Removal of Methylene Violet from Aqueous Solutions Using BaSr2NbO5.5
Received: 22-Sep-2017 / Accepted Date: 26-Oct-2017 / Published Date: 30-Oct-2017 DOI: 10.4172/2155-9872.1000385
Abstract
The removals of Methyl violet from aqueous solutions using the double perovskites BaSr2NbO5.5 are reported. The oxides were prepared using solid state reaction in which the raw materials were mixed initially by either acetone or water. The X-ray diffraction measurements demonstrated that the preparation methods have influenced the cell volume, crystal size and surface area of the oxides. As consequence, the adsorption properties were also affected. The study showed that the prepared oxides have a cubic structure with crystallite size is less than 82 nm. However, the crystallite size of the oxide obtained by wet method (W) is higher than that of the oxide obtained by dry method (D). Consequently, the amounts of the dye adsorbed by BaSr2NbO5.5 (D) were higher than those adsorbed by BaSr2NbO5.5 (W). The maximum removal capacities of Methyl violet are found to be 8.71 and 9.39 mg/g using BaSr2NbO5.5 (W) and BaSr2NbO5.5(D) respectively. The removals of the dye have positive relationship with pH, temperature, the specific surface area and the mass of the oxide.
Keywords: Removal; Methylene violet; Perovskite oxides
Introduction
Organic dyes play a vital role in industry. They are used in textile dyeing, paper printing, photography, pharmaceutical, leather, food products etc. After usage, dyes are discharged to the water sources like river, seas and oceans etc. [1,2]. The discharge of such substances into the water is dangerous since many of these are toxic, mutagenic and/or carcinogenic to living beings [3]. For instances, methylene blue, organic dye usually used in cotton and wool manufacturing, can cause serious health problems for human such as vomiting, hard breathing and mental disorder. Dyes can also affect biodegradation, light penetration and photosynthesis producing imbalance in the ecosystem [4]. Hence, the development of an effective treatment technique that can remove or convert such pollutants into non-toxic or less harmful materials is highly required. Examples of wastewater treatment methods are filtration, chemical precipitation, electrolysis, sedimentation, oxidation, photo degradation and adsorption. Adsorption is considered as an effective method in term of initial cost, simplicity of construction and insensitivity of toxics. Thus, a wide variety of natural and synthetic materials such as activated carbon, peat, various silica, activated clay, banana pith, natural manganese mineral, oil ash, goat hair, alum sludge, natural zeolite, mixtures of flash, soil and Nano materials have been investigated as dye adsorbents.
Nanomaterials exhibits unusual properties compared to their bulk counterpart due to the large surface area to volume ratio in the nanoscale. Naturally, their material properties and applications largely depend on size, distribution and morphology of the nanomaterial, essentially allowing them to exhibit completely new or improved properties. Nanomaterials find applications to address technological and environmental challenges including of solar energy conversion, catalysis, medicine, and water treatment. Among Nano materials, perovskite oxides have been proven to be of interest due to their efficiency in adsorbing and degrading recalcitrant organic compounds [5]. Generally, perovskite type oxides are containing of two different cations, each surrounded by oxygen anions. The larger cation which can be alkali, alkaline earth, or rare earth has dodecahedral symmetry into the framework. Whilst the smaller cation which can be a transition metal ion, is six coordinates. This structure can tolerate significant non-stoichiometry and partial substitution. Perovskite can also accommodate different combinations of cations, so long as the crystal charge is neutral [6].
This work investigates the removal of Methyl violet 10 B (MV) from aqueous solutions using the ordered deficient perovskite BaSr2NbO5.5. The oxide has been synthesized by solid state reaction but used different slurry media, namely acetone and water. The purpose of the solvents is to enhance the homogeneity of the mixtures, and to create dry (D) and wet (W) media for the initial reaction. The main aim of the study is to examine the impact of an aqueous slurry medium on the adsorption properties of the oxides [7-9].
The ordered defect double perovskites of the type A2B*BO5.5 where A and B*=Ca2+, Sr2+ or Ba2+ and B=Sb5+, Nb5+ or Ta5+ are of current interest, since the mechanism of water interaction and the proton diffusion in their structure are critical in determining the physical properties of such systems [10,11]. The title double perovskites exhibit ionic conductivity [12] and can absorb signi icant amounts of water to become proton conductors [13,14]. Unlike stoichiometric ABO3 perovskites, in which all the B-type cations are six coordinates, the effective coordination number of the cations in anion-de icient A2B*BO5.5 perovskites are reduced from six, so that locally some Btype cations will be five, or even four, coordinate [15]. Water can be incorporated into the lattice by occupying either the vacancies or nearby interstitial sites. The site which the incorporated water occupies will be dependent on the differences in the coordination tendencies of the B* and B cations [15].
Methyl violet 10B (MV) is known in medicine as Gentian violet and is the active ingredient in a Gram stain, used to classify bacteria [16]. It is used as a pH indicator, with a range between 0 and 1.6. Compounds related to methyl violet are potential carcinogens. Methyl violet 10B inhibits the growth of many Gram positive bacteria, except streptococci. It is soluble in water, ethanol, diethylene glycol and dipropylene glycol [17]. Methyl violet is a mutagen and mitotic poison, therefore concerns exist regarding the ecological impact of the release of methyl violet into the environment. Methyl violet has been used in vast quantities for textile and paper dyeing, and 15% of such dyes produced worldwide are released to environment in wastewater [18].
Experimental
Sample preparation
The preparation of samples involved different stoichiometric compositions of Nb2O5 (Merck, 99.99%) and SrCO3 or BaCO3 (BDH, 99.98-99.99%). The mixtures were initially ground, as either an acetone or aqueous slurry, and preheated at 850°C for 12 h, and then reground and heated at 1100°C for 48 h.
Instrumentations
The crystallography of the samples was examined by a PANalytical X’Pert X-ray powder diffraction using Cu Kα radiation (1.5400 ?) and a PIXcel solid-state detector. he operating voltage was 40kV and the current was 30 mA. he samples were measured in lat plate mode at room temperature with a scan range of 10°<2θ<80° and a scan length of 10 mins were used. The microstructure of the powder samples was examined by scanning electron microscopy using an Intellection Quemscan.
The absorbance of solutions was determined using ultraviolet visible spectrophotometer (UV/Vis, model Spect-21D) and (190-900 Perkin- Elmer) at maximum wavelength of absorbance (590 nλ). The concentrations of solutions were estimated from the concentration dependence of absorbance fit. The pH measurements were carried out on a WTW720 pH meter model CT16 2AA (LTD Dover Kent, UK) and equipped with a combined glass electrode.
Batch mode
Batch mode removal studies were carried out by varying several parameters such as contact time, pH, temperature and mass of prepared oxide (adsorbent). Essentially, a 50 ml of dye solution with concentration of 50 ppm was taken in a 250 ml conical flask in which the initial pH was adjusted using HCl/NaOH. Optimized amount of adsorbent was added to the solution and stirred using magnetic stirrer for specific time. The oxide samples were separated from solutions using centrifuge 3500 CPM for 5 minutes.
Results And Discussion
Characterization of oxides
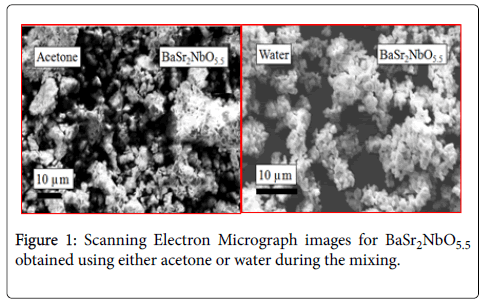
Visually, it was observed that the colour of the powdered compounds obtained using either acetone or water during the mixing is slightly different. For example, powders of BaSr2NbO5.5 are dark yellow when prepared using acetone whereas it is light yellow if water is used in the initial mixing. The hardness of the powders was found to be dissimilar; generally, samples are much smoother after mixing with water. SEM analysis also revealed that the particle sizes were not equal. These surprising observations indicate that the presence of water in the initial stage of the reaction leads to larger crystallites and this is probably the basis for the differences in appearance and texture. Figure 1 demonstrates SEM images for BaSr2NbO5.5. The grains are monophasic and apparently free of impurities.
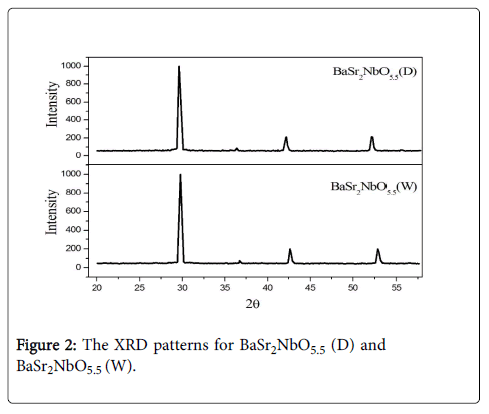
X-ray diffraction measurements showed the oxides had a faced cubic structure with space group (Fmm). Figure 2 illustrates the XRD patterns of the oxides. The Average Crystallite size Dp, specific surface area S, lattice strain φ, Lattice parameter a and Cell volume V estimated from X-ray diffraction data are summarised in Table 1. The crystallite size can be calculated using sheerer formula [19] (Equation. 1) where the specific surface area can be calculated using Sauter formula [20] (Equation.2) in which ρ is the density of the synthesised material. It is clearly seen that the average crystallite size and the cell volumes of the oxide obtained using water (W) in initial mixing is larger than those of the oxide obtained using acetone (D) reflecting the importance of synthesis method.
Dp= (0.94λ)/(β1/2×cosΘ) (1)
S = 6000/ (Dp×ρ) (2)
| Formula | Dp (ηm) | (g/cm3) | S (m2/g) | φ | a (Å) | V (Å3) |
|---|---|---|---|---|---|---|
| BaSr2NbO5.5 (D) | 50.24 | 5.419 | 22.04 | 0.0028 | 8.4599(1) | 605.476(2) |
| BaSr2NbO5.5 (W) | 72.80 | 5.384 | 15.31 | 0.0019 | 8.4733(5) | 608.554(7) |
Table 1: Average Crystallite size Dp, Specfic surface area S, lattice strain φ, Lattice parameter a and Cell volume V. estimated from X-ray diffraction data.
Batch mode
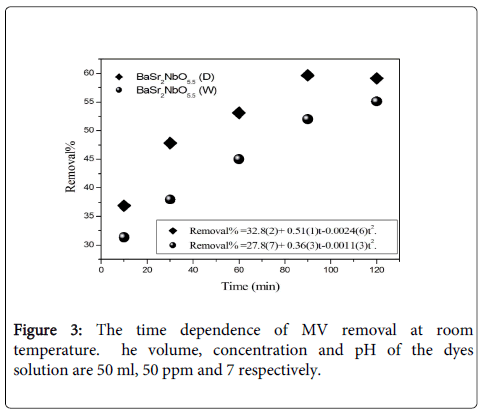
Effect of contact time: The removal percentage of dyes over the adsorbents can be calculated as: R%=[(Ci-Ct)/Ci]×100, where R% is the removal percentage, Ci=50 ppm is initial concentration of dye solutions, Ct is the concentration of dye at contact time estimated from the concentration dependence of absorbance fit. The effect of contact time on the MV removal was observed at the range of (0-120 min). Figure 3 shows the time dependence of MV removal at room temperature. There is no finite time was observed for the dye removal up to 120 min. The removals of the dye increase as the contact time increases. The removals of MV using BaSr2NbO5.5 (D) were larger than those using BaSr2NbO5.5 (W). This is due to the differences in the crystallite size and the specific surface area of the two oxides. The surface area of BaSr2NbO5.5 (D) is found to be larger than that of BaSr2NbO5.5 (W) [21].
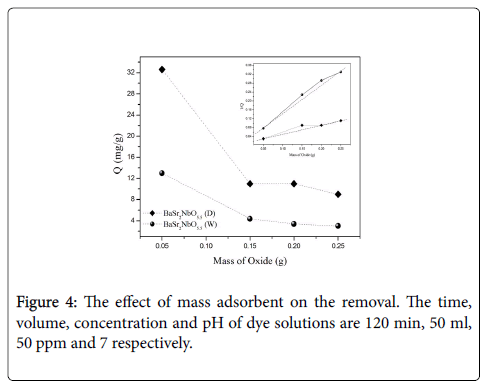
Effect of adsorbent mass: The amount of the dye adsorbed by one gram of the oxides (Q) was calculated as following: Q(mg/g)=[(Ci-Ct) ×V]/W, where t=120 min is the contact time, V=50 ml is the volume of dye solution and W is the mass of oxides. As shown in Figure 4. Q decreases as the mass of adsorbents increased.
The maximum capacity of adsorbent Qmax can be estimated from the intercept of the liner it of 1/Q at Y axis. It was found that BaSr2NbO5.5 (D) has Qmax (57.40 mg/g) higher than that for BaSr2NbO5.5(W) (47.52 mg/g). This result is consisted with the surface area of the oxides (Table 1). Qmax increased as the surface area of the oxides increased. The amounts of MV adsorbed by BaSr2NbO5.5 (W) which has the larger crystallite size (72.8 nm) and the smaller speci ic surface area (15.31 m2/g) are much lower than those adsorbed by BaSr2NbO5.5 (D) which has the smaller crystallite size (50.24 nm) and the larger speci ic surface area (22.04 m2/g).
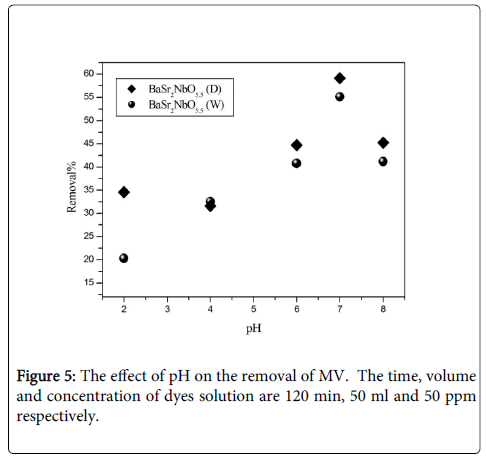
Effect of pH: To study the effect of pH, experiments were carried out at various pH values, ranging from 2 to 8 for constant dye concentration (50 ppm) and adsorbent mass (0.1 g). Figure 5 shows the removal of dyes as a function of pH. It was observed that the removal of MV on the oxides increases as pH increased. The highest removal of MV was recorded f or BaSr2NbO5.5 (D) at pH=7 around 59% where the lowest removal of MV was recorded for BaSr2NbO5.5 (W) at pH=2 around 21%. The interpretation of pH effects on the efficiency of the adsorption process is a very difficult task, because of its multiple roles. It is related to the acid base property of both the metal oxide and the organic dye. The adsorption of water molecules at metal sites is followed by the dissociation of OH− groups, leading to coverage with chemically equivalent metal hydroxyl groups (M-OH). Due to amphoteric behaviour of both the metal oxide and the organic dye, the equilibrium reactions below are considered. The electrostatic interactions between the positive catalyst surface and dye anions leading to strong adsorption of the last on the oxide support.
M-OH+H+ ↔ M-OH2+.
M-OH ↔ M-O-+H+.
Dye-OH+H+ ↔ Dye++H2O.
H-Dye+OH- ↔ Dye-+H2O.
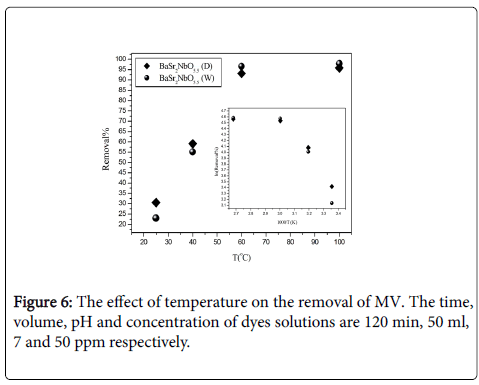
Effect of temperature: Temperature has an important impact on the adsorption process. An increase in temperature helps the reaction to compete more efficiently with e–/H + recombination. The removal of two dyes was investigated at 25, 40, 60 and 100°C. The obtained results are illustrated below in Figure 6.
The removal of MV dye increased as temperature increased. For instance, the removal of MV increased from ~55% at 25°C to ~95% at 100°C. This result is agreed with normal expectations, and is a consequence of the increase of adsorption strength and the concentration of active intermediates with temperature. The energy of activation (Ea), was calculated from the Arrhenius plot of ln (Removal %) vs 1000/T . Arrhenius plot shows that the activation energies of the removal of MV are positive and equal to 6.03 and 7.17 kJ/mole for BaSr2NbO5.5 (D) and BaSr2NbO5.5 (W) respectively. This realects the differences in the strength of the interaction forces between the two dyes and the oxides.
Conclusion
The removal of Methyl Violet from aqueous solution by the defected double perovskite BaSr2NbO5.5 has been studied. The oxides were synthesised by solid state reaction but using two different slurry media namely water and acetone. The BaSr2NbO5.5 (W) oxide obtained by wet method have smaller speci ic surface area than the BaSr2NbO5.5 (D) oxide obtained by dry method. Consequently, the amounts of MV adsorbed by BaSr2NbO5.5(D) were higher than those adsorbed by BaSr2NbO5.5(W).
In general, the study showed no finite time for the two dyes removal up to 120 min. The removals of both MV dyes increased as the physical parameters: time, temperature, pH, adsorbent mass increase. The highest removal efficiency was recorded for MV dye using BaSr2NbO5.5 (W) at pH=6 where the lowest removal was observed BaSr2NbO5.5 (D) at pH=2. The study indicated that the preparation methods has in luenced the texture and adsorption properties of the oxide.
References
- Baban A, Yediler A, Ciliz NK (2010) Integrated Water Management and CP Implementation for Wool and Textile Blend Processes. CLEAN – Soil, Air, Water 1: 84-90.
- Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technology 3: 247-255.
- Carmen Z (2012) Textile Organic Dyes–Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents – A Critical Overview. InTech.
- Hao OJ, Kim H, Chiang PC (2000) Decolorization of Wastewater. Critical Reviews in Environmental Science and Technology 4: 449-505.
- Ruthven DM (1984) Principles of Adsorption and Adsorption Processes. Wiley.
- Ishihara T (2009) Structure and Properties of Perovskite Oxides. Springer, US, Perovskite Oxide for Solid Oxide Fuel Cells.
- Wenk HR, Bulach AG (2005) Minerals: Their Constitution and Origin. Cambridge University Press, Cambridge.
- Sporn D, Merklein S, Grond W, Seifert S, Wahl S, et al. (1995) Sol-gel processing of perovskite thin films. Microelectronic Engineering 29: 161-168.
- Browall KW, Muller O, Doremus RH (1976) Oxygen ion conductivity in oxygen-deficient perovskite-related oxides. Materials Research Bulletin 12: 1475-1481.
- Ross NL, Angel RJ, Finger LW, Hazen RM, Prewitt CT (1987) Oxygen-Defect Perovskites and the 93-K Superconductor. Journal of American Chemical Society 351: 164-172.
- Lecomte J, Loup JP, Hervieu M, Raveau B (1981) Non-stoichiometry and electrical conductivity of strontium niobates with perovskite structure. Physica Status Solidi 2: 743-752.
- Nowick AS, Du Y (1995) High-temperature protonic conductors with perovskite-related structures. Solid State Ionics 77: 137-146.
- Glöckner R, Neiman A, Larring Y, Norby T (1999) Protons in Sr3(Sr1+xNb2-x)O9-3x/2 perovskite. Solid State Ionics 125: 369-376.
- Animitsa I, Neiman A, Sharafutdinov A, Nochrin S (2000) Strontium tantalates with perovskite-related structure. Solid State Ionics pp: 136-137.
- Zhou Q, Kennedy BJ, Avdeev M (2011) Thermal expansion behaviour in the oxygen deficient perovskites Sr2BSbO5.5 (B=Ca, Sr, Ba). Competing effects of water and oxygen ordering. Journal of Solid State Chemistry 9: 2559-2565.
- Books H (2011) Articles on Triarylmethane Dyes, Including: Phenolphthalein, Methyl Violet, Bromothymol Blue, Coomassie Brilliant Blue, Bromophenol Blue. Malachite Gr, Hephaestus Books.
- Langford JI, Wilson AJC (1978) Scherrer after sixty years: A survey and some new results in the determination of crystallite size. Journal of Applied Crystallography 2: 102-113.
- Nogi K, Hosokawa M, Naito M, Yokoyama T (2012) Nanoparticle Technology Handbook. Elsevier.
- Michael Mozurkewich SWB (1984) Negative activation energies and curved Arrhenius plots. 1. Theory of reactions over potential wells. Journal of Physics and Chemistry 88: 6429.
Citation: Awin LA, El-Rais MA, Baghni EI, Etorki AM (2017) Removal of Methylene Violet from Aqueous Solutions Using BaSr2NbO5.5. J Anal Bioanal Tech 8:385. DOI: 10.4172/2155-9872.1000385
Copyright: © 2017 Awin LA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4911
- [From(publication date): 0-2017 - Jun 11, 2025]
- Breakdown by view type
- HTML page views: 4091
- PDF downloads: 820