57: 23-30.
- Kocaoba S, Akcin G (2002) Removal and Recovery of Chromium and Chromium Speciation with MINTEQ2A2. Talanta. 57: 23-30.
- Vlyssides AG, Israilides CJ (1997) Detoxification of tannery waste liquors with an electrolysis system. Environmental Pollution 97: 1–2, 147–52.
- USPHS (1997) Toxicological Profile for Chromium. Agency for Toxic Substances and Disease Registry. U.S. Public Health Service.
- Asio VB (2009) Heavy metals in the environment and their health effects, Soil & Environment.
- Fonseca MG, de Oliveira MML, Arakaki NH (2006) Removal of cadmium, zinc, manganese and chromium cations from aqueous solution by a clay mineral. J Hazardous Materials 137: 288–92.
- Williams NH, Yandell, JK (1982) Outer-sphere electron-transfer reaction of adsorbete anions. Aust. J. Chem 1982: 1133 –44.
- Chakir AJ Bessiere KE, Kacemi B (2002) Comparative study of the removal of trivalent chromium from aqueous solutions by bentonite and expanded perlite, J Hazardous Materials 95: 29– 46.
- Junior OK, Gurgel LVA (2006) Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse, Bioresource Technology, Article in press.
- Agaje B (2007) Removal of Chromium from Waste Water Using Locally Available Adsorbents. Addis Ababa University Science Faculty Chemistry department. Addis Ababa.
- Varsha, M, Apurba D (2008) Biological Treatment of Tannery Wastewater for Sulfide Removal. Department of Chemical and Bio Engineering, National Institute of Technology, Int. J. Chem. Sci.: JALANDHAR - 144 011 (Punjab) INDIA. 6:Â 472-86.Â
- Jochimsen JC, Jekel MR (1997) Partial oxidation effects during the combined oxidative and biological treatment of separated streams of tannery wastewater. Water Science and Technology 35: 337–45.
- Hanna H (2010) Developing and evaluating performance of sequencing batch reactor for the Perk MVD, (2006) Soil and Water Contamination: From Molecular to Catchments Scale. London; JK.
- treatment of sulfur rich modjo tannery effluent. A Thesis Submitted to the School of Graduate Studies Addis Ababa University. In Partial Fulfillment of the Requirement for the Degree of Master of Science in Environmental Science. Addis Ababa Ethiopia.
- Motzer WE,Testa SM, Guertin J,Stanin FT (2005) Independent Environmental Technical Evaluation Group, Chromium(VI) Hand Book. 1stedition, CRC Press, New York.
- Gajanar k, Chandra k (1999) A new method for Dissolved chromium(VI) Analysis in natural chromite minerals obtained after organic treatment. Chemcon.-05, New Delhi.
- Sarin V, Pant KK (2006) Removal of chromium from industrial waste by using eucalyptus bark. Bioresource Technology 97: 15– 20.
- Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: A review of sources, chemistry, risk and best available strategies for remediation, ISRN Ecology
- James BR (1996) The Challenge of Remediating Chromium-Contaminated Soil. Environ, Sci. Techn.30: 248A-51A.
- Huffman EWD, Allaway HW (1973) Growth of Plants in Solution Culture Containing Low levels of Cr. Plant Physiolog 52: 72-5.
- Skeffington R, Shwery PA, Peterson PJ (1976) Chromium Uptake and Transport in Barely Seedlings (HordeumvulgareL). Plantrta (Ber) 132: 209-14.
- Mehari Alebachew (2006)Uptake and Phytoaccumulation of Chromium at Seedling Stage for Three Commonly Grown Tree Species in Ethiopia. (Unpublished Thesis). Addis Ababa University. Addis Ababa Ethiopia.
- Quevauviller Ph, Maier E, Griepink B (1995) Quality Assurance for Environmental Analysis, ELSEVIER. Amsterdam, 17
- Bes-Piá A, Cuartas-Uribe B, Mendoza-Rocan JA, Galiana-Aleixandre MV, Iborra-Clar MI, et al. (2008) Pickling wastewater reclamation by means Nano filtration. Desalination. 221: 225–33.
- KolomaznÃk K, Janácová D, Prokopová Z(2005)Modeling of Raw Hide Soaking. WSEAS Transactions on Information Science and Applications. Athens, Greece, WSEAS. 11: 4
- Tesfaye Minuta (2006)Biological nitrogen removal from tannery wastewater using alkaliphilic sludge. Addis Ababa University School of graduate studies. Addis Ababa Ethiopia.
- Agency for Toxic Substances and Disease Registry (2004) U.S department ofhealth and human services Public Health Service. Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, Mailstop F-32 Atlanta, Georgia 30333.
- Stuart M, Levit MSJD (2010) Effects of Ammonia on Fish Center for Science Participation Bozeman, Montana substances in water: sulfide. Medmenham. Water Research Centre, (ESSL,TR 257).
- Randall DJ, Tsui TKN (2002)  Ammonia Toxicity In Fish. Mar Pollut Bull 45: 1
- Speijers GJA (1989) Integrated criteria document nitrate; effects. Appendix to RIVMÂ Report No. 758473012. Bilthoven, National Institute for Public Health and the Environment) (RIVM Report No. A758473012).
- FAO and WHO (1996) Toxicological evaluation of certain food additives and contaminants. Geneva, World Health Organization, Joint FAO/WHO Expert Committee on Food Additives (WHO Food Additives Series 35).
- NAS (1981) The health effects of nitrate, nitrite, and N-nitroso compounds. Part 1 of a two-part study by the Committee on Nitrite and Alternative Curing Agents in Food. Report by the United States National Research Council, National Academy of Sciences. Washington, DC, National Academy Press.
- Höring H, Nagel M, Haerting J (1991) [The nitrate-dependent endemic thyroid areas.] In:. Quantitative Methoden in der Epidemiologie. Berlin, I. Guugenmoos-Holzmann, (MedizinischeInformatik und Statistik, in  German). 72: 147–53
- Höring H (1992) The influence of environmental chemicals on the thyroid. Bundesgesundheitsblatt, (in German). 35: 194–7.
- Grabda E, Einszporn OT, Felinska C, Zbanysek R (1974) Experimental methemoglobinemia in trout. Acta Ichthyol. Piscat. 4: 43–71.
- Teodora Bagarinao (1992) Sulfide as an Environmental Factor and Toxicant: Tolerance and Adaptation in Aquatic Organisms. Scripps Institution of Oceanography. University of California at San Diego, La Jolla. CA 92 093. USA.
- WHO (1996) Health criteria and other supporting information. In: (2nd ed.) Guidelines for drinking-water quality 2, WHO, Geneva, 940–9.
- USEPA (1999) Constructed Wetlands Treatment of Municipal Wastewaters. EPA/625/R/99/010. Cincinnati, Ohio.
- Kornarios M, Lyberato G (2006) Biological Treatment of Wastewater from a Dye Manufacturing Company Using a Trickling Filter. Journal of Hazardous Material. 95 –102.
- Del Pozo R, Diez V (2003) Organic Matter Removal in Combined Anaerobic–aerobic fixed-film Bioreactors. Wat. Res. 37: 3561 – 8.
- Apaydin O, Kurt U, Gönüllü, MT (2009) An investigation on the treatment of tannery wastewater by electrocoagulation. Global Nest Journal 11: 546–55.
- Doan H, Lohi A (2001) Intermittent aeration in biological treatment of wastewater. American J. of Engineering and Applied Sciences 2: 260 - 7.
- Cloete TE, Muyima NYO (1997) Microbial community analysis: The key for the design of biological wastewater treatment systems. IAWQ No. 5, England. 2 - 7.
- Haydar S, Aziz JA, Ahmad MS (2007) Biological Treatment of Tannery Wastewater Using Activated Sludge Process. Pakistan Journal of Engineering and Applied Science 1: 61 – 6.
- Asaye K (2009) Evaluation of Selected Plant Species for the Treatment of Tannery Effluent in a Constructed Wetland System. (Unpublished Thesis), AAU, Addis Ababa.
- Hiba N, Izat N (2012) Nitrate and Nitrite Ion Removal from Aqueous Solutions by Activated Carbon Prepared from Olive Stones. This Thesis Submitted in Partial Fulfilment of the Requirements for the Degree of Master of Science in Chemistry, Faculty of Graduate Studies, An-Najah National University, Nablus, Palestine.
- Fahim NF, Barsoumb BN, Eid AE, Khalil MS (2005) Removal of chromium(III) from tannery wastewater using activated carbon from sugar industrial waste.
- Azharit AH, Noor NZA, Normah A, Anuar IM, Sham O, et al. (2011) Ammonia and cod removal from synthetic leachate  using rice husk composite adsorbent. Environmental Health Program, Faculty of Health Sciences, University Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur.
- Mohan D, Singh KP (2001) Single-and multi component adsorption of cadmium and zinc using activated carbon derived from bagasse-an agricultural waste. Water Res 36: 2304-11.
- Bansal RC, Donnet JB, Stoekli F (1988) Active Carbon. Marcel Dekker. New York
- Khadija Q, Inamullah B, Rafique K, Abdul KA (2008) International Journal of Chemical and Biological Engineering 1: 3.
- APHA (1998) Standard Methods for the Examination of Water and Wastewater: Twentieth ed. American Public Health Association/ American Water Works Association/Water Environment Federation; Washington DC; USA.
- Dagmawi M (2012) Chromium removal from tannery wastewater using moringa stenopetala seed powder as an adsorbent. A Thesis Submitted to the School of Graduate Studies of Addis Ababa University In Partial Fulfillment of the Requirement for the Degree of Master of Science in Environmental Science. Addis Ababa Ethiopia.
- Dinesh M, Kunwar PS (2002) Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse an agricultural waste. Environmental Chemistry Division, Industrial Toxicology Research Centre, Post Box No. 80, Mahatma Gandhi Marg, Lucknow 226001 UP, India
- Moore W, Johnson D (1967) Procedures for the Chemical Analysis of Wood and Wood Products. Madison, WI: U.S. Forest Products Laboratory, U.S. Department of Agriculture.
- Achaw OW, Afrane G (2008) The evolution of pore structure of coconut shells during the preparation of cocnut shell based activated carbons. Microporous and Mesoporous Materials. 112: 284 – 90.
- Okieimen CO, Okieimen FE (2001) Enhanced metal sorption by groundnut (Arachis hypogea) husks modified with thioglycollic acid. Bull.Piere Appl. Sci., 20: 13-20.
- Burrage LJ, Allmand AJ (1938) The effect of moisture on the sorption of carbon tetrachloride from an airstream by activated charcoal. J SocChemInd 57: 424–31.
- Stoeckli HF, Ballerini L (1991)‘‘Evolution of Micro porosity During Activation of Carbon,’’ Fuel. 70: 557.
- Fahim NF, Barsoumb BN, Eid AE, Khalil MS (2005) Removal of chromium(III) from tannery wastewater using activated carbon from sugar industrial waste.
- Demir A, Günay A, Debik E (2002) Ammonium removal from aqueous solution by ion exchange using packed bed natural zeolite. Water SA 28: 329-36.Â
- Berhanu G (2007) Constructed wetland system for domestic wastewater treatment: A case study in Addis Ababa, Ethiopia. Unpublished MSc thesis in Environmental science. Addis Ababa University.
- Moinuddin G, Muhammad T, Tauqeer A, Shehzad M, Khurram.KS (2012) Adsorption studies for the removal of ammonia by thermally activated carbon. Department of Chemical Engineering, COMSATS Institute of Information Technology Lahore, Pakistan.
- Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J. Hazard. Mater 164: 1012–8.
- Namasivayam C, Sangeetha, D (2005) Removal and recovery of nitrate from water by ZnCl2 activated carbon from coir pith, an agricultural solid waste. Ind. J. Chem. Techno 12: 513–21.  Nano filtration. Desalination. 221: 225–33.
- Moonis AK, Yong -Tae A, Mahindra K (2011) Adsorption Studies for the Removal of Nitrate Using Modified Lignite Granular Activated Carbon. Department of Environmental Engineering, Yonsei University, Wonju, Gangwon do, South Korea, Chemistry Department, College of Science, King Saud University, Riyadh, Saudi Arabia Separation Science and Technology, 46: 2575–84.
- Blanco J, Torrades F, De la Varga  M, GarcÃa-Montaño (2012)  Fenton and biological-Fenton coupled processes for textile wastewater treatment and reuse. Desalination. 286: 394–9.
- Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. Criteria For Ammonia. 30:38–70
- De Gisi S, Galasso M, De Feo G (2009) Treatment of tannery wastewater through the combination of a conventional activated sludge process and reverse osmosis with a plane membrane. Desalination, 249: 337–342.
- Deepali KK, Gangwar R, Joshi BD (2009) Comparative study of physico-chemical properties of effluent from tannery industries. Indian Journal of Environmental Sciences 3: 49–52.
- Dogruel S, Genceli EA, Babuna FG,  Orhon D (2004) Ozonation of non- biodegradable organics in tannery wastewater. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances & Environmental Engineering. 39: 1705–15.
- EEPA (1997) Â Environmental Protection Authority. Annual Report; Addis Ababa, Ethiopia.
- EEPA (2003) Â Environmental Protection Authority. Annual Report; Addis Ababa, Ethiopia.
- Ellouze E, Tahri N, Ben Amar R, (2012) Enhancement of textile wastewater treatment process using Nano-filtration.Desalination. 286: 16–23.
- Elouear ZJ, Bouzid BN, Feki M, Jamoussi F, Montiel A, et al. (2008) Heavy metal removal from aqueous solutions by activated phosphate rock. J. Hazard. Mater 156: 412–20.
- EPAÂ 542-F-12-001 (2012) Environmental Protection Emergency Response Agency (5102G).
- Fababuj RM, Mendoza-Roca JA, Galiana-Aleixandre MV, Bes-Piá A, Cuartas-Uribe B, et al. (2007) Reuse of tannery wastewaters by combination of  ultrafiltration and reverse osmosis after a conventional physical–chemical treatment.   Desalination, 204: (1–3), 219–226.
- Feng JW, Sun YB, Zheng Z, Zhang JB, Li S, et al. (2007) Treatment of tannery wastewater by electrocoagulation. Journal of Environmental Sciences, 19: 1409–15.
- Goltara A, Martinez J, Mendez R (2003) Carbon and nitrogen removal from tannery wastewater with a membrane bioreactor. Water Science and Technology. 48: 207–14.
- Kabdasli I, Tünay, O, Orhon, D (1993) The treatability of chromium tannery wastes. Water Science and Technology, 28(2), 97–105.
- Koteswari YN, Ramanibai R (2003) The effect of tannery effluent on the colonization rate of plankters: a microcosm study. Turk J Biol, 27: 163–70.
- Masud M, Baig M, Hassan I, Qazi A, Malik M, et al. (2001) Textile wastewater characterization and reduction of its COD & BOD by oxidation. Electronic Journal of Environment, Agriculture and Food chemistry
- McKenzie DJ, Shingles A, Claireaux G, Domenic P (2008) Sublethal Concentrations of Ammonia Impair Performance of the Teleost Fast Start Escape Response. Physiological and Biochemical Zoology (by The University of Chicago). 82: 353–62.
- Mitsch WJ, Gosselink JG (1993) Wetlands, 2nd Edition. Van Nostar and Reinhold, New York.
- NAS (National Academy of Science) (1974) Chromium-NAC.Washingten DC.USA.
- Noll KE, Gounaris V, Hou WSÂ (1991) Adsorption Technology for Air and Water Pollution Control, Lewis Pub, Michigan.
- Palmer CD, Wittbrodt PR (1991) Processes Affecting The Remediation Of Chromium Contaminated Sites. Environ. Health Perspect 92: 25–40.
- Prabhavathy C, De S (2010) Treatment of fat liquoring effluent from a tannery using membrane separation process: experimental and modeling. Journal of Hazardou Materials, 176: 1–3), 434–43.
- Preethi V, ParamaKalyani KS, Iyappan K, Srinivasakannan,C, Balasubramaniam, N, et al. (2009)    Ozonation of tannery effluent for removal of cod and color. Journal of Hazardous Materials, products 166: 150–4.
- Rajamanickam SV (2000) A Successful and Sustainable Hazardous Waste Management Technology Chromium Recovery and Reuse System in Tanneries of South Asia and Africa. Pollution Prevention/ Waste Minimization. India.
- Rameshraja D, Suresh S (2011) Treatment of tannery wastewater by various oxidation and combined processes. Int J Environ Res 5: 349–60.
- Reeves RD (2004) Nickel and Zinc Accumulation by Species of Thlapsi L, Cochlearia L, and other Genera of the Brassicaceae. Taxon 37: 309-18.
- Scholz WG, Rougeä P, Boädalo A, Leitz U(2005)  Desalination of mixed tannery effluent with membrane bioreactor and reverse osmosis treatment. Environmental Science and Technology. 39: 8505–11
- Singanan M, Alemayehu A, Singanan V (2007) Studies of the Removal of Hexavalent Chromium from Industrial Wastewater by Using Biomaterials. EJEAFChe. 6: 2557 - 64.
- Srinivasan S, Mary V, Kalyanaraman GPS, Sureshkumar PS, Sri Balakameswari K, et al. (2012) Combined advanced oxidation and biological treatment of tannery effluent. Clean Technologies and Environmental policy, 14: 251–6.
- Sundarapandiyan S, Chandrasekar R, Ramanaiah B. Krishnan S, Saravanan P, et al. (2010) Electrochemical oxidation and reuse of tannery saline wastewater. Journal of Hazardous Materials 180: 197–203.
- Suzuki M(1996) “Application of Adsorption Technology for Environmental Controlâ€, in Fundamentals of Adsorption. MD. LeVan. 3-14.
- Tiravanti G, Petruzzelli D, Passino R (1997) Pre-treatment of tannery wastewaters by an ion exchange process for Cr(III)removal and recovery. Water Science and Technology. 36: 2–3, 197–207.
- US 1999 Environmental Protection Agency (EPA). Update of Ambient Water Quality.
- USEPA (1993) Subsurface Flow Constructed Wetland for Wastewater Treatment. Technology Assessment. EPA/832/R/93/008.
- USEPA (2000) Introduction to Phytoremediation; Office of Research and Development, EPA/600/R99/107.
- USEPA (2004) Primer for Municipal Wastewater Treatment Systems. Office of Wastewater Management, Washington DC.
- USEPA (2008) Onsite Wastewater Treatment Technology Fact Sheet 9, Enhanced Nitrogen Removal - Nitrogen. EPA/625/R/00/008. Accessed on 45 - 52.
- Vidal G, Nieto J, Mansilla HD, Bornhardt C (2004) Combined oxidative and biological treatment of separated streams of tannery wastewater. Water Science and Technology. 49: 287–92.
- WHO (2011) Nitrate and Nitrite in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality.
Citation: Husen AA, Khwairakpam G (2021) Removal of Chromium and Some Selected Nutrients from Tannery Waste Water by Adsorption Methods of Sugar Bagasse Activated Carbon: Case Study on Addis Ababa Tannery Share Company. Environ Pollut Climate Change. 5: 223. DOI: 10.4172/2573-458X.1000223
Copyright: © 2021 Husen AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4390
- [From(publication date): 0-2021 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 3328
- PDF downloads: 1062